Abstract
Arabidopsis exocyst subunit SEC3A has been reported to participate in embryo development. Here we report that SEC3A is involved during pollen germination. A T-DNA insertion in SEC3A leads to an absolute, male-specific transmission defect that can be complemented by the expression of SEC3A coding sequence from the LAT52 promoter or SEC3A genomic DNA. No obvious abnormalities in the microgametogenesis are observed in the sec3a/SEC3A mutant, however, in vitro and in vivo pollen germination are defective. Further studies reveal that the callose, pectin, and cellulose are apparently not deposited at the germination site during pollen germination. SEC3A is expressed ubiquitously, including in pollen grains and pollen tubes. Notably, SEC3A-GFP fusion proteins are specifically recruited to the future pollen germination site. This particular localization pattern is independent of phosphatidylinositol 4,5-bisphosphate (PI-4,5P2), although SEC3-HIS fusion proteins are able to bind to several phosphoinositols in vitro. These results suggest that SEC3A plays an important role in the establishment of the polar site for pollen germination.
Pollen germination is a very important event among a series of pollination processes by which pollen tube delivers the sperm cells into the ovule to complete fertilization. In Arabidopsis, once a desiccated, pollen grain contacts a papilla cell on the stigmatic surface, it became hydrated within a short period of time. The presence of a Ca2+ gradient beneath the potential germination site1,2, the reorganization of F-actin cytoskeleton3,4, and the massive deposition of callose, pectin, and cellulose at the germination plaque5,6,7 are key events taking place before tube emergence. Until now, it is not yet clear how the the germination site is established.
In order to satisfy pollen germination and the rapid pollen tube tip growth, cell wall material, proteins and other membrane components, are transported to the growing tip of the pollen tube via the vesicle trafficking system8,9. Compared to intensive researches on pollen tube growth, limited number of players functioning from the onset of pollen germination have been identified. These included proteins involved in cell wall material synthesis and modification. For example, BUP encodes a novel Golgi-located glycosyltransferase, the bup is affected during pollen germination and pollen tube growth by affecting pectin synthesis or delivery7. Pollen grains from homozygous plants mutated in PECTIN METHYLESTERASE48 gene developed multiple germinating sites due to the presence of more abundant highly methylesterified pectin in the intine wall10. A T-DNA insertion line mutated in the Callose Synthase 9 gene produced pollen grains able to in situ precociously germinate inside the anther6, and mutations in CSLD1 and CSLD4 caused a significant reduction in cellulose deposition and an alteration of the cell wall organization leading to defective pollen germination and tube growth11. These data suggested that tight regulation of cell wall synthesis and modification is crucial for the germination site establishment and pollen tube emergence. Additionally, components involved in vesicle trafficking, an intimately related process for cell wall material delivery, have also been implicated during pollen germination12. For example, a mutation in AtSYT2, a homolog of mammalian Synaptotagmins implicated in regulating membrane fusion during exo/endocytosis, affected pollen germination and pollen tube elongation13,14,15. Pollen-specific GNL2 was shown to be essential for pollen germination and pollen tube tip growth based on its necessary role in polar recycling. GNL2 is localized to the germination site and pollen tube tip, and absolutely no pollen germination was observed in gnl2 mutants16,17. Furthermore, proteins involved in intracellular signaling18, and dynamic actin regulation3,4 have also been shown to play a role during pollen germination.
Vesicle tethering is required after vesicle delivery but preceding the SNARE-mediated docking/fusion steps at the target membrane19. The exocyst, composed of SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70, and EXO84 subunits, is an evolutionally conserved octameric protein complex that tethers secretory vesicles to specific domains of the plasma membrane20. Spatial regulation of membrane trafficking by the exocyst complex is fundamental to epithelial cell polarization, neuronal synaptogenesis and the polar growth of budding yeast21, which suggested a role of exocyst in polar exocytosis. Several Arabidopsis exocyst subunits have been implicated in biological processes that rely on regulated vesicle trafficking. For example, mutations in SEC5, SEC6, SEC8, and SEC15A dramatically reduced pollen germination and pollen tube growth which led to a male-specific transmission defect22,23. Mutations in SEC8 and EXO70A1 locus reduced pectin deposition in the seed coat24. exo70a1, exo84b, and pollen rescued sec6 mutants (PRsec6) display cytokinesis defects25,26. In maize roothairless1 where SEC3 was mutated, root hairs could not elongate properly27.
Previous investigations of the sec3a mutation (SALK_145185) indicated that the deletion of SEC3A gene resulted in embryo-lethality. The authors showed that in the interphase cells, SEC3A-GFP is present in the cytosol and at the plasma membrane where it accumulates as immobile punctate structures over the cell surface of the root hairs and the root epidermal cells, furthermore its recruitment to the plasma membrane is not mediated by the conventional secretory pathway28.
In this report, with another T-DNA insertion mutant of the SEC3A gene (GK_652H12), we provided genetic, molecular, and cellular evidence that SEC3A is crucial for male gametophytic transmission. The mutation in SEC3A gene led to the lack of pollen germination along with perturbations in the deposition of cell wall material. SEC3A-GFP fusion protein was found to accumulate at the future site of pollen germination, which is independent on PI-4,5P2 content. These results suggest that the polar localization of SEC3A at the bulge of the germinating pollen grain is essential for pollen germination.
Results
The expression pattern of SEC3A and SEC3B genes
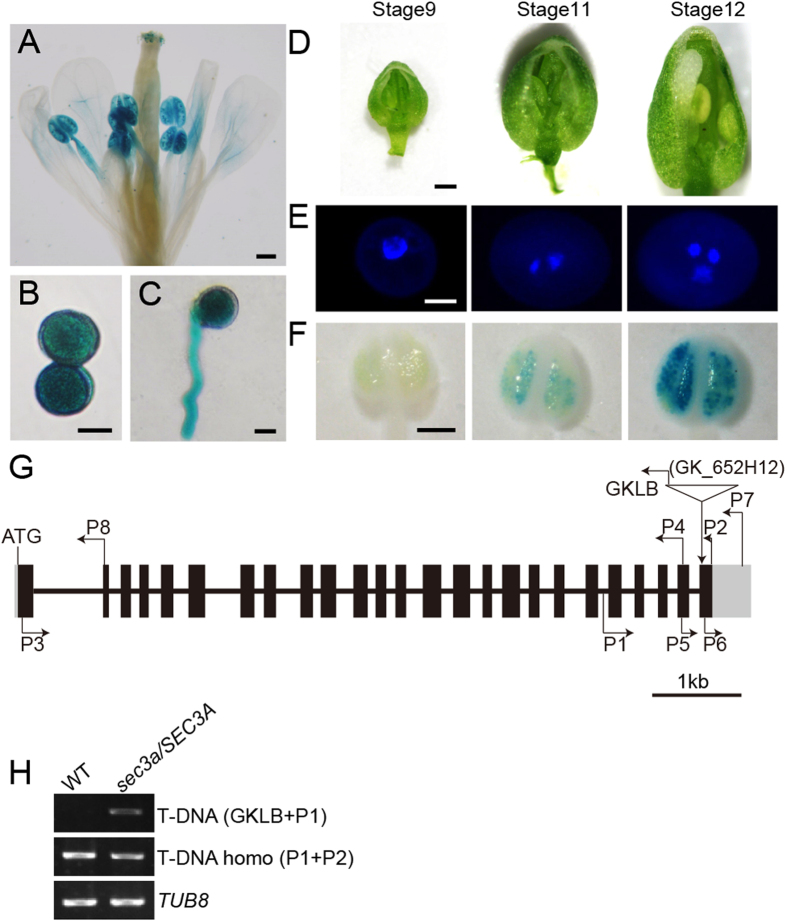
The time and location of gene expression often implies its function. To investigate the expression pattern of SEC3A in detail, a 1024 bp promoter region upstream from the ATG start codon was fused with a GUS reporter gene and introduced into wild type Arabidopsis plants. Fifteen independent pSEC3A:GUS transgenic plants were obtained and six of them were chosen randomly and examined further. Strong GUS activities were detected in flowers (Fig. 1A), mature pollen (Fig. 1B) and pollen tubes (Fig. 1C). Notably, SEC3A started its expression only in pollen from the bicellular stage (Fig. 1D,F), which corresponded to the stage 11 flower onwards29 (Fig. 1E,F). In addition, SEC3A expression was detected in seedlings, root columella cells, vascular bundles of root maturation zone, and embryos (Supplementary Fig. S1), suggesting a role of SEC3A in sporophytic development as well.
Figure 1. SEC3A expression pattern in pollen and the genotyping of sec3a/SEC3A mutant.
(A) The flower, (B) Mature pollen grains, and (C) Pollen tube showed strong SEC3A expression. (D) Flowers at stage 9, 11, 12 were used for anther dissection. (E) DAPI staining showed that pollen from stage 9, 11, 12 flowers was at unicellular, bicellular, and the tricellular stages, respectively. (F) GUS staining of anthers from flowers of (D). (G) A schematic representation of SEC3A transcript and the site of T-DNA insertion. The T-DNA insert is located in the last Exon. The black boxes represent exons, lines in between represent introns. GKLB, P1, P2 are the primers used in the PCR assays. (H) Genotyping of sec3a/SEC3A by PCR analysis. TUB8 was used as an internal control. Bars = 0.2 mm for (A,F), 10 μm for (B,C), 0.4 mm for (D), and 5 μm for (E).
In Arabidopsis, SEC3B shares 96.6% sequence identity to SEC3A in the coding region, and the two genes are arranged in tandem. It is therefore very important to explore the expression pattern of SEC3B to predict whether they are functionally redundant in a particular tissue. In four pSEC3B:GUS lines obtained, the GUS staining of SEC3B looked much weaker than that of SEC3A which is consistent with the RT-PCR results generated with gene-specific primers (Supplementary Fig. S2, Supplementary Table S1). SEC3B expression was not detected in the pollen and pollen tube, although it was noticed in tissues such as the cotyledon, the root vascular bundles, and the mature leaves (Supplementary Fig. S3). Furthermore, homozygotes obtained from the progeny of two sec3b/SEC3B mutants (SALK_071060, SALK_124458) showed no observable phenotype. These results indicate that SEC3A is the major SEC3 paralog in pollen.
A mutation in SEC3A gene causes male sterility
SEC3A gene contains 25 exons and 24 introns. One T-DNA line of the SEC3A gene (sec3a/SEC3A, GK_652H12) was obtained where the insertion was in the last exon (Fig. 1G). PCR-based genotyping revealed that no homozygous sec3a mutant plants could be identified (n > 180) (Fig. 1H). The progeny from the self-pollinated sec3a/SEC3A plants segregated in a ratio of roughly 1:1 (n = 243) instead of the expected 3:1 (Table 1), indicating a disorder in gametophytic transmission rather than a zygotic lethality of the mutation. To investigate if the mutation in SEC3A affected male or female gametophytic development, the sec3a/SEC3A plants were used as male or female donors to cross with the wild type. When the sec3a/SEC3A plants were crossed as female parents, approximately 49% (n = 271) of the resulting F1 progenies were heterozygotes as expected for normal transmission. In contrast, when pollen grains from the sec3a/SEC3A were pollinated to wild type plants, none (n = 224) of the F1 seedlings were heterozygous plants (Table 1). These results indicated that genetic transmission of sec3a mutation through the male was abolished in the mutant, while female gametophytic transmission was normal.
Table 1. Genotyping of the sec3a/SEC3A mutant.
| No. of Progeny | Genotypes of Progeny | χ2 | P | |||
|---|---|---|---|---|---|---|
| SEC3A/SEC3A | sec3a/SEC3A | sec3a/sec3a | ||||
| Self-cross | 25 (%) | 50 (%) | 25 (%) | Expected | ||
| 243 | 48 | 52 | 0 | 125.7 | <0.001a | |
| Out-cross (♀ × ♂) | 50 (%) | 50 (%) | 0 (%) | Expected | ||
| WT × sec3a/SEC3A | 224 | 100 | 0 | 0 | 224 | <0.001a |
| sec3a/SEC3A × WT | 271 | 51 | 49 | 0 | 0.1328 | NS |
P, values were calculated using the χ2 test.
aSignificant difference between the observed and the expected ratios.
NS, Not significantly different.
The sec3a mutant is defective during pollen germination
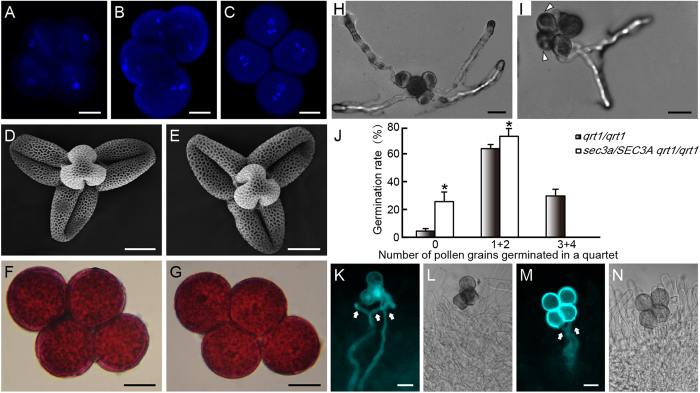
Male gametophytic lethal might be due to two possible defects. One is that haploid cells inherited the mutant allele after meiosis do not develop into mature pollen, or they developed normally, but are defective during pollen germination and/or tube growth, or later stages of double fertilization. To address these two possibilities, sec3a was introgressed into the quartet 1 (qrt1) mutant background where four microspores from a microsporocyte fail to separate after meiosis, but their functions are virtually unaffected30,31. Within a quartet, two microspores are mutant (sec3a) and the other two are wild type (SEC3A). Pollen grain development was examined using the quartets from sec3a/SEC3A qrt1/qrt1 plants. No difference in nuclear composition was observed in quartets at the unicellular, bicellular and tricellular stages using DAPI staining (Fig. 2A–C). In addition, sec3a pollen grains were morphologically comparable to wild type under the scanning electron microscope (Fig. 2D,E), and they are viable as revealed by the Alexander staining (Fig. 2F,G). Together, these results indicated that the mutation in SEC3A did not affect pollen development.
Figure 2. sec3a pollen is defective during pollen germination.
Quartets from sec3a/SEC3A qrt1/qrt1 plants was stained with DAPI at the unicellular (A), bicellular (B) and tricellular (C) stages. Scanning electron microscopy (SEM) analysis of qrt1/qrt1 (D) and sec3a/SEC3A qrt1/qrt1 quartets (E). Alexander staining of quartets from qrt1/qrt1 (F) and sec3a/SEC3A qrt1/qrt1 (G). No abnormality was observed for sec3a/SEC3A qrt1/qrt1 quartets in above assays. In vitro germination of quartets of qrt1/qrt1 (H) and sec3a/SEC3A qrt1/qrt1 (I) plants on solid germination medium. (J) Statistic analysis of qrt1/qrt1 and sec3a/SEC3A qrt1/qrt1 quartets showing 0, 1 + 2, 3 + 4 pollen tube(s) 6 h after germination. Values represent the means ± SD. *Means P < 0.05 by Student’s t test (n = 600 pollen grains for each genotype). In vivo germination of pollen grains from qrt1/qrt1 (K,L) and sec3a/SEC3A qrt1/qrt1 (M,N) quartets. (L,N) are corresponding bright-field images of (K,M), respectively. Arrowheads in (I) indicated non-germinated pollen grains. Arrows in (K,M) indicated pollen tubes. Bars = 10 μm for (A–G), 20 μm for (H,I,K,M).
Pollen germination in vitro and in vivo were carried out with quartets of sec3a/SEC3A qrt1/qrt1 (Fig. 2). Due to the 2:2 ratio of the wild type versus mutant within a quartet from a heterozygote, germination of three or four pollen grains in the quartet requires the germination of one or both mutant pollen grains. Therefore, a relative low percentage of quartets with three or four grains germinated would indicate a pollen germination defect. When cultured on a solid medium, approximately 31.7% (n = 600) of quartets from qrt1/qrt1 plants had three or four pollen grains germinated (Fig. 2H,J), while no quartet from sec3a/SEC3A qrt1/qrt1 (n = 600) plants could do the same (Fig. 2I,J). In vivo pollination assay was carried out using male-sterile plants (ms1) as pollen recipient to avoid the potentially complicating effects of stigma maturity and emasculation stresses32. While approximately 47% (n = 108) of qrt1/qrt1 quartet could germinate three or four pollen tubes into the pistil as revealed by aniline blue staining (Fig. 2K,L), none of the sec3a/SEC3A qrt1/qrt1 (n = 60) quartets was able to do that (Fig. 2M,N). These results suggested that sec3a mutation significantly inhibited pollen germination in vitro and in vivo.
Phenotype of sec3a/SEC3A is rescued by pLAT52:SEC3A, pLAT52:SEC3A-GFP and gSEC3A transgenes, respectively
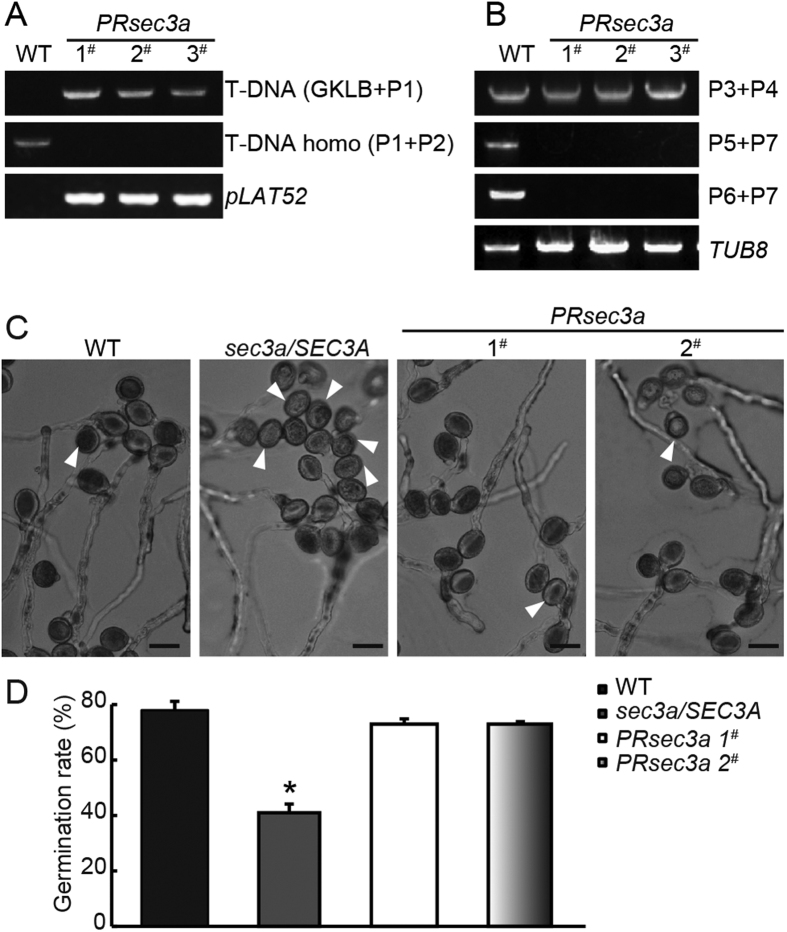
To demonstrate that the mutant phenotype is caused by the disruption of SEC3A gene, sec3a/SEC3A plants were transformed with SEC3A coding sequence driven by the pollen-specific LAT52 promoter33 in a vector that confers resistance to hygromycin. Transgenic plants hemizygous for pLAT52:SEC3A (20 lines) and pLAT52:SEC3A-GFP (12 lines) loci in sec3a/SEC3A mutant were obtained, and several lines of each genotype were chosen randomly for further characterization. The male transmission defects were complemented, with sec3a/sec3a, sec3a/SEC3A and wild type progeny appearing at the expected Mendelian ratio (Table 2). Moreover, sec3a homozygotes, named as PRsec3a (Pollen-Rescued sec3a: sec3a/sec3a pLAT52:SEC3A/pLAT52:SEC3A), were identified using PCR-based genotyping with progeny lines in which all seedlings showed hygromycin resistant (pLAT52:SEC3A transgene selection marker) (Fig. 3A). RT-PCR analysis of PRsec3a mutants indicated that the transcripts spanning (P5 + P7) or behind (P6 + P7) the T-DNA insertion sites were not expressed, while the transcript (P3 + P4) before the insertion was detected (Figs 1A and 3B). However, it should be unstable due to the lack of Poly(A) tail. Similarly, PRsec3a-GFP lines (sec3a/sec3a pLAT52:SEC3A-GFP/pLAT52:SEC3A-GFP) were obtained (Table 2, Supplementary Fig. S4). Furthermore, twelve sec3a/SEC3A lines bearing hemizygous SEC3A genomic DNA were generated (Supplementary Fig. S4). In two randomly selected lines, the male transmission efficiency increased to approximately 2:1, indicating a complete complementation (Supplementary Table S2).
Table 2. Complementation analysis of sec3a/SEC3A mutants.
| Complemented T1 | No. of Progeny | Genotypes of Progeny | χ2 | P | ||
|---|---|---|---|---|---|---|
| SEC3A/SEC3A | sec3a/SEC3A | sec3a/sec3a | ||||
| 33.3 (%) | 50 (%) | 16.7 (%) | Expected | |||
| pLAT52:SEC3A 1# | 249 | 35.3 | 42.1 | 22.6 | 8.42 | 0.0148 |
| pLAT52:SEC3A 2# | 194 | 29.4 | 54.1 | 16.5 | 1.56 | NS |
| pLAT52:SEC3A 3# | 201 | 27.9 | 48.8 | 23.3 | 7.31 | 0.0258 |
| pLAT52:SEC3A-GFP 1# | 204 | 37.7 | 50.5 | 11.8 | 2.34 | NS |
| pLAT52:SEC3A-GFP 2# | 166 | 32.5 | 52.4 | 15 | 0.08 | NS |
pLAT52:SEC3A represents pLAT52:SEC3A hemizygous transgene in sec3a/SEC3A mutant background.
pLAT52:SEC3A-GFP represents pLAT52:SEC3A-GFP hemizygous transgene in sec3a/SEC3A mutant background.
P, values were calculated using the χ2 test.
NS NS, Not significantly different.
Figure 3. Complementation of sec3a mutants with pLAT52:SEC3A transgene.
(A) Genotyping of PRsec3a. (B) RNAs were extracted from true leaves, and P3 + P4, P5 + P7 or P6 + P7 primer pairs were used to analyze different SEC3A transcripts. (C) In vitro germination of pollen grains from wild type, sec3a/SEC3A, and PRsec3a plants after 6 h on the solid medium. Arrowheads indicated ungerminated pollen. (D) Statistic analysis showed that pLAT52:SEC3A transgene restored in vitro germination rate of sec3a/SEC3A pollen to normal. Values represent the means ± SD. *Means P < 0.05 by Student’s t test. Bars = 20 μm.
In vitro pollen germination of two randomly selected PRsec3a lines were evaluated on the solid media. Under the same conditions, 78% of wild type pollen grains (n = 672) and 41% (n = 638) of that from sec3a/SEC3A plants were able to germinate (Fig. 3C,D). Remarkably, in PRsec3a mutant, the ratio was restored to that of the wild type (73%, n = 683) (Fig. 3C,D). These date demonstrated that the male gametophytic defects were indeed due to T-DNA insertion in the SEC3A gene.
SEC3A-GFP decorates the pollen germination site, and the localization is independent on PI-4, 5P2
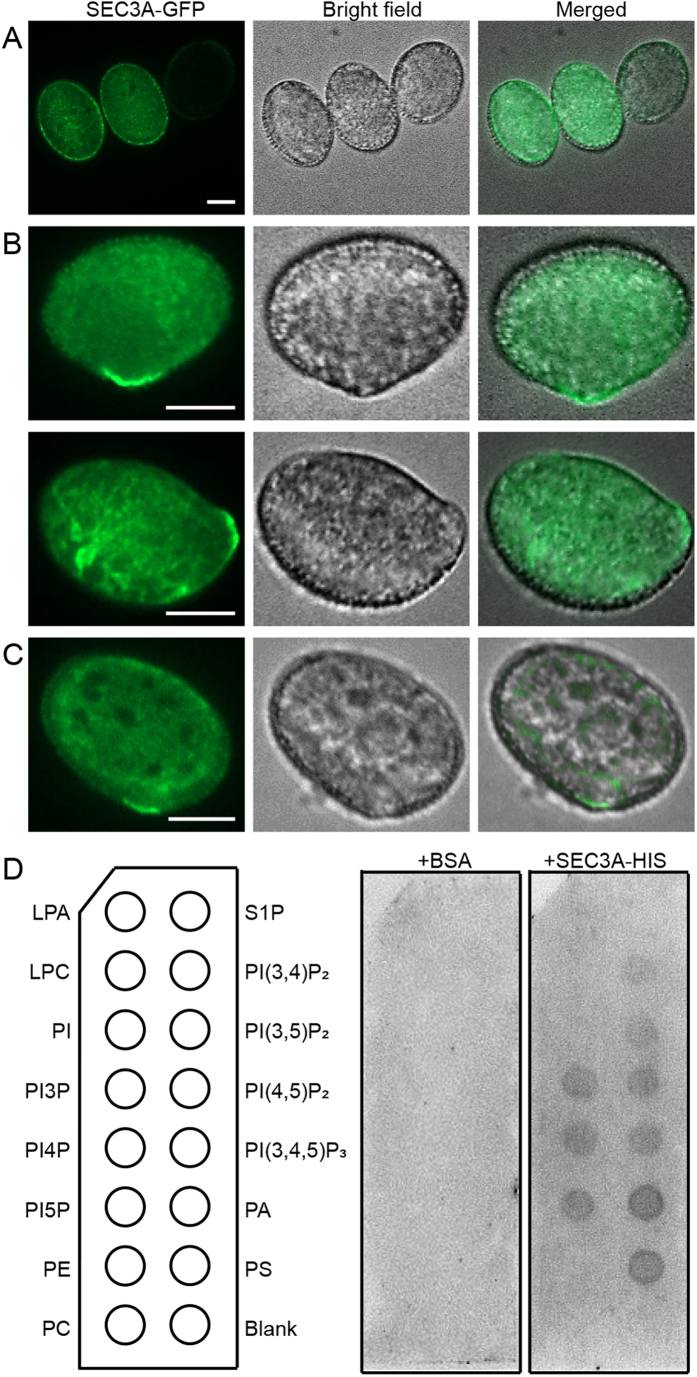
To analyze the role of SEC3A more precisely, the dynamic localization of SEC3A in the germinating pollen grains were explored. Our studies showed that the male transmission was restored to normal in sec3a/SEC3A mutant with hemizygous pLAT52:SEC3A-GFP (Table 2), and PRsec3a-GFP lines could be identified (Supplementary Fig. S4). These results indicated that the SEC3A-GFP protein is functional in pollen, and therefore should display its correct subcellular localization. In pLAT52:SEC3A-GFP transgenic plants, SEC3A proteins appeared from the binucleated pollen stage onwards (Supplementary Fig. S5), consistent with the GUS analysis results (Fig. 1). In mature pollen prior to activation, SEC3A-GFP was dispersed in the cytoplasm (Fig. 4A). Interestingly, after being placed in liquid germination medium for 30 min, SEC3A-GFP was shift to the cortex of the future germination site before any visible change of pollen morphology was noticed (Fig. 4B, Supplementary Video. S1), and the signals oscillated at this position throughout the tube emerging process (Supplementary Videos S1 and S2).
Figure 4. SEC3A proteins marks the pollen germination site, and this localization is independent on PI4, 5-P2.
pLAT52:SEC3A-GFP was transformed into wild type (Col-0) (A,B), and pip5k4 mutant plants (C), respectively, and pollen grains were collected and germinated in liquid medium. (A) SEC3A-GFP localization before pollen germination. (B) SEC3A-GFP localization during pollen germination. (C) SEC3A-GFP localization during pollen germination in pip5k4 mutant background. (D) SEC3A protein lipid overlay binding assay. Bars = 10 μm.
In yeast, Sec3p directly interacted with PI-4,5P2 and marked the exocytic site at the bud34. To test whether SEC3A could bind to PIPs directly in vitro, SEC3A was fused to HIS and expressed in E. Coli. Purified proteins were used in PIPs strip overlay assays. As shown in Fig. 4D, SEC3A bound to several membrane phosphoinositides including PI-4,5P2, while the control with BSA alone could not (Fig. 4D). In plants, PI-4,5P2 could be generated through the phosphorylation of phosphatidylinositol-4-phosphate (PI4P) by phosphatidylinositol-4-phosphate 5-kinases (PIP5K)35,36. In pip5k4 mutant, the production of PI-4,5P2 and the membrane recycling was decreased in pollen tubes37,38. pLAT52:SEC3A-GFP was introduced into the pip5k4 homozygous mutant (SALK_001138, Supplementary Fig. S6), and its localization was examined. Interestingly, SEC3A proteins still accumulated at the bulge of the germinating pip5k4 pollen grain with bright fluorescent labeling (Fig. 4C), just as in the wild type (Fig. 4B), indicating that the localization of SEC3A in pollen was not dependent on PI-4,5P2.
Polar accumulation of cell wall material was not observed in the germinating sec3a pollen grains
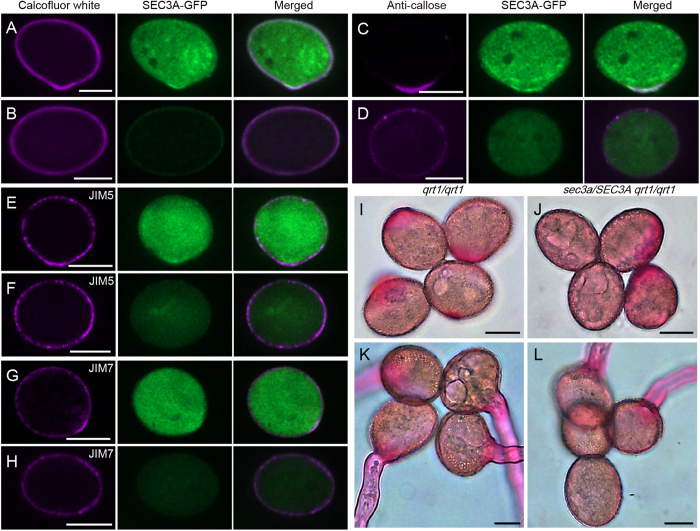
Pollen germination started with hydration, which might act as a triggering signal. Following hydration, plaque formation starts and is completed within 1 h, by then the pollen tube has emerged39. Polar deposition of callose, cellulose and pectin is observed in the incipient bulge of germinating pollen grains5,7,40. The phenotypes of sec3 mutant and the localization pattern of SEC3A prompted us to look into the cell wall deposition at the germination site. Pollen grains from sec3a mutant hemizygous for pLAT52:SEC3A-GFP were used since mutant pollen (sec3a) can easily distinguished from the complemented one (sec3a pLAT52:SEC3A-GFP) by the absence or presence of the GFP signal. Calcofluor white which labels cellulose and callose41, appeared at the germination site in the complemented pollen grain, colocalized with the SEC3A-GFP signals (Fig. 5A). Similarly, antibodies against callose (anti-callose, Fig. 5C), low methylestified pectins (JIM 5, Fig. 5E), and high methylestified pectins (JIM7, Fig. 5G) labeled the germination plaques in the complemented pollen grains, respectively. In contrast, mutant pollen exhibited an even staining of the cell wall with no sign of germination (Fig. 5B,D,F,H). In addition, qrt1/qrt1 and sec3a/SEC3A qrt1/qrt1 quartets were stained with ruthenium red, which labels a broad range of methylesterified pectins. Ruthenium red strongly labeled the germination plaques of all four pollen grains of qrt1/qrt1 (Fig. 5I) while only two pollen grains of sec3a/SEC3A qrt1/qrt1 quartet could be labeled (Fig. 5J). Even after 6 h of germination when a quartet from qrt1/qrt1 plant produced four pollen tubes (Fig. 5K), sec3a/SEC3A qrt1/qrt1 quartet still had two non-germinated pollen grains that show no sign of polar pectin deposition (Fig. 5L). Thus pectin distribution revealed by ruthenium red is consistent with the JIM5 and JIM7 staining (Fig. 5E,F,G,H). Together, these data demonstrated that polar accumulation of several types of cell wall materials was not observed in the germinating sec3a pollen.
Figure 5. Accumulation of Cell wall materials are not observed in sec3a germinating pollen.
(A,B) Calcofluor white staining. (C,D) Pollen grains labeled with anti-callose monoclonal antibody. (E,F) Pollen grains labeled with JIM5 monoclonal antibody. (G,H) Pollen grains labeled with JIM7 monoclonal antibody. sec3a pLAT52:SEC3A-GFP (A,C,E,G) and sec3a (B,D,F,H) pollen were used for the assays. (I–L) Ruthenium red staining of tetrads from qrt1/qrt1 (I,K) and sec3a/SEC3A qrt1/qrt1 (J,L) plants. Bars = 10 μm.
Discussion
In this report, we demonstrated that a mutation in SEC3A led to the failure of pollen germination with no polar accumulation of cell wall materials. SEC3A is expressed in pollen and pollen tube, and SEC3A marked the pollen germination site. Thus, SEC3A is a key player during pollen germination, and its recruitment to the germination site is not dependent on PI4,5-P2.
Mutations in distinct exocyst subunit caused polar growth defects in processes such as root hair elongation, hypocotyl elongation, and pollen tube growth22,23,27,42. In this study, we found that in sec3a mutant (GK_652H12), germination of the pollen grains was hindered, resulting in an absolute male-specific transmission defect which could be rescued by pLAT52:SEC3A, pLAT52:SEC3A-GFP or genomic SEC3A transgene (Figs 2 and 3, Table 2, Supplementary Fig. S4, Supplementary Table S2). The sec3a pollen grains developed normally, however, no pollen germination was observed in in vitro and in vivo germination assays (Fig. 2), let alone pollen tube growth. In a recent paper43, the authors showed that among 1769 sec3a heterozygous tetrads, only one with three pollen, and zero with four pollen germinated in an in vitro assay; Among 73 sec3a heterozygous tetrads, only one with three pollen, and zero with four pollen germinated in an in vivo assay. Therefore, we believed that pollen germination defect, rather than pollen tube growth, is the major physiological aberrance revealed by the sec3a/SEC3A mutant, and our data is consistent with the report43. Given the fact that mutations in SEC6, SEC8, SEC15A, and SEC5 dramatically affect pollen germination (1% to 7% germination rate in respective mutants) as well22,23, it is most likely that SEC3A acts together with other exocyst subunits, but as a more crucial player, in tethering secretory vesicles containing newly synthesized pectin, callose or cellulose synthase to establish and consolidate the germination aperture. It is therefore interesting to explore in the future the dynamic localization of other exocyst subunits at the germination aperture. However, other possibilities cannot be totally excluded. For example, the failure of pollen germination in sec3a/SEC3A mutant might be caused by defects in synthesis of some cell wall material or by an abnormal rapid degradation of cell wall materials. SEC3A appeared in a dynamic cone-shaped area and at the extreme plasma membrane at the tip of elongating pollen tube (Supplementary Video S3 and Supplementary Fig. S8) confirming recent findings43 and implying a role of SEC3A in pollen tube growth as well. This localization is however in contrast with an earlier report of SEC3A being localized to immobile puncta at the plasma membrane of root hairs28.
The expression pattern of SEC3A (Supplementary Fig. S1) is suggestive of a possible role of SEC3A in embryo development. Previous investigations of sec3a mutant line (SALK_145185) indicated that disruption of the SEC3A gene caused embryo lethality28. However, in our hand, PCR-based genotyping (Supplementary Table S1) of the SALK_145185 line did not yield any T-DNA specific band. Therefore we could not confirm the reported embryo lethality of this particular line. PRsec3a generated in our study represented sporophytic sec3a homozygotes according to the genotyping results (Fig. 3), but its embryo development was normal (Supplementary Fig. S7). Our results was supported by the recent report where sec3a mutant was shown to have a male transmission defect43.
How pollen germination site is established and pollen tube elongation initiated are not well known. In S. cerevisiae, bud tip localized Sec3p is thought to be a spatial landmark for polarized exocytosis and for the recruitment of other exocyst subunits to the exocytic sites44,45. The polarized localization of Sec3p is depended on its interaction with PI4,5-P2 and the Rho family of small GTPases34,46,47. In this study, SEC3A has been shown to be recruited from the cytosol to the cell cortex which marks the future germination site (Fig. 4, Supplementary Videos S1 and S2). There are two scenarios under which SEC3A could be recruited to this particular site. In plant, Rac/Rop small GTPases accumulate specifically at the plasma membrane of the tip of elongating pollen tubes and are key regulators of polar cell expansion48. Interestingly, Rop1 GTPase effector ICR1/RIP1, an interacting protein of SEC3A49, has been shown to localize and oscillate at the germination site50 in a similar manner to that of SEC3A (Supplementary Videos S1 and S2). Moreover, the icr1 mutant were reported to be partially male sterile, although the details of pollen abnormality remained to be studied49. Under this scenario, ROP GTPase might be involved in the recruitment of exocyst to the site of pollen germination through the interaction between SEC3A and ICR1. PI4,5-P2 has been shown to accumulate exclusively at the apex of elongating pollen tube to control polar secretion by modulating actin organization and membrane traffic51. In pip5k4 mutant where PI4,5-P2 level was reduced, but the position of SEC3A at the germination pore is unaffected (Fig. 4). Hence, the alternative scenario that SEC3A binds directly to PI4,5-P2 to achieve its polar localization is not true. Given the fact that SEC3A labeled pollen germination site (Fig. 4), mutant pollen from sec3a/SEC3A plant could not germinate, and partial complementation of sec3a resulted in multiple germination sites43, we suggest that SEC3A is required for germination site selection and/or establishment.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana Columbia ecotype (Col-0) was used as the wild type. T-DNA insertion lines of SEC3A (GK_652H12) and PIP5K4 (SALK_001138) were obtained from the Nottingham Arabidopsis Stock Center (NASC) and Arabidopsis Biological Resource Center (ABRC), respectively. Seeds were surface sterilized and plated on half-strength Murashige and Skoog medium with 0.8% agar, imbibed at 4 °C for 3 days, and then placed in a growth chamber at 22 °C with a 16-h-light/8-h-dark cycle. Seven-day-old seedlings were then transferred to soil and maintained under the same condition.
GUS staining
For GUS staining, different tissues of transgenic Arabidopsis plants were vacuum infiltrated for 15 min in GUS staining solution of 100 mM phosphate buffer (pH 7.0), 0.1% Triton X-100, 0.5 mM [K3Fe(CN)6], 0.5 mM [K4Fe(CN)6], 10 mM EDTA and 0.5 mg ml−1 bromochloroindoyl-β-glucuronide (X-Gluc). Samples were then incubated overnight at 37 °C and cleared in acetic acid:ethanol (1:3 v/v).
Phenotypic analysis of mutants
To determine different pollen development stages, pollen grains were stained in a DAPI solution (0.1 M phosphate buffer solution pH 7.0, 1 mM EDTA, 0.1% Triton X-100 and 1 μg ml−1 DAPI) for 15 min before observation. The viability of pollen grains was assessed using Alexander staining52. For SEM, mature pollen grains were coated directly with gold particles (EIKO IB-3) and observed on HITACHI S-3000N scanning electron microscope.
In vitro pollen germination was conducted essentially according to described previously53. Pollen harvested from newly fully opened flowers was placed onto pollen germination medium (PGM) consisting of 1 mM CaCl2, 1 mM Ca(NO3)2, 1 mM MgSO4, 0.01% (w/v) H3BO3, 18% (w/v) sucrose, pH 7.0, which was solidified with 0.8% (w/v) agar, and grown in a growth chamber in the dark at 22 °C.
For in vivo pollen germination assay, mature pistils of the male-sterile mutant ms132 were pollinated with a limited number (3–5) of quartets from sec3a/SEC3A qrt1/qrt1 and qrt1/qrt1 plants. The pollen tubes in the pistils were stained with aniline blue and viewed with a confocal microscope Zeiss LSM710 system.
Complementation experiments
For the pollen-rescue experiment, the LAT52 promoter and the coding sequence (CDS) of SEC3A were amplified by PCR using the primer pairs LAT52-S (SacI)/LAT52-A (KpnI) and SEC3A1-S (BamHI)/SEC3A1-A (NheI), respectively (Supplementary Table S1). The resulting DNA fragments were cloned into the pCAMBIA1300 vector (CAMBIA, http://www.cambia.org) and sequencing validated. For genomic DNA complementation experiment, about 1.1 kb promoter region along with 8230 bp genomic sequence of SEC3A was PCR-amplified using the primer pair SEC3A2-S/SEC3A2-A (Supplementary Table S1). The amplified fragment was cloned into the vector pCAMBIA1300221 (CAMBIA, http://www.cambia.org) using in-fusion HD cloning kit according to the manufacturer’s instructions (Clontech). The above constructs were introduced into the sec3a/SEC3A plants using the Agrobacterium (strain EHA105)-mediated infiltration method54, followed by hygromycin selection.
Confocal microscopy
Confocal images of pollen grains or pollen tubes were collected by UltraView spinning-disc confocal scanner unit (Perkin Elmer). The wavelength was 488 nm for GFP excitation and 505–530 nm for detection. The excitation and detection wavelengths for DAPI and Calcofluor white were 359 nm and 385–405 nm for excitation, 461 nm and 437–445 nm for detection,
PIP strip overlay binding assay
For the PIP strip binding assays, phospholipid membranes (Echelon) were blocked with 3% bovine serum albumin (BSA) in PBS-T (0.1% v/v Tween-20) for 1 h at room temperature. After that, membranes were incubated in a buffer with or without SEC3A-HIS fusion proteins (0.5 μg ml−1) in 3% BSA/PBS-T for another hour. Unbound protein was washed away with PBST for three times, 10 min each. The membranes were then incubated with anti-HIS antibodies to detect the bound proteins.
Immunofluorescence and cytochemical staining
Pollen grains were adhered to poly-L-lysine-covered glass slides after germination in liquid PGM for 30 min. Samples were fixed in 4% (w/v) polyformaldehyde in PIPES buffer (50 mM PIPES, 1 mM EGTA, 5 mM MgSO4, 0.5 mM CaCl2, 0.1% TritonX-100, pH 7) for 1 h. After washing with PBS (100 mM potassium phosphate, 138 mM NaCl, and 2.7 mM KCl, pH 7.3), samples were incubated in the blocking buffer (0.8% BSA, 0.1% gelatin, and 2 mM NaN3 in PBS) at room temperature for 30 min and then incubated with the primary antibodies (1:200 diluted in blocking buffer) for 1 h. Pectins with low and high degrees of methylesterification were labeled with JIM5 and JIM755,56, respectively (Plant Probes). Callose was labeled with anti-callose57 (Biosupplies Australia Pty Ltd.). The pollen grains were washed three times with PBS and incubated for 30 min with Alexa Fluor 594 conjugated secondary antibodies (1:100 dilution in blocking buffer). The samples were washed for five times with PBS before analysis using an UltraView spinning-disc confocal scanner unit (Perkin Elmer). For cytochemical staining, the pollen tubes were stained without fixation after germination in liquid PGM. Calcofluor white (0.001%, w/v) and ruthenium red (0.01%, w/v) in PGM were used to stain β-glucans (both cellulose and callose) and pectins, respectively. Chemicals used are from Sigma-Aldrich.
Additional Information
How to cite this article: Li, Y. et al. Exocyst subunit SEC3A marks the germination site and is essential for pollen germination in Arabidopsis thaliana. Sci. Rep. 7, 40279; doi: 10.1038/srep40279 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This research was supported by grant 31200236 from the National Science Foundation of China (NSFC); Grants KYTZ201402 and KJQN201534 from the Fundamental Research Funds for the Central Universities in China; Grant 2014ZX0800925B from the Ministry of Agriculture of China for Transgenic Research; A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Author Contributions Y.L. and X.Y.T. performed most of the experiments. Y.L. and Y.Q.B. wrote, and revised this manuscript. M.R.W., B.X.L., Y.X.Z., C.Y.W. and Q.C.R. helped analyze data. J.X.W. and Z.Y.L. contributed to manuscript modification. All authors have participated in this research and approved the final manuscript.
References
- Preuss D., Lemieux B., Yen G. & Davis R. W. A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes & development 7, 974–985 (1993). [DOI] [PubMed] [Google Scholar]
- Iwano M. et al. Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol 136, 3562–3571 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. J. et al. LlSR28 is involved during pollen germination by affecting filamentous actin dynamics. Molecular plant 6, 1163–1175 (2013). [DOI] [PubMed] [Google Scholar]
- Chang M. & Huang S. Arabidopsis ACT11 modifies actin turnover to promote pollen germination and maintain the normal rate of tube growth. The Plant journal 83, 515–527 (2015). [DOI] [PubMed] [Google Scholar]
- Johnson S. A. & McCormick S. Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiology 126, 685–695 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B., Wang X. & Hong Z. Precocious pollen germination in Arabidopsis plants with altered callose deposition during microsporogenesis. Planta 231, 809–823 (2010). [DOI] [PubMed] [Google Scholar]
- Hoedemaekers K. et al. BURSTING POLLEN is required to organize the pollen germination plaque and pollen tube tip in Arabidopsis thaliana. New Phytologist 206, 255–267 (2015). [DOI] [PubMed] [Google Scholar]
- Krichevsky A. et al. How pollen tubes grow. Developmental Biology 303, 405–420 (2007). [DOI] [PubMed] [Google Scholar]
- Chebli Y., Kroeger J. & Geitmann A. Transport logistics in pollen tubes. Molecular plant 6, 1037–1052 (2013). [DOI] [PubMed] [Google Scholar]
- Leroux C. et al. PECTIN METHYLESTERASE48 Is Involved in Arabidopsis Pollen Grain Germination. Plant Physiol 167, 367–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. et al. Arabidopsis CSLD1 and CSLD4 are required for cellulose deposition and normal growth of pollen tubes. J Exp Bot 62, 5161–5177 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F. & Nielsen E. Targeting and regulation of cell wall synthesis during tip growth in plants. J Integr Plant Biol 55, 835–846 (2013). [DOI] [PubMed] [Google Scholar]
- Jahn R., Lang T. & Sudhof T. C. Membrane fusion. Cell 112, 519–533 (2003). [DOI] [PubMed] [Google Scholar]
- Craxton M. Synaptotagmin gene content of the sequenced genomes. BMC genomics 5, 43 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Arabidopsis Synaptotagmin 2 participates during pollen germination and tube growth and is delivered to plasma membrane via conventional secretion. Molecular plant 8, 1737–1750 (2015). [DOI] [PubMed] [Google Scholar]
- Jia D. J. et al. GNOM-LIKE 2, encoding an adenosine diphosphate-ribosylation factor-guanine nucleotide exchange factor protein homologous to GNOM and GNL1, is essential for pollen germination in Arabidopsis. J Integr Plant Biol 51, 762–773 (2009). [DOI] [PubMed] [Google Scholar]
- Richter S. et al. Polarized cell growth in Arabidopsis requires endosomal recycling mediated by GBF1-related ARF exchange factors. Nature cell biology 14, 80–86 (2012). [DOI] [PubMed] [Google Scholar]
- Golovkin M. & Reddy A. S. A calmodulin-binding protein from Arabidopsis has an essential role during pollen germination. Proceedings of the National Academy of Sciences of the United States of America 100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G. & Pfeffer S. R. Membrane tethering in intracellular transport. Curr Opin Cell Biol 11, 453–459 (1999). [DOI] [PubMed] [Google Scholar]
- Heider M. R. & Munson M. Exorcising the exocyst complex. Traffic 13, 898–907 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B. & Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21, 537–542 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. A., Synek L., Zarsky V. & Fowler J. E. SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol 138, 2005–2018 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hala M. et al. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20, 1330–1345 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I. et al. Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytologist 188, 615–625 (2010). [DOI] [PubMed] [Google Scholar]
- Fendrych M. et al. The Arabidopsis Exocyst Complex Is Involved in Cytokinesis and Cell Plate Maturation. Plant Cell 22, 3053–3065 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. et al. Regulation of cytokinesis by exocyst subunit SEC6 and KEULE in Arabidopsis thaliana. Molecular plant (2013). [DOI] [PubMed] [Google Scholar]
- Wen T. J., Hochholdinger F., Sauer M., Bruce W. & Schnable P. S. The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol 138, 1637–1643 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Immink R., Liu C. M., Emons A. M. & Ketelaar T. The Arabidopsis exocyst subunit SEC3A is essential for embryo development and accumulates in transient puncta at the plasma membrane. New Phytologist 199, 74–88 (2013). [DOI] [PubMed] [Google Scholar]
- Sanders P. M. et al. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sexual Plant Reproduction 11, 297–322 (1999). [Google Scholar]
- Preuss D., Rhee S. Y. & Davis R. W. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science 264, 1458–1460 (1994). [DOI] [PubMed] [Google Scholar]
- Rhee S. Y. & Somerville C. R. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. The Plant journal 15, 79–88 (1998). [DOI] [PubMed] [Google Scholar]
- Zinkl G. M., Zwiebel B. I., Grier D. G. & Preuss D. Pollen-stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 126, 5431–5440 (1999). [DOI] [PubMed] [Google Scholar]
- Twell D., Yamaguchi J. & McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109, 705–713 (1990). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. The Journal of cell biology 180 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Roeber B. & Pical C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol 130 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. et al. Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. The Plant journal 52, 803–816 (2007). [DOI] [PubMed] [Google Scholar]
- Ischebeck T., Stenzel I. & Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell 20, 3312–3330 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa E., Kost B. & Malho R. Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell 20, 3050–3064 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi. D. Q. & Yang W. C. Pollen Germination and Tube Growth. Plant Developmental Biology – Biotechnological Perspectives 1 (2010). [Google Scholar]
- Lalanne E. et al. SETH1 and SETH2, two components of the glycosylphosphatidylinositol anchor biosynthetic pathway, are required for pollen germination and tube growth in Arabidopsis. Plant Cell 16, 229–240 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. J. & Fulcher R. G. Dye interactions. A basis for specific detection and histochemistry of polysaccharides. The journal of histochemistry and cytochemistry 31, 823–826 (1983). [DOI] [PubMed] [Google Scholar]
- Synek L. et al. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. The Plant journal 48, 54–72 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch D. et al. Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger F. P., Hughes T. E. & Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92, 559–571 (1998). [DOI] [PubMed] [Google Scholar]
- Boyd C., Hughes T., Pypaert M. & Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. Journal of Cell Biology 167, 889–901 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Tamanoi F. & Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nature cell biology 3, 353–360 (2001). [DOI] [PubMed] [Google Scholar]
- Yamashita M. et al. Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nature structural & molecular biology 17, 180–186 (2010). [DOI] [PubMed] [Google Scholar]
- Zheng Z. L. & Yang Z. The Rrop GTPase switch turns on polar growth in pollen. Trends Plant Sci 5, 298–303 (2000). [DOI] [PubMed] [Google Scholar]
- Lavy M. et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Current biology: CB 17, 947–952 (2007). [DOI] [PubMed] [Google Scholar]
- Li S., Gu Y., Yan A., Lord E. & Yang Z. B. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Molecular plant 1, 1021–1035 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. et al. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. The Journal of cell biology 145, 317–330 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. P. Differential staining of aborted and nonaborted pollen. Stain technology 44, 117–122 (1969). [DOI] [PubMed] [Google Scholar]
- Ye J. et al. Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell 21, 3868–3884 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J. & Bent A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant journal 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- Willats W. G. et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J Biol Chem 276, 19404–19413 (2001). [DOI] [PubMed] [Google Scholar]
- Dardelle F. et al. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol 153, 1563–1576 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y., Kaneda M., Zerzour R. & Geitmann A. The cell wall of the Arabidopsis pollen tube spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160, 1940–1955 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.