Abstract
Background
Low-normal thyroid function within the euthyroid range may contribute to increased atherosclerosis susceptibility. The leptin/adiponectin (L/A) ratio is associated with cardiovascular disease and reflects adipose tissue dysfunction. Relationships of the L/A ratio with low-normal thyroid function are unknown.
Methods
Relationships of thyroid stimulating hormone (TSH) and free thyroxine (free T4) with leptin, adiponectin and the L/A ratio in euthyroid subjects were documented in 67 fasting subjects with metabolic syndrome (Mets) and 86 euthyroid subjects without MetS (TSH and free T4 levels within the institutional reference range).
Results
Neither plasma leptin nor adiponectin was significantly correlated with TSH or free T4 in subjects with and without MetS. In the whole group, high sensitivity C-reactive protein (hs-CRP) was positively correlated with the L/A ratio (r = 0.485, P < 0.001). Notably, the L/A ratio was positively correlated with TSH in subjects with MetS (r = 0.252, P = 0.040) but not in subjects without MetS (r = −0.068, P = 0.54; interaction term, P = 0.027). In MetS subjects, the L/A ratio remained positively related with TSH after adjustment for age, sex, diabetes status, hs-CRP and the use of antihypertensive and glucose lowering medication (β = 0.283, P = 0.018), as well as after adjustment for individual MetS components (β = 0.294, P = 0.020).
Conclusions
In the context of MetS, a higher TSH within the euthyroid range confers an increased L/A ratio, a proposed marker of atherosclerosis susceptibility and adipocyte dysfunction.
Keywords: Adiponectin, Leptin, Leptin/adiponectin ratio, Metabolic syndrome, Thyroid function
Background
The concept is emerging that low-normal thyroid function, as inferred from a higher thyroid stimulating hormone (TSH) or a lower free thyroxine (free T4) within the euthyroid range, may adversely impact several health issues including the development of cardiovascular disorders [1, 2]. In line with this concept, low-normal thyroid function is associated with a greater increased intima media thickness (cIMT), an established biomarker of subclinical atherosclerosis [3, 4]. Furthermore, low-normal thyroid function is associated with increased coronary artery calcification [5] and progression thereof [6], although an association of a high-normal TSH level with increased coronary heart disease risk has been variably reported [7, 8].
Several factors are likely to contribute to the association of (subclinical) atherosclerosis with low-normal function. Low-normal thyroid function relates to higher plasma levels of total cholesterol and atherogenic apolipoprotein B-containing lipoproteins [9], and contributes to enhanced cholesteryl ester transfer from HDL to triglyceride-rich lipoproteins, a pro-atherogenic process [10, 11]. Low-normal thyroid function conveys changes in high density lipoprotein (HDL) anti-oxidative function as well, which conceivably contribute to impaired oxidative stress defense [12]. Interestingly, thyroid function status affects circulating levels of leptin and adiponectin, adipokines which exert pro- and anti-atherogenic properties, respectively [13–15]. Thus, leptin has been reported to decrease and adiponectin to increase after levothyroxine supplementation in subclinical hypothyroidism [16]. These findings provide a rationale to hypothesize that the plasma leptin/adiponectin (L/A) ratio is higher in subjects with low-normal thyroid function. Of note, the L/A ratio may represent a preferential marker compared to leptin and adiponectin alone in predicting incident cardiovascular disease [17, 18]. The L/A ratio is also considered to represent a biomarker of adipocyte dysfunction [19]. Higher plasma leptin and lower adiponectin levels are well known features of the metabolic syndrome (MetS) [20]. As a result, the L/A ratio is elevated in MetS [21–23], which supports the potential clinical relevance to determine relationships of low-normal thyroid function with the L/A ratio in subjects with MetS.
We initiated the present study to determine possible relationships of plasma leptin, adiponectin and the L/A ratio with TSH and free T4 in euthyroid subjects with and without MetS.
Methods
Subjects
The study protocol was approved by the medical ethics committee of the University Medical Center Groningen, the Netherlands, approved the study. Written informed consent was obtained from the participants, who were aged > 18 years. Type 2 Diabetes Mellitus (T2DM) and non-diabetic subjects were approached by advertisement in local newspapers. T2DM had been diagnosed previously by primary care physicians applying a fasting plasma glucose ≥ 7.0 mmol/l and/or non-fasting plasma glucose ≥11.1 mmol/l as diagnostic criteria. MetS was defined according to NCEP-ATP III criteria [24]. Three or more of the following criteria were required for categorization of subjects with MetS: waist circumference >102 cm for men and >88 cm for women; hypertension (blood pressure ≥ 130/85 mmHg or use of antihypertensive drugs); fasting plasma triglycerides ≥ 1.7 mmol/l; HDL cholesterol < 1.0 mmol/l for men and <1.3 mmol/l for women; fasting glucose ≥ 5.6 mmol/l.
Serum TSH and free T4 levels had to be within the institutional reference range, and anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-Tg) auto-antibodies had to be absent (see below).
Subjects with a history of cardiovascular disease (CVD), chronic kidney disease (estimated glomerular filtration rate < 60 ml/min/1.73 m2 or micro/macroalbuminuria), liver disorders (serum transaminase levels >two times the upper reference limit), as well as current smokers, subjects who used lipid lowering drugs or insulin were also excluded from participation as were pregnant women. The use of anti-hypertensive medication and oral contraceptives was allowed.
Physical examination did not reveal pulmonary or cardiac abnormalities. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Blood pressure was measured after 15 min of rest at the left arm using a sphygmomanometer. The participants were evaluated between 0800 and 1000 h after an overnight fast.
Laboratory analyses
Serum and EDTA-anticoagulated plasma samples, prepared by centrifugation at 1400 g for 15 min at 4 °C, were stored at −80 °C until analysis. Plasma glucose was measured shortly after blood collection.
Serum TSH (sandwich principle; Roche Diagnostics GmbH., Mannheim, Germany, cat. no. 117314591; reference range 0.5–4.0 mU/l) and free T4 (competition principle; Roche Diagnostics GmbH., Mannheim Germany, cat. no. 11731297; reference range 11.0–19.5 pmol/l) were measured with a electrochemiluminescence immunoassay on a Modular Analytics immunoassay analyzer. Anti-TPO and anti-Tg) auto-antibodies were measured with enzyme-linked immunoassays (ImmunoCap cat nos. 14-4508-35 and 14-4507-35, respectively; Phadia, Freiburg, Germany), and were considered to be positive using cut-off values provided by the supplier (anti-TPO antibodies > 60 IU/ml and anti-Tg antibodies > 289 IU/ml).
Plasma leptin and total adiponectin were assayed using commercially available assays (Luminex xMAP technology; Linco Research Inc., St Charles, MO, USA; Lincoplex panel A cat. no. HADK1-61 K-A and panel B cat. no. HADK2-61 K-B) [25]. All intra-assay and inter-assay coefficients of variation were <6 and <8%. High sensitivity C-reactive protein (hs-CRP) was determined was by nephelometry (BNII N; Dade Behring, Marburg Germany) [17].
Plasma total cholesterol and triglycerides were assayed by routine enzymatic methods (Roche/Hitachi cat nos 11875540 and 11876023, respectively; Roche Diagnostics GmbH, Mannheim, Germany). HDL cholesterol was measured with a homogeneous enzymatic colorimetric test (Roche/Hitachi, cat no 04713214; Roche Diagnostics GmbH, Mannheim, Germany). Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL cholesterol. Glucose was measured on an APEC glucose analyzer (APEC Inc., Danvers, MA, USA).
Statistical analysis
SPSS 22 (version 22.0, SPSS Inc., Chicago, IL, USA) was used for data analysis. Data are expressed as mean ± SD, median (interquartile ranges) or in numbers. Differences between people with and without MetS were determined by unpaired T-tests or Chi-square tests. Because of skewed distribution, triglycerides, hs-CRP, leptin, adiponectin and the L/A ratio were logarithmically transformed to compare between-group differences, and to perform correlation analyses.
Univariate relationships were calculated using Pearson correlation coefficients. Multivariable linear regression analyses were carried out to disclose the independent relationships of the L/A ratio with thyroid function parameters. To determine whether the relationships of thyroid function parameters with the L/A ratio were different between subjects with and without MetS, interaction terms were calculated as the product term between the thyroid function variable of interest and the presence of MetS. Two-sided P-values < 0.05 indicated statistical significance.
Results
We included 67 subjects with MetS and 86 subjects without MetS (Table 1). Forty nine subjects with MetS and 24 subjects without MetS were diagnosed with T2DM (P < 0.001). Twenty three subjects with MetS and seven subjects without MetS were taken anti-hypertensive drugs (mostly angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and diuretics, alone or in combination) (P < 0.001). Metformin and sulfonylurea were used, either alone or in combination, by 28 and 27 subjects with MetS, respectively. Of the subjects without MetS, 10 used metformin and 12 used sulfonylurea. Other glucose lowering drugs were not used. Estrogens were taken by two post-menopausal woman with MetS and by one pre-menopausal woman without MetS.
Table 1.
Clinical characteristics, glucose, lipids, leptin, adiponectin, the leptin/adionectin (L/A) ratio and thyroid function parameters in 67 subjects with metabolic syndrome (MetS) and in 86 subjects without MetS
| MetS (n = 67) |
No MetS (n = 86) |
P-value | |
|---|---|---|---|
| Age (years) | 58 ± 9 | 56 ± 9 | 0.10 |
| Sex (men/women) | 40/27 | 52/34 | 0.92 |
| Systolic blood pressure (mm Hg) | 144 ± 18 | 131 ± 20 | <0.001 |
| Diastolic blood pressure (mm Hg) | 89 ± 9 | 81 ± 10 | <0.001 |
| BMI (kg/m2) | 29.8 ± 4.4 | 25.1 ± 3.3 | <0.001 |
| Waist (cm) | 105 ± 12 | 87 ± 11 | <0.001 |
| Glucose (mmol/l) | 8.6 ± 2.7 | 6.2 ± 1.4 | <0.001 |
| Total cholesterol (mmol/l) | 5.54 ± 0.97 | 5.62 ± 0.97 | 0.60 |
| Non-HDL cholestrerol (mmol/l) | 4.38 ± 0.98 | 4.07 ± 1.05 | 0.068 |
| HDL cholesterol (mmol/l) | 1.16 ± 0.34 | 1.55 ± 0.38 | <0.001 |
| Triglycerides (mmol/l) | 1.96 (1.70-2.52) | 1.15 (0.85-1.55) | <0.001 |
| hs-CRP (mg/l) | 2.03 (1.26-4.22) | 1.02 (0.48-2.25) | <0.001 |
| Leptin (μg/l) | 11.5 (5.5-31.9) | 4.8 (3.0-11.6) | <0.001 |
| Adiponectin (mg/l) | 14.7 (9.2-26.8) | 18.2 (14.2-38.8) | <0.001 |
| L/A ratio (μg/mg) | 0.83 (0.37-1.85) | 0.23 (0.12-0.62) | <0.001 |
| TSH (mU/l) | 1.55 ± 0.70 | 1.66 ± 0.68 | 0.37 |
| Free T4 (pmol/l) | 13.9 ± 1.59 | 13.8 ± 1.47 | 0.82 |
Data in mean ± SD or in median (interquartile range). BMI body mass index, hs-CRP high sensitivity C-reactive protein, TSH thyroid stimulating hormone, free T4 free thyroxine. Logarithmically transformed values of triglycerides, hs-CRP, leptin, adiponectin and the L/A ratio are used for statistical comparisons
Age, sex distribution, TSH and free T4 levels were not significantly different between subjects with and without MetS (Table 1). Blood pressure, BMI, waist, plasma glucose and triglycerides were expectedly higher, whereas HDL cholesterol was lower in MetS subjects. Total cholesterol and non-HDL cholesterol were not significantly different between the groups. Plasma hs-CRP was elevated in MetS subjects. Furthermore, leptin was increased and adopinectin was decreased in MetS subjects (Table 1). As a result, the L/A ratio was approximately three-fold higher in MetS subjects.
In the whole group, hs-CRP was correlated positively with leptin (r = 0.435, P < 0.001) and inversely with adiponectin in univariate analysis (r = −0.289, P < 0.001). Consequently, hsCRP was correlated positively with the L/A ratio (r = 0.485, P < 0.001). In the whole group, neither plasma leptin nor adiponectin or the L/A ratio were significantly correlated with TSH or with and free T4 (P > 0.40 for all; data not shown). Furthermore, neither plasma leptin nor adiponectin were significantly correlated with TSH and free T4 in subjects with or without MetS (Table 2). However, the L/A ratio was positively correlated with TSH in MetS subjects, contrasting the lack of such a relationship in subjects without MetS (Table 3; Fig. 1). Indeed, the relationship of the L/A ratio with TSH was different between subjects with and without MetS (interaction term for between group difference: β = 0.201, P = 0.027). The positive relation of the L/A ratio with TSH was also present in MetS subjects who did not use antihypertensive drugs (n = 43; r = 0.329, P = 0.034) or metformin (n = 39; r = 0.285, P = 0.070), again without such relationships being found in subjects without MetS (n = 79; r = −0.026, P = 0.82 and n = 76, r = −0.047, P = 0.69, respectively).
Table 2.
Univariate relationships of leptin, adiponectin and the leptin/adiponectin (L/A) ratio with thyroid stimulating hormone (TSH) and free thyroxine (free T4) in 67 subjects with metabolic syndrome (MetS) and in 86 subjects without MetS
| MetS (n = 67) |
No MetS (n = 86) |
|||
|---|---|---|---|---|
| TSH | free T4 | TSH | free T4 | |
| Leptin | 0.168 | 0.020 | −0.013 | −0.113 |
| Adiponectin | −0.090 | −0.046 | 0.154 | 0.056 |
| L/A ratio | 0.252* | 0.031 | −0.068 | −0.106 |
Pearson correlation coefficients are shown. Logarithmically transformed values of leptin, adiponectin, the L/A ratio are used for statistical comparisons. *P = 0.04
Table 3.
Multivariable linear regression analysis demonstrating the relationship of the leptin/adiponectin (L/A) ratio with thyroid stimulating hormone (TSH), age, sex and diabetes status and high sensitivity C-reactive protein (hs-CRP) in 67 subjects with metabolic syndrome (MetS; A) and in 86 subjects without MetS (B)
| L/A ratio | ||
|---|---|---|
| β | P-value | |
| A MetS (n = 67) | ||
| Age | −0.130 | 0.25 |
| Sex (women vs. men) | 0.278 | 0.018 |
| T2DM | −0.049 | 0.74 |
| hs-CRP | 0.150 | 0.21 |
| TSH | 0.289 | 0.018 |
| B No MetS (n = 86) | ||
| Age | −0.076 | 0.39 |
| Sex (women vs. men) | 0.431 | <0.001 |
| T2DM | 0.002 | 0.98 |
| hs-CRP | 0.430 | <0.001 |
| TSH | −0.119 | 0.22 |
T2DM: type 2 diabetes mellitus; β: standardized regression coefficient. The L/A ratio and hs-CRP are logarithmically transformed. Models are additionally adjusted for the use of antihypertensive medication, sulfonylurea and metformin
Fig. 1.
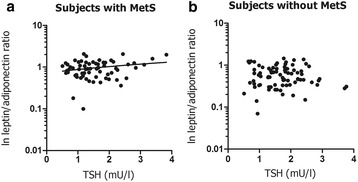
Relationship of the leptin/adionectin (L/A) ratio with thyroid stimulating hormone (TSH in 67 subjects with metabolic syndrome (MetS) (a: r = 0.252, P = 0.040) and 86 subjects without MetS (b: r = −0.068, P = 0.54). The L/A ratio is logarithmically transformed
Multivariable linear regression analysis demonstrated that the positive relationship of the L/A ratio with TSH in MetS subjects remained present after adjustment for age, sex, diabetes status and the use of antihypertensive and glucose lowering medication (Table 3A). No such relationship was found in subjects without MetS (Table 3B). In alternative analysis, the L/A ratio was also positively related to TSH in MetS subjects when taking the individual MetS components into account and thus being associated with TSH independent of an enlarged waist (Table 4A). Additionally, the L/A ratio was positively related to an enlarged waist in subjects without MetS (Table 4A and 4B). In the whole group, the L/A ratio was independently associated with female sex (β = 0.338, P < 0.001), hs-CRP (β = 0.262, P < 0.001) and an enlarged with waist (β = 0.204, P = 0.005) without an independent association with TSH (β = 0.079, P = 0.214; data not shown).
Table 4.
Multivariable linear regression analysis demonstrating the relationship of the leptin/adiponectin (L/A) ratio with thyroid stimulating hormone (TSH), age, sex, metabolic syndrome (MetS) components and high sensitivity C-reactive protein (hs-CRP) in 67 subjects with MetS (A) and in 86 subjects without MetS (B)
| L/A ratio | P-value | |
|---|---|---|
| β | ||
| A MetS (n = 67) | ||
| Age | −0.154 | 0.19 |
| Sex (women vs. men) | 0.214 | 0.082 |
| Elevated glucose | 0.053 | 0.69 |
| Hypertension | 0.138 | 0.25 |
| Enlarged waist | 0.201 | 0.12 |
| Elevated triglycerides | −0.064 | 0.61 |
| Low HDL cholesterol | 0.172 | 0.17 |
| hs-CRP | 0.090 | 0.47 |
| TSH | 0.294 | 0.020 |
| B No MetS (n = 86) | ||
| Age | −0.028 | 0.76 |
| Sex (women vs. men) | 0.53 | <0.001 |
| Elevated glucose | 0.097 | 0.31 |
| Hypertension | 0.090 | 0.38 |
| Enlarged waist | 0.310 | <0.001 |
| Elevated triglycerides | 0.177 | 0.047 |
| Low HDL cholesterol | 0.072 | 0.38 |
| hs-CRP | 0.299 | <0.001 |
| TSH | −0.060 | 0.48 |
HDL high density lipoproteins, β standardized regression coefficient. The L/A ratio and hs-CRP are logarithmically transformed. Models are additionally adjusted for the use of sulfonylurea and metformin
Discussion
This study reveals to our knowledge for the first time that the plasma L/A ratio is positively related to a higher TSH level in euthyroid subjects with MetS but not in subjects without MetS. This relationship in MetS subjects remained present when taking relevant covariates into account, including the presence of diabetes, hs-CRP and the use of antihypertensive and oral glucose lowering drugs, and was also present in analysis in which we adjusted for individual MetS components, including an enlarged waist circumference. The current results, therefore, suggest that the L/A ratio, an alleged predictor of cardiovascular disease and biomarker of adipocyte dysfunction [17, 19] associates with low-normal thyroid function. Altogether, the present findings add to accumulating evidence which underscores the possibility that low-normal thyroid function may confer increased atherosclerosis susceptibility [1, 2, 9].
We enrolled strictly euthyroid subjects, as inferred from TSH and free T4 levels within the institutional reference range. With this selection criterion, TSH and free T4 were similar in subjects with MetS compared to subjects without MetS. This entirely agrees with our previous findings in a small group of non-diabetic subjects [26], although mild thyroid function changes in MetS have been documented in another study [9]. Leptin and adiponectin play an important role in obesity-associated metabolic risk by modulating inflammatory processes and affecting insulin sensitivity [19, 20, 27]. In agreement, we found that the L/A ratio was positively related to hs-CRP in univariate analysis. Given the associations of plasma leptin and adiponectin with (central) obesity, the strong elevations in the L/A ratio in MetS subjects as demonstrated here is not surprising [21–23]. Accordingly, waist circumference predicted the L/A ratio in the present study, even independent of hyperglycemia and other MetS components. We consider this finding reassuring because a considerable number of participants had been diagnosed with type 2 diabetes, making that the number subjects without diabetes was too low to allow for meaningful subgroup analysis.
Clinical observations showing that plasma leptin decreases whereas adiponectin increases after levothyroxine substitution in subjects with subclinical hypothyroidism [16] prompted us to delineate the relationship of the L/A ratio with low-normal thyroid function. In concert with these human findings [16], thyroid hormone upregulates adiponectin gene expression in rat adipose tissue [28]. In contrast, leptin gene expression in rat epididymal fat is downregulated after experimental hyperthyroidism, although lower circulating leptin levels in response to high thyroid hormone exposure are at least in part attributable to a decrease in body fat [29]. It remains to be more precisely determined why there was only a relation of the L/A ratio with TSH in the MetS subjects. In comparison, the relationship with low-normal thyroid function with other pro-atherogenic biomarkers have been demonstrated previously to be particularly evident in diabetic or MetS subjects [2, 9, 10]. Our present observation that this relationship remained present after adjustment for waist circumference would be consistent with a contribution of thyroid function status on this ratio.
A number of other limitations and methodological aspects of our study need to be discussed. First, we performed a cross-sectional study, making that cause-effect relationships cannot be established with certainty. However, we are not aware of published data indicating that the leptin or adiponectin directly affect thyroid hormone regulation. Second, we relied on a single set of thyroid function parameters. In this regard it is noteworthy that each individual probably has a narrow set-point of thyroid function status, underscoring the pathophysiological relevance of once measured thyroid function status [30]. Third, circulating adiponectin increases in response to angiotensin II, making that an effect of the antihypertensive medication used cannot by excluded [31]. Fourth, metformin lowers of the TSH level in hypothyroid subjects and thus could alter the set-point of the pituitary-thyroid axis [32, 33]. Notably, metformin treatment does not elicit TSH changes in euthyroid subjects [33, 34]. For these reasons, we adjusted for the use of antihypertensive and glucose lowering drugs in multivariable regression analysis, confirming the independent relation of the L/A ratio with TSH in MetS. In addition, the positive relation of the L/A ratio with TSH in MetS was also present after exclusion of subjects taking antihypertensive medication or metformin, indicating that the use of these medications did not confound the interpretation of our data.
The interest for a neutraceutical approach to improve the cardiometabolic risk profile besides well-established pharmacological treatment modalities is growing [35]. In this respect it is noteworthy that a combination of several compounds including red yeast rice extract, berberine, policosanol, astaxanthin, coenzyme Q10 and folic acid has been shown to reduce the L/A ratio besides low density lipoprotein (LDL) cholesterol lowering [36]. Given the elevated L/A ratio in MetS, additional studies with respect to a neutraceutical approach to ameliorate pro-atherogenic biomarkers appear to be warranted.
Conclusions
A higher TSH level within the euthyroid range confers an increased L/A ratio in MetS subjects, which is likely to contribute to an adverse cardiometabolic profile in this patient category.
Acknowledgments
Dr. A.C. Muller-Kobold, PhD, Laboratory Center, University Medical Center Groningen, The Netherlands, determined thyroid function parameters. Dr. L.D. Dikkeschei, PhD, Laboratory of Clinical Chemistry, Isala Clinics, Zwolle, The Netherlands, determined plasma lipids. Miss B. Haandrikman, University Medical Center Groningen, The Netherlands, carried out the leptin and adiponectin assays.
Funding
No financial support from an external agency was used for this study.
Availability of data and material
Proprietary algorithms used to generate the data in this manuscript will not be made available for general use. Data may be made available by request to the corresponding author.
Author’s contributions
RPFD performed the statistical analyses. RPFD and LJNvanT-W interpreted the data. LJNvanT-W wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
This study is investigator initiated. The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the medical ethics committee of the University Medical Center Groningen.
Disclosure statement
The authors declared no conflict of interests.
Abbreviations
- anti-Tg
Anti-thyroglobulin
- anti-TPO
Anti-thyroid peroxidase
- BMI
Body mass index
- cIMT
Intima media thickness
- CVD
Cardiovascular disease
- free T4
Free thyroxine
- HDL
High density lipoprotein
- hs-CRP
High sensitivity C-reactive protein
- L/A ratio
Leptin/adiponectin
- LDL
Low density lipoprotein
- MetS
Metabolic syndrome
- T2DM
Type 2 diabetes mellitus
- TSH
Thyroid stimulating hormone
Contributor Information
Lynnda J. N. van Tienhoven-Wind, Phone: +31 50 361 3731, Email: L.J.N.van.Tienhoven-Wind@umcg.nl
Robin P. F. Dullaart, Email: R.P.F.Dullaart@umcg.nl
References
- 1.Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98:3562–71. doi: 10.1210/jc.2013-1315. [DOI] [PubMed] [Google Scholar]
- 2.van Tienhoven-Wind LJ, Dullaart RP. Low-normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur J Clin Invest. 2015;45:494–503. doi: 10.1111/eci.12423. [DOI] [PubMed] [Google Scholar]
- 3.Dullaart RP, de Vries R, Roozendaal C, Kobold AC, Sluiter WJ. Carotid artery intima media thickness is inversely related to serum free thyroxine in euthyroid subjects. Clin Endocrinol (Oxf) 2007;67:668–73. doi: 10.1111/j.1365-2265.2007.02943.x. [DOI] [PubMed] [Google Scholar]
- 4.Takamura N, Akilzhanova A, Hayashida N, et al. Thyroid function is associated with carotid intima-media thickness in euthyroid subjects. Atherosclerosis. 2009;204(2):e77–81. doi: 10.1016/j.atherosclerosis.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Kim BK, Chang Y, et al. Thyroid hormones and coronary artery calcification in euthyroid men and women. Arterioscler Thromb Vasc Biol. 2014;34:2128–34. doi: 10.1161/ATVBAHA.114.303889. [DOI] [PubMed] [Google Scholar]
- 6.Park HJ, Kim J, Han EJ, et al. Association of low baseline free thyroxin levels with progression of coronary artery calcification over four years in euthyroid subjects: The Kangbuk Samsung Health Study. Clin Endocrinol (Oxf) 2016;84:889–95. doi: 10.1111/cen.12946. [DOI] [PubMed] [Google Scholar]
- 7.Åsvold BO, Vatten LJ, Bjøro T, Thyroid Studies Collaboration et al. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA Intern Med. 2015;175:1037–47. doi: 10.1001/jamainternmed.2015.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue K, Tsujimoto T, Saito J, Sugiyama T. Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid. 2016;26:1457–1465. doi: 10.1089/thy.2016.0156. [DOI] [PubMed] [Google Scholar]
- 9.van Tienhoven-Wind LJ, Dullaart RP. Low-normal thyroid function and novel cardiometabolic biomarkers. Nutrients. 2015;7:1352–77. doi: 10.3390/nu7021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triolo M, Kwakernaak AJ, Perton FG, de Vries R, Dallinga-Thie GM, Dullaart RP. Low normal thyroid function enhances plasma cholesteryl ester transfer in Type 2 diabetes mellitus. Atherosclerosis. 2013;228:466–71. doi: 10.1016/j.atherosclerosis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Kappelle PJ, Perton F, Hillege HL, Dallinga-Thie GM, Dullaart RP. High plasma cholesteryl ester transfer but not CETP mass predicts incident cardiovascular disease: a nested case–control study. Atherosclerosis. 2011;217:249–52. doi: 10.1016/j.atherosclerosis.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Triolo M, de Boer JF, Annema W, Kwakernaak AJ, Tietge UJ, Dullaart RP. Low normal free T4 confers decreased high-density lipoprotein antioxidative functionality in the context of hyperglycaemia. Clin Endocrinol (Oxf) 2013;79:416–23. doi: 10.1111/cen.12138. [DOI] [PubMed] [Google Scholar]
- 13.Dallinga-Thie GM, Dullaart RPF. Do genome-wide association scans provide additional information on the variation of plasma adiponectin concentrations? Atherosclerosis. 2010;208:328–9. doi: 10.1016/j.atherosclerosis.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Diekman MJ, Romijn JA, Endert E, Sauerwein H, Wiersinga WM. Thyroid hormones modulate serum leptin levels: observations in thyrotoxic and hypothyroid women. Thyroid. 1998;8:1081–6. doi: 10.1089/thy.1998.8.1081. [DOI] [PubMed] [Google Scholar]
- 15.Bossowski A, Sawicka B, Szalecki M, Koput A, Wysocka J, Zelazowska-Rutkowska B. Analysis of serum adiponectin, resistin and leptin levels in children and adolescents with autoimmune thyroid disorders. J Pediatr Endocrinol Metab. 2010;23:369–77. doi: 10.1515/jpem.2010.058. [DOI] [PubMed] [Google Scholar]
- 16.Yildiz BO, Aksoy DY, Harmanci A, et al. Effects of L-thyroxine therapy on circulating leptin and adiponectin levels in subclinical hypothyroidism: a prospective study. Arch Med Res. 2013;44:317–20. doi: 10.1016/j.arcmed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Kappelle PJ, Dullaart RP, van Beek AP, Hillege HL, Wolffenbuttel BH. The leptin/adiponectin ratio predicts first cardiovascular event in men: a prospective nested case–control study. Eur J Intern Med. 2012;23:755–9. doi: 10.1016/j.ejim.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Seven E, Husemoen LL, Sehested TS, Ibsen H, Wachtell K, Linneberg A, Jeppesen JL. Adipocytokines, C-reactive protein, and cardiovascular disease: a population-based prospective study. PLoS One. 2015;10:e0128987. doi: 10.1371/journal.pone.0128987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finucane FM, Luan J, Wareham NJ, European Group for the Study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular Disease Risk Study Group) Savage DB, et al. Correlation of the leptin: adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52:2345–9. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blüher M, Mantzoros CS. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–145. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Dullaart RP, Kappelle PJ, Dallinga-Thie GM. Carotid intima media thickness is associated with plasma adiponectin but not with the leptin:adiponectin ratio independently of metabolic syndrome. Atherosclerosis. 2010;211:393–6. doi: 10.1016/j.atherosclerosis.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Cicero AF, Magni P, Moré M, Ruscica M, Borghi C, Strollo F, Brisighella Heart Study Staff Metabolic syndrome, adipokines and hormonal factors in pharmacologically untreated adult elderly subjects from the Brisighella Heart Study historical cohort. Obes Facts. 2012;5:319–26. doi: 10.1159/000339575. [DOI] [PubMed] [Google Scholar]
- 23.López-Jaramillo P, Gómez-Arbeláez D, López-López J, et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18:37–45. doi: 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Dullaart RP, de Vries R, van Tol A, Sluiter WJ. Lower plasma adiponectin is a marker of increased intima-media thickness associated with type 2 diabetes mellitus and with male gender. Eur J Endocrinol. 2007;156:387–94. doi: 10.1530/EJE-06-0681. [DOI] [PubMed] [Google Scholar]
- 26.Dullaart RP, van den Berg EH, van der Klauw MM, Blokzijl H. Low normal thyroid function attenuates serum alanine aminotransferase elevations in the context of metabolic syndrome and insulin resistance in white people. Clin Biochem. 2014;47:1028–32. doi: 10.1016/j.clinbiochem.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Vega GL, Grundy SM. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes. 2013;2013:409679. doi: 10.1155/2013/409679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifi S, Tabandeh MR, Nazifi S, Saeb M, Shirian S, Sarkoohi P. Regulation of adiponectin gene expression in adipose tissue by thyroid hormones. Physiol Biochem. 2012;68:193–203. doi: 10.1007/s13105-011-0131-1. [DOI] [PubMed] [Google Scholar]
- 29.Syed MA, Thompson MP, Pachucki J, Burmeister LA. The effect of thyroid hormone on size of fat depots accounts for most of the changes in leptin mRNA and serum levels in the rat. Thyroid. 1999;9:503–12. doi: 10.1089/thy.1999.9.503. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JP. Setpoints and susceptibility: Do small differences in thyroid function really matter? Clin Endocrinol (Oxf) 2011;75:158–159. doi: 10.1111/j.1365-2265.2011.04036.x. [DOI] [PubMed] [Google Scholar]
- 31.Lely AT, Krikken JA, Bakker SJ, et al. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab. 2007;92:1821–6. doi: 10.1210/jc.2006-2092. [DOI] [PubMed] [Google Scholar]
- 32.Rotondi M, Pirola I, Agosti B, et al. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care. 2009;32:1589–90. doi: 10.2337/dc09-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Díez JJ, Iglesias P. Relationship between serum thyrotropin concentrations and metformin therapy in euthyroid patients with type 2 diabetes. Clin Endocrinol (Oxf) 2013;78:505–11. doi: 10.1111/j.1365-2265.2012.04468.x. [DOI] [PubMed] [Google Scholar]
- 34.Lupoli R, Di Minno A, Tortora A, Ambrosino P, Lupoli GA, Di Minno MN. Effects of treatment with metformin on TSH levels: a meta-analysis of literature studies. J Clin Endocrinol Metab. 2014;99:E143–8. doi: 10.1210/jc.2013-2965. [DOI] [PubMed] [Google Scholar]
- 35.Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 2014;6:11–32. doi: 10.1016/j.jff.2013.12.006. [DOI] [Google Scholar]
- 36.Ruscica M, Gomaraschi M, Mombelli G, et al. Nutraceutical approach to moderate cardiometabolic risk: results of a randomized, double-blind and crossover study with Armolipid Plus. J Clin Lipidol. 2014;8:61–8. doi: 10.1016/j.jacl.2013.11.003. [DOI] [PubMed] [Google Scholar]


