Abstract
Objective
The effects of type 2 resistant starch from high-amylose maize (HAM-RS2) in rodents fed with low-fat diets were demonstrated in previous studies. Fish oil is also reported to reduce body fat. In the current study, the effects of high fat and fish oil on HAM-RS2 feeding in rats were investigated.
Design and Methods
Rats were fed 0 or 27% (weight) HAM-RS2 with low (15% energy) or high fat (42% energy) diets that included 0 or 10% (energy) tuna oil to test the effect of HAM-RS2 in diet-induced obesity and effects of tuna oil. Data were analyzed as 2 × 2 × 2 factorial.
Results
Rats fed HAM-RS2 had decreased cecal contents pH, increased cecal and cecal contents weight, increased cecal contents acetate, propionate, and butyrate, increased GLP-1 and PYY, and decreased abdominal fat. However, high fat partially attenuated effects of HAM-RS2, but increased GLP-1 active. Dietary tuna oil had limited effects at concentration used.
Conclusions
Results demonstrated that a high fat diet partially attenuates the response to HAM-RS2. The mechanism may center on reduced levels of cecal contents propionate and butyrate and reduced serum PYY. This study demonstrated that with consumption of high fat, HAM-RS2 produces fermentation but results in partial attenuation of effects.
Introduction
We have previously demonstrated that the addition of a specific type 2 resistant starch from high-amylose maize (HAM-RS2) to a low-fat (7% of weight, 18% energy) diet was associated with increased fermentation and reduced abdominal body fat (1,2). The reduced body fat was associated with increased gene expression of pro-opiomelanocortin in the arcuate nucleus of the hypothalamus (3); and increased oxidation of fat as demonstrated by a decreased respiratory quotient (4). However, this reduction in body fat was not associated with a decrease in energy intake (1,3,4). Use of a moderate-fat diet (11% by weight, 28% energy) in mice (4) and an endocrine model of obesity, ovariectomized (OVX) rats (5), and a diabetic model, GK rats (6), fed a low fat diet also resulted in reduced body fat with the feeding of HAMRS2. In the current study, we investigated the effects of HAMRS2 in a rat model of diet-induced obesity using a high fat diet (20% by weight, 42% energy). Fish oil is reported to have an opposite effect on body fat as other types of fat resulting in reduced body fat (7), and, therefore tuna oil (4% by weight, 8.4% energy) was tested in low and high fat diets. Other fermentable carbohydrates have been reported to be effective in promotion of fermentation with consumption of high fat diets (8,9). Based on the results from these past studies, it was hypothesized that fermentation and other effects associated with dietary HAM-RS2 in low and moderate-fat diets and OVX rats would be observed, would likely be observed with feeding of a high-fat diet; and tuna oil should further increase fermentation and decrease body fat in rats fed with low- and high-fat diets.
Methods
Rats and diets
Ninety-six male Sprague-Dawley rats (6 weeks-old) were purchased from Harlan Co. (Indianpolis, IN). Upon arrival, the rats were in quarantine for one week in multi-housed in shoebox cages and fed chow. After quarantine, the rats were housed another week singly in wire-bottom cages and acclimated to a powdered, low-fat control diet. The rats were weighed after the week of acclimation and then stratified by weight and assigned to eight treatment groups (n = 12). During the 12-week study, the rats were weighed and food intake and spillage were measured two times per week. The study protocol was approved by the Louisiana State University Institutional Animal Care and Use Committee.
The rats were assigned to eight treatment diets (Table 1) as a 2 × 2 × 2 factorial with two concentrations of HAM-RS2 (0 and 27% weight of diet), two concentrations of fat (6 and 20% weight of diet or 14 or 42% of energy), and two concentrations of tuna oil (0 or 4% weight of diet, 8.4% energy). The four low-fat diets had a metabolizable energy value of 3.4 kcal/g and the high-fat diets had a value of 4.2 kcal/g. The concentration of HAM-RS2 used in the current study is the same as used in previous proof-of-concept studies with low- (1,3) and moderate-fat (4) diets, GK diabetic rats (6), OVX rats (5), and a study investigating glycemic index (10). All low fat diets and high fat diets were made isocaloric using cellulose in the control diets without HAM-RS2. This has also been done in previous research. Purified cellulose is considered not fermentable in rats and, thus, has a metabolizable energy of 0 kcal/g (11). HAMRS2 has a metabolizable energy of 2.8 kcal/g (12). Using this energy value, we have estimated that about 50% of the HAM-RS2 is fermented at dietary concentrations used, and this allows us to estimate that control and HAM-RS2 diets are similar in non-fermentable fiber and to test the effects of fermentable fiber of HAMRS2.
TABLE 1.
| Ingredients (g) | LC | LR | HC | HR |
|---|---|---|---|---|
| Amioca® cornstarchc | 531.9 | 171.9 | 391.9 | 31.9 |
| Hi-maize® cornstarchc | 0 | 480.0 | 0 | 480.0 |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 |
| Casein | 140.0 | 140.0 | 140.0 | 140.0 |
| Cellulose | 120.0 | 0 | 120.0 | 0 |
| Corn oil | 60.0 | 60.0 | 100.0 | 100.0 |
| BHT (0.0002 g/g oil) | 0.012 | 0.012 | 0.040 | 0.040 |
| Lard | 0 | 0 | 100.0 | 100.0 |
| Mineral mix | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 | 10.0 | 10.0 |
| Choline chloride | 1.3 | 1.3 | 1.3 | 1.3 |
| l-Cystine | 1.8 | 1.8 | 1.8 | 1.8 |
| Total | 1,000.0 | 1,000.0 | 1,000.0 | 1,000.0 |
| kcal/g | 3.40 | 3.47 | 4.16 | 4.22 |
Acronym letters for the diets include: L = low-fat, H = high-fat, C = control, R =resistant starch (high amylose maize type 2, HAM-RS2).
Each of the four diets listed in the table were replicated (total of eight diets) with the inclusion of 83.3 g/1,000 g of diet of an encapsulated powder product of tuna oil (Nu-MegaVR was a gift from Clover Corporation, Gymea, N.S.W., Australia). Components of the product included 12.5 g protein, 14.2 g dextrose, 13.3 g glucose, 40 g fat as tuna oil, 2.5 g minerals, and 0.8 g vitamins. Diet components were reduced accordingly to compensate for components of the encapsulated product: casein 127.5 g, sucrose 72.5, corn oil 20 g, mineral mix 32.5 g, and vitamin mix 9.2 g.
Amioca® cornstarch and Hi-maize® cornstarch were gifts from Ingredion Incorporated (Bridgewater, NJ). The former is 100% amylopectin (waxy cornstarch) and essentially 100% digestible; while the latter was estimated by Ingredion Incorporated to contain 56% resistant starch. Thus, diets with Hi-maize® cornstarch are estimated to contain 480 g Hi-maize® × 0.56 = 26.9% HAM-RS2.
Study procedures
At the end of the 12-week study, several samples were taken from the rats. Blood serum was obtained from non-fasting rats by cardiac puncture (5% isoflurane anesthesia). A dipeptidyl peptidase IV inhibitor was added to the collection tubes for blood serum for proper assay of active glucagon-like peptide 1 (GLP-1). The GI tract was removed from the end of the esophagus to the anus, cleaned of attached fat, and weighed. Then the stomach, small intestine, cecum, and the rest of the large intestine were weighed individually with their contents and with their contents removed. The final body weight of the rats was obtained the day rats were killed and the GI tract weight with contents was subtracted from that body weight to obtain a disemboweled body weight (DBW). Then the individual GI tract component weights without contents (empty) were added to the DBW to calculate an emboweled body weight (EBW). The EBW was used to determine the percent of body weight of the abdominal fat pads. Epididymal, perirenal, and retroperitoneal fat pads were removed from the abdominal cavity and weighed. For measurements of short-chain fatty acids and pH, the cecal contents were collected and frozen for later analysis. Thawed cecal contents were homogenized in distilled water (0.5 g wet sample to 5 ml of water) and pH was measured using a combination electrode. Samples were then acidified with 1 ml of a 25% (wt/wt) solution of metaphosphoric acid that contained 2 g/l 2-ethyl-butyric acid as an internal standard for short-chain fatty acid contents. Solids in the homogenized samples were separated by centrifugation at 8,000g for 10 min. Before use, the sample was filtered through a Millipore filter (MILX HA 33 mm, 0.45 μm MCE STRL; Fisher SLHA 033SS). Short-chain fatty acids in the effluent were analyzed by gas–liquid chromatography by a method similar to Barry et al. (13). Briefly, the column was an Alltech (Nicholasville, KY) Econo-cap EC-1000, 100% polyethylene glycol acid modified with dimensions of 15 m × 0.53 mm with a film thickness of 1.20 μm. The program for temperature control was: 115°C for 0.1 min, increase rate of temperature 10°C per minute up to 150°C and held for 0.1 min, then increased the temperature at 11°C per minute up to 170°C and held for 2 min. The injector temperature was 250°C.
Assays
Plasma assays were conducted using commercial kits. Serum active (7-36) and total GLP-1 (7-36 and 9–36) were measured using ELISA kits (ALPCO Diagnostics, Salem, NH). The human kits are effective in measuring the rodent hormone and the intra-assay variations for the kits are CVs of 2.5–5.4% and 3.7–4.7%, respectively. Rat/mouse PYY (1–36 and 3–36) from serum was measured using a radioimmunoassay kit (Millipore Corporation, St. Charles, MO) with an intraassay variation of 3.2–3.9%. Serum insulin (rat sensitive) was measured using an ELISA kit (Millipore) with an intra-assay variation of 2.7–5.8%, and glucose with an intra-assay variation of 3% was analyzed by a spectrophotometric method (QuantiChrom Assay; BioAssay Systems, Hayward, CA).
Statistical analysis
Data from the study were analyzed as a 2 × 2 × 2 factorial with the three factors described above in “Rats and Diets.” All dependent variables except for EBW and energy intake were log10 transformed for statistical analyses as original data demonstrated they were not normally distributed with significance at w < 0.05 with the Shapiro–Wilk test. Results were considered statistically significant at P < 0.05 and the analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Data are presented in their original form as least square means (lsmeans) ± pooled SE.
Results
Food and energy intakes
There was no main effect of HAM-RS2 on either food or energy intakes (Table 2). However, main effects of fat and fish oil were observed. High fat in the diet promoted reduced food intake, but this still resulted in increased energy consumption due to the increased energy density of the high fat diet. Fish oil promoted increased food and energy intakes. There was a significant interaction between HAM-RS2 and fat for both food and energy intakes. Rats fed HAM-RS2 in a low-fat diet consumed greater food and energy intake than rats not fed HAM-RS2. However, rats consumed less food and energy with a high fat diet when HAM-RS2 was included in the diet compared to rats not fed HAM-RS2.
TABLE 2.
Cumulative food and energy intakes for the 12-week studya
| Groupb | LC | LR | LFO | LRFO | HC | HR | HFO | HRFO | Pooled SEM |
|---|---|---|---|---|---|---|---|---|---|
| Food intake (g) | 1501.1 | 1537.1 | 1562.5 | 1570.6 | 1394.0 | 1370.3 | 1433.8 | 1365.1 | 19.3 |
| Energy intake (kcal) | 5253.9 | 5379.9 | 5468.6 | 5497.1 | 5854.9 | 5755.4 | 6022.1 | 5733.5 | 73.3 |
Acronym letters for the groups include: L = low-fat, H = high-fat, C = control, R = resistant starch (high amylose maize type 2, HAM-RS2), FO = fish oil.
Data were analyzed as a 2 × 2 × 2 factorial with fat as low or high, RS as ±, and FO as ±. Significant effects for both food intake and energy intake included fat, P < 0.0001; FO, P < 0.02; RS*Fat, P < 0.01. Food intake data were log10 transformed for statistical analysis.
Fermentation variables
Fermentation variables are collapsed from eight to four groups and listed for the independent variables fat and HAM-RS2 in Table 3 as these variables were not affected by the independent variable fish oil. The presence of HAM-RS2 in the diet resulted in significant increases in most of the fermentation variables measured that included increased amount of cecal contents, reduced pH of cecal contents, increased empty cecal weights, and increased short chain fatty acid (SCFA) amounts in the total amount of cecal contents. Note that the concentrations of SCFA in cecal contents were not different between RS and non-RS groups (data not shown).
TABLE 3.
| Groupc | LC | LR | HC | HR | Pooled SEM | RS | Fat | RS*Fat |
|---|---|---|---|---|---|---|---|---|
| Cecal contents (g) | 2.46 | 14.00 | 2.57 | 9.95 | 0.64 | 0.0001 | 0.02 | 0.002 |
| pH cecal contents | 7.05 | 6.17 | 7.09 | 6.23 | 0.06 | 0.0001 | NS | NS |
| Empty cecum (g) | 0.60 | 2.25 | 0.58 | 1.65 | 0.09 | 0.0001 | 0.002 | 0.02 |
| Acetate (mmol) | 0.17 | 0.85 | 0.17 | 0.81 | 0.06 | 0.0001 | NS | NS |
| Propionate (mmol) | 0.03 | 0.16 | 0.02 | 0.12 | 0.01 | 0.0001 | 0.04 | NS |
| Butyrate (mmol) | 0.04 | 0.24 | 0.02 | 0.16 | 0.02 | 0.0001 | 0.07 | NS |
| Total C2-C8 (mmol) | 0.25 | 1.28 | 0.23 | 1.13 | 0.07 | 0.0001 | NS | NS |
Acronym letters for the groups include: L = low-fat, H = high-fat, C = control, R = resistant starch (high amylose maize type 2, HAM-RS2), and RS = resistant starch (HAM-RS2).
Data are shown collapsed on two factors, fat (low and high) and RS (±) from the 2 × 2 × 2 factorial because there were no significant effects of the third factor, fish oil (±). All markers of fermentation had significant effects of RS and several had significant effects of fat. P values are shown in last three columns on right. All data were log10 transformed for statistical analyses.
Samples analyzed for the original eight groups for the short-chain fatty acids range from 8 to 12 per group.
The level of fat in the diet did not affect the pH of the cecal contents as far as the effect of HAM-RS2 in lowering the pH. However, the presence of high fat in the diet reduced the amount of cecal contents, empty cecal weights, and amounts of propionate and butyrate (P = 0.07) in the cecal contents. There also were significant interactions for these dependent variables as the decreases with high fat were observed only in the groups that were fed HAM-RS2.
Plasma assays
There were no differences among the groups for plasma glucose and insulin when the rats were killed in the fed state (data not shown).
The presence of HAM-RS2 in the diet resulted in increased amounts of serum GLP-1 active and total GLP-1 (Figure 1A,B). The effect of high fat on serum GLP-1 active was an overall increase. Regarding total GLP-1, there was a two-way interaction between fat and fish oil that approached significance (P = 0.09). Rats fed with the high fat diet with fish oil had numerically higher concentrations than rats fed with the low fat diet with added fish oil. Several individual and interactive effects of fish oil on GLP-1 active and total before conversion of the data to log10 looked interesting, but after conversion only one interaction remained as approaching significance.
Figure 1.
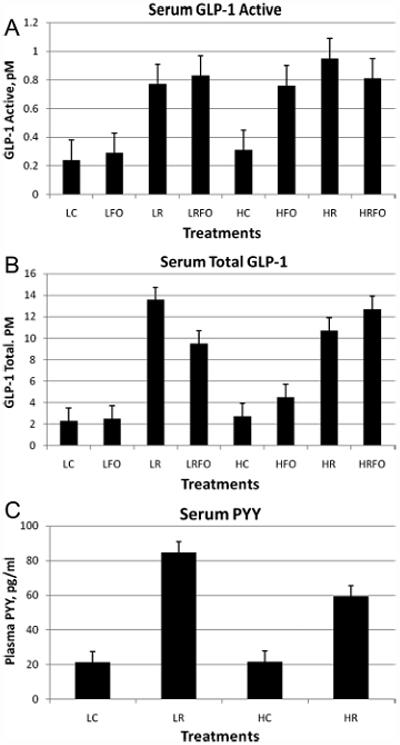
(A) Serum GLP-1 active (n = 12 for non-fish oil groups and n = 7–8 for fish oil groups), (B) Serum GLP-1 total (n = 11 all groups), and (C) Serum PYY (n = 12 all groups). Data were log10 transformed for statistical analyses. Significant or approaching significant effects of results for (A) were RS (HAM-RS2) P < 0.0001, fat P < 0.02; for (B) were RS P < 0.0001, fat*fish oil P < 0.09; and for (C) were RS P < 0.0001, RS*fat P < 0.08. Acronym letters for the figures include: L = low-fat, H = high-fat, C = control, R = HAM-RS2, and FO = fish oil.
Plasma PYY was increased in the groups that were fed HAM-RS2, and tended (RS*fat P = 0.08) to be reduced in the groups fed with high fat (Figure 1C). There were no effects or effects approaching significance of fish oil, so the data were collapsed on RS and fat.
Abdominal fat and body weight
Abdominal fat and abdominal fat as a percentage of the EBW were reduced in rats that fed HAM-RS2 (Figure 2A,C). However, the effect was partially attenuated numerically by including high fat in the diet. The two-way interaction for the percentage of abdominal fat approached significance (P = 0.07). The effect of resistant starch on reduction of the EBW approached significance (P = 0.07), but rats that fed the high fat diet had greater EBWs (Figure 2B).
Figure 2.
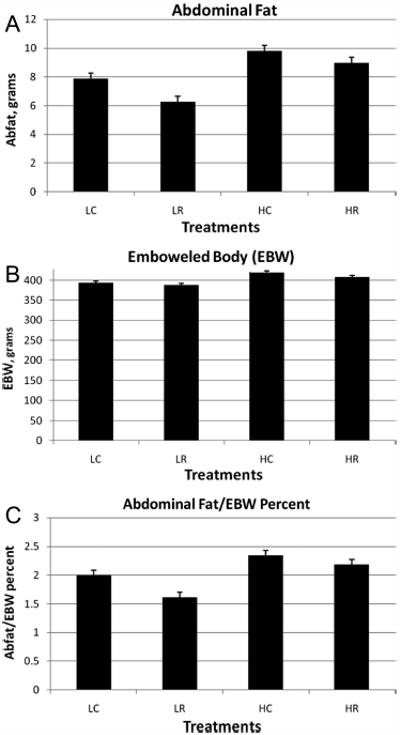
(A) Abdominal cavity fat, (B) EBW (body weight with GI tract present and contents removed), and (C) abdominal cavity fat/EBW × 100. Data for (A) and (C) were log10 transformed for statistical analyses. LSmeans were collapsed on RS (HAM-RS2) and fat as there were no significant effects for fish oil with these dependent variables. Significant or approaching significant effects of results for (A) were RS P < 0.003, fat P < 0.0001; for (B) were RS P = 0.07, fat P < 0.0001; and for (C) were RS P < 0.0004, fat P < 0.0001, RS*fat P = 0.07. Acronym letters for the figures include: L = low-fat, H = high-fat, C = control, and R = HAM-RS2.
Discussion
In our previous studies, we have demonstrated several beneficial effects of HAM-RS2 in rodents that fed a low fat diet. Such benefits included the following: improvement in fermentation, increased production of plasma GLP-1 and PYY, improved insulin sensitivity, increased gene expression of proopiomelanocortin in the hypothalamic arcuate nucleus in the brain, a decreased respiratory quotient, improvements in diabetic rats, and decreased abdominal fat (1,3,4,6,14). Additionally, we have reported that dietary HAM-RS2 was associated with lower body fat in mice that were fed a moderate fat diet (4) and in a low fat diet fed to OVX rats (5), a model of endocrine obesity. In the present study, we investigated the effects of HAM-RS2 included in a diet-induced obesity study comparing the low-fat diet with a high-fat diet. Tuna oil was also tested as fish oil is reported to have an opposite effect on body fat compared to other sources of fat (7). Several studies have reported using fructoligosaccharide (FOS), a fermentable carbohydrate, in conjunction with a high fat diet. Such studies report that FOS is effective in producing beneficial health effects similar to those we have previously published for HAM-RS2 in a low fat diet (8,15). The prebiotic, FOS, added to a high fat diet also reduces the metabolic endotoxemia reported with the feeding of a high fat diet (15). Thus, we anticipated that HAM-RS2 would also be associated with reduced body fat with the feeding of a high fat diet, and that tuna oil should also enhance the fermentation and the reduction of body fat associated with the feeding of HAM-RS2 in both low- and high-fat diets.
However, results of this study show that the effects of HAM-RS2 were partially reduced when rats were fed a high fat compared to a low fat diet with resistant starch. Reductions included several fermentation variables (weight of cecal contents, empty cecal weight, total amounts of propionate and butyrate (P = 0.07) in cecal contents), plasma PYY (RS*fat P = 0.08), and abdominal fat (RS*fat P = 0.07). Such findings indicate that rats respond differently to HAM-RS2 with a high fat diet, and consideration should be given to this partial attenuation and difference in response (16). The specific mechanism by which HAM-RS2 reduces body fat may be a reflection of the amount of propionate and butyrate in the cecal contents, which were partially attenuated with the feeding of HAM-RS2 in a high fat diet (17).
While the rats fed the high fat diet still responded to the HAM-RS2, the positive health implications were partially attenuated when compared to the low fat resistant starch groups. There was still evidence of fermentation, but the rats that fed the resistant starch in a high fat diet consumed greater energy over the study than the rats that fed the resistant starch in a low fat diet (Table 2). This likely inhibited the numerical effect of HAM-RS2 on reducing body fat (RS*fat P = 0.07). Because only partial attenuation occurred with HAM-RS2 in a high fat diet, such findings indicate the consumption of HAM-RS2 may remain beneficial for many Americans that consume diets with fat levels above recommendations and often very high in saturated fat sources. From 1999 through 2008, average intakes of total fat and saturated fat were 34% and 11% of the energy, respectively (18). The high fat diets in the present study were 20% of the weight of the diet ingredients and 42% of the energy of the diet. Also, 50% percent of the high fat diet was saturated fat by weight of the diet. This level of fat is within the upper ranges of human consumption of Americans (19). In a previous study, consumption of a diet with moderate fat (11% by weight and 28% by energy) did not attenuate reduction of body fat with feeding of HAM-RS2 to rodents (4). These results with moderate dietary fat and HAM-RS2 may indicate that most Americans would not have attenuation of effects associated with the consumption of HAM-RS-2.
There were basically no positive effects associated with addition of tuna oil to the low and high-fat diets. In a previous study, rats that fed fish oil and HAM-RS2 demonstrated increase in SCFA production and a lower colonic pH when compared to a control diet with sunflower oil and HAM-RS2 (20). However, the concentration of tuna oil was only 4% of the weight of the diet (10% energy) in the current study. Conlon et al. used 10% tuna oil of weight of diet in their study to observe improved fermentation effects (20); but in the present study the major aim was to compare low fat diets with high fat diets and to have the same weight of the diet as tuna oil in both low and high fat diets.
On the other hand, tuna oil consumption increased both food and energy intake. This, however, did not result in an increase in abdominal fat or body weight (Figure 2). A review by Buettner et al. reported that fish oil has a different effect on body weight than other types of fat—a reduced body weight (7). The current study results may indicate that higher intake concentrations of tuna oil may be required to observe decreases in body weight and body fat.
In summary, the presently reported research study demonstrated a partial attenuation of the beneficial effects of HAM-RS2 in rats that were fed a high fat diet. This partial attenuation reflects rats not responding as well to HAM-RS2 when fed on a high-fat diet as compared to HAM-RS2 in a low-fat diet. The high-fat concentration used in the present study was within the range of human consumption, but well above the average fat intake for the American population. A previous study demonstrated no attenuation of reduced body fat associated with dietary HAM-RS2 with feeding of a moderate-fat diet. However, the current data indicate that those American's consuming a high fat-diet may benefit from the consumption of prebiotics like HAM-RS2, but warrant further research into reduction in health benefits when coupled with the consumption of a high fat diet.
Acknowledgments
Amioca® cornstarch and Hi-maize® cornstarch were gifts from Ingredion Incorporated (Bridgewater, NJ). Nu-Mega encapsulated tuna oil was a gift from Clover Corporation (Gymea, N.S.W., Australia).
Funding agencies: This study was supported with funding from Ingredion Incorporated, the Louisiana State University Agricultural Center, and the Gordon Cain Professorship in the School of Human Ecology of the College of Agriculture of Louisiana State University.
Footnotes
Disclosure: Michael Keenan, the corresponding author, and Roy Martin have received funding from Ingredion Incorporated for research. Christine Pelkman is an employee of Ingredion Incorporated and Ian Brown is an employee of Clover Corporation.
References
- 1.Keenan MJ, Zhou J, McCutcheon KL, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring) 2006;14:1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 2.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 3.Shen L, Keenan MJ, Martin RJ, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17:40–45. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Martin RJ, Tulley RT, et al. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J Agric Food Chem. 2009;57:8844–8851. doi: 10.1021/jf901548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan MJ, Robert J, Martin RJ, et al. Resistant strach from high amylose maize (HAM-RS2) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity, accepted for publication. doi: 10.1002/oby.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakazaki rat. Mol Nutr Food Res. 2011;55:1499–1508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 9.Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51–59. doi: 10.1016/j.jnutbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Scribner KB, Pawlak DB, Aubin CM, Majzoub JA, Ludwig DS. Long-term effects of dietary glycemic index on adiposity, energy metabolism, and physical activity in mice. Am J Physiol Endocrinol Metab. 2008;295(5):E1126–E1131. doi: 10.1152/ajpendo.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JMFGJ, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- 12.Tulley RT, Appel MJ, Enos TG, et al. Comparative methodologies for measuring metabolizable energy of various types of resistant high amylose corn starch. J Agric Food Chem. 2009;57:8474–8479. doi: 10.1021/jf900971c. [DOI] [PubMed] [Google Scholar]
- 13.Barry KA, Wojcicki BJ, Bauer LL, et al. Adaptation of healthy adult cats to select dietary fibers in vivo affects gas and short-chain fatty acid production from fiber fermentation in vitro. J Anim Sci. 2011;89:3163–3169. doi: 10.2527/jas.2010-3445. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Martin RJ, Tulley RT, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295(5):E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 2008;56:305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Reid G, Gaudier E, Guarner F, et al. Reponders and non-responders to probiotic interventions: how can we improve the odds? Gut Microbes. 2010;1:200–204. doi: 10.4161/gmic.1.3.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HV, Frassetto A, Kowalik EJ, Jr, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JD, Wang C-Y. NCHS data. National Center for Health Statistics; Hyattsville, MD: 2010. Trends in Intake of Energy and Macronutrients in Adults From 1999–2000 Through 2007–2008. [PubMed] [Google Scholar]
- 19.Panel on Macronutrients . Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein, and Amino Acids (Macronutrients) The National Academy Press; Washington, DC: 2002/2005. [Google Scholar]
- 20.Conlon MA, Bird AR. Interactive and individual effects of dietary non-digestible carbohydrates and oils on DNA damage, SCFA and bacteria in the large bowel of rats. Br J Nutr. 2009;101:1171–1177. doi: 10.1017/S0007114508056031. [DOI] [PubMed] [Google Scholar]


