Abstract
The monolayer of endothelial cells lining the vessel wall forms a semi-permeable barrier (in all tissue except the relatively impermeable blood-brain and inner retinal barriers) that regulates tissue-fluid homeostasis, transport of nutrients, and migration of blood cells across the barrier. Permeability of the endothelial barrier is primarily regulated by a protein complex called adherens junctions (AJs). AJs are not static structures: they are continuously remodeled in response to mechanical and chemical cues in both physiological and pathological settings. Here we discuss recent insights into the post-translational modifications of junctional proteins and signaling pathways regulating plasticity of AJs and endothelial permeability. We also discuss in the context of what is already known and newly defined signaling pathways that mediate endothelial barrier leakiness (hyper-permeability) that are important in the pathogenesis of cardiovascular and lung diseases and vascular inflammation.
Keywords: adherens junctions, signal transduction, mechanosensing, small RhoGTPases, vascular disorders
Subject codes: Cell Signaling, Endothelium, Vascular Biology
1. Endothelial barrier integrity in health and disease
The endothelium lining the intima of all blood and lymphatic vessels forms a semi-permeable barrier between circulating plasma and the interstitium. Inter-endothelial junctions connect endothelial cells into a contiguous monolayer to restrict the transport of proteins across the endothelial barrier in a size-selective manner 1–3. Molecules of 3 nm radii or less passively diffuse through junctions while high molecular weight proteins such as albumin (67 kD), constituting 75% of protein in the plasma, and blood cells are largely retained in the circulation 2–4.
Inter-endothelial junctions are the main structures maintaining tissue-fluid homeostasis. The plasma oncotic pressure derived from the circulating albumin is the main factor contributing to passive re-absorption of fluid and solutes back into the circulation 5. Loss of inter-endothelial junctions as the result of an acute or chronic process leads to flux of proteinaceous fluid into the interstitium causing tissue edema. This is a common cause of a broad range of pathological conditions in humans including systemic capillary leak syndrome 6, angioedema 7, anaphylaxis 8, acute respiratory distress syndrome 9, 10, and age-related and diabetic-associated eye diseases 11–13, various disorders of central nervous system 14–17. Hence, elucidating signaling mechanisms responsible for control of junction integrity is of fundamental importance to developing novel therapeutic strategies for treating edema.
2. Role of inter-endothelial junctions in regulating endothelial barrier function
Inter-endothelial junctions are composed of protein complexes of adherens junctions (AJs), tight junctions (TJs), and gap junctions (GJs) 18–21 (Figure 1a). Both AJs and TJs form pericellular zipper-like structures along endothelial cell borders through adhesion of distinct adhesive proteins 18–20. In contrast, gap junctions are intercellular channels enabling direct electrical and chemical communication between endothelial cells through the passage of ions and signaling molecules with a size of 1kD or less 22.
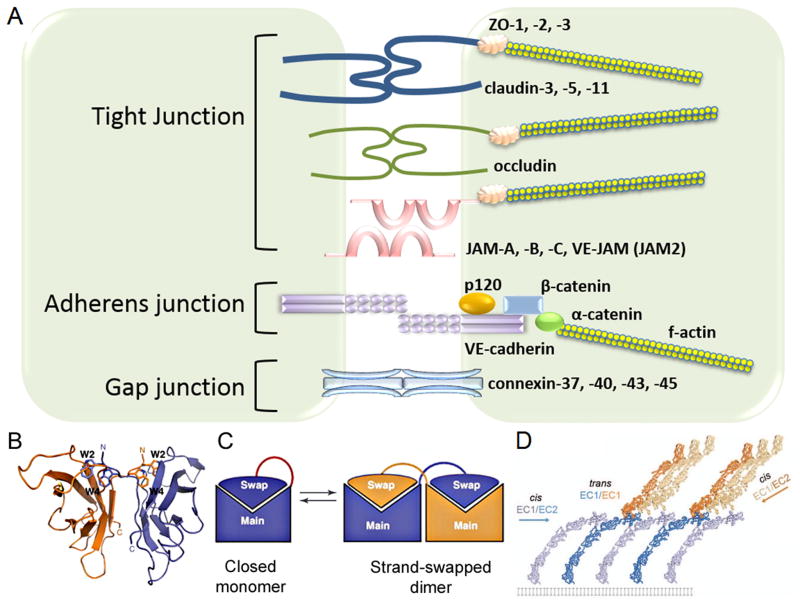
Figure 1. Composition of inter-endothelial junctions.
A) Schematic representation of inter-endothelial junctions comprised of tight junctions (TJs), adherens junctions (AJs), and gap junctions (GJs). TJs are mediated by adhesion proteins such as claudins, occludin, and junctional adhesion molecules (JAMs) whereas the zona occludin proteins (ZO-1, ZO-2 and ZO-3) connect adhesion molecules to the actin cytoskeleton. AJs are comprised of VE-cadherin and associated β- and p120-catenins. α-catenin binds β-catenin to connect AJs to the actin cytoskeleton. GJs are comprised of two connexin hexamers forming hemichannels.
B) VE-cadherin mediates adhesion by trans-dimerization of tryptophan 2 and tryptophan 4 residues in a hydrophobic pocket of the opposing VE-cadherin molecule. Ribbon presentations of VE-cadherin trans-dimer. Adapted with permission from Brasch et al., Trends in Cell Biology, 2012.
C) Strand swap dimerization occurs through the insertion of a tryptophan residue into the hydrophobic binding pocket of the opposing cadherin. Adapted with permission from Brasch et al., Trends in Cell Biology, 2012.
D) Trans dimerization (between EC1 and EC1 of opposing cadherins) orients VE-cadherin molecules and facilitates cis interactions (between EC1 and EC2 of neighboring cadherins). Adapted with permission from Brasch et al., Trends in Cell Biology, 2012.
Gap junctions
A functional gap junction is comprised of two hemichannels aligned in the plasma membrane of adjacent endothelial cells 23. Each hemichannel consists of six connexin molecules, assembled within the ER or trans-Golgi 24–26. The hemichannel can be either homomeric or a heteromeric; i.e., assembled by the same or distinct connexin isoforms, respectively 27. Channels comprised of different isoforms might exhibit altered activities in respect to ion selectivity and permeability as compared to homomeric channels 28–30.
The three major connexin isoforms expressed in systemic arteriolar endothelial cells are Cx37, Cx40, Cx43 31, 32. These gap junctions are responsible for communication between endothelial and endothelial-smooth muscle cells 33, 34. In animal models, deletion of Cx43 in endothelial cells causes hypotension 35, whereas deletion of Cx40 leads to hypertension associated with dysregulation of renin system 36–38. Interestingly, Cx43-mediated gap junctions elicited distinct functions in pulmonary circulation 39. These junctions contribute to conduction of Ca2+ between endothelial cells in lung capillaries and induce the expression of P-selectin, the cell surface adhesion molecule involved in the recruitment of leukocytes to sites of injury in post-capillary venules 39. Hence, Cx43-mediated gap junctions are critical for regulation of vascular tone in the systemic circulation and contribute to the propagation of pro-inflammatory signaling in pulmonary capillary beds.
Tight junctions
The architecture and composition of endothelial TJs varies in different vascular beds 19, 40–42. For example, TJs are more developed in small arterioles whereas AJs are more predominant in post-capillary venules 19, 40. TJs are localized at the outermost part of inter-endothelial junctions but can also be intermingled with AJs 40, 43–45. In contrast to the peripheral microcirculation, highly specialized vascular beds such as the blood brain barrier (BBB) and the inner blood–retinal barrier (iBRB) where exchange of solutes between microvessel and brain is restricted 18, 46, TJs are predominant in forming extensive networks at the apical side of inter-endothelial junctions (for reviews, see 47). Disruption of TJs is associated with BBB and iBRB leakage, a characteristic of multiple human diseases including diabetic and oxygen-induced retinopathy 48 and disorders of the central nervous system such as stroke 49–51.
TJs are comprised of several adhesive proteins including occludin, claudins, and junctional adhesion molecules (JAMs) 52–57 (for reviews, see 58). Claudin 5 is ubiquitously expressed in all vascular beds whereas claudin 1, 3, and 12 are specific to the brain microvasculature 59–61. Claudin-1, -2, and -5 are found in TJs of retinal vessels 48. Claudins and occludins, in association with cytosolic Zonula occludens (ZO)-1, 2 and 3 proteins assemble “zipper-like” structures along the rim of endothelial cells 62, 63. The role of JAM-A in the organization of TJs is less understood.
The integrity of the BBB is crucial to the proper functioning of the central nervous system. Disruption of the BBB associated with trauma, hemorrhagic stroke, rupture of cerebral aneurysm, and inflammation leads to serious consequences ranging from progressive neuronal dysfunction, sclerosis, brain edema, paralysis, and death 64 (for reviews, see 65, 66). Studies in animal models demonstrate that deletion of the claudin-5 gene cldn5 causes death in newborn animals due to increased permeability of the BBB in a size-selective manner 59. Claudin-5 is targeted for degradation by matrix metalloproteinase after an ischemic insult, and loss of claudin-5 is responsible for disruption of the BBB in ischemic stroke 51. In contrast, loss of claudin-3 but not claudin-5 or occludin accounted for breakdown of the BBB in experimental models of allergic encephalomyelitis and glioblastoma multiforme 60 suggesting that claudin proteins might have distinct and indispensable roles in regulating the organization of TJs in brain circulation. Deletion of the occludin gene ocln causes no apparent defects in the organization or strength of TJs 67 suggesting that its function is compensated by other adhesive proteins.
In contrast to the BBB, neither claudin nor ocludin proteins are downregulated in the iBRB using experimental models of diabetic and oxygen-induced retinopathy 48, 68. In fact, expression of claudin-2 and -5 is upregulated in oxygen-induced retinopathy 48 suggesting that breakdown of the iBRB is associated with post-translational modifications of adhesion proteins of TJs. PKCζ is known to facilitate the formation of TJs through phosphorylation of occludin 69. Hyper-activation of PKCζ observed in type 2 diabetic induced the mis-localization of occludin and disruption of TJs resulting in increased leakage of plasma proteins into the retina 70, 71.
TJs are linked to the actin cytoskeleton through the Zonula Occludens proteins ZO-1, ZO-2, and ZO-3 expressed in endothelial cells 62, 63. They interact with adhesive proteins of TJs and anchor the actin cytoskeleton with TJs (62, 63). ZO-1 plays a crucial role in the assembly of functional TJs and AJs 62, 72, 73. As discussed below, ZO-1 might regulate the cross-interaction between TJs and AJs through control of intracellular tension and assembly of the VE-cadherin mechanosensory complex 73. Decreased expression of ZO-1 is associated with severe plasma leakage observed in multiple sclerosis 74 and diabetic rats 75, 76.
In a study in mice with inducible endothelial cell-restricted disruption of β-catenin it is shown that endothelial β-catenin signaling was essential for maintaining BBB integrity through regulation of claudin-1 and claudin -3 in adult brain endothelial cells 77. These mice developed multiple brain petechial hemorrhages accompanied by neuronal injury and CNS inflammation. Thus, nuclear β-catenin is an essential mechanism in regulating BBB via the expression of claudin-1 and claudin-3. This conclusion is supported by the evidence that Wnt /β-catenin signaling regulates expression of claudin-3 78.
Adherens junctions
AJs are comprised of Vascular Endothelial (VE)-cadherin and associated α-, β- and p120-catenin adhesion complexes 79–81. In addition, there is also a variety of other recently described junctional proteins, i.e. vinculin, N-WASP and Arp2/3, which interact with catenins involved primarily in stabilizing VE-cadherin-mediated adhesion (discussed below). Multiple lines of evidence showed that VE-cadherin adhesion is the primary adhesion event during vascular development 82. VE-cadherin-mediated adhesion promotes activation of forkhead box transcriptional factor FoxO1, which is also required for claudin-5 expression 83. Knockout of β-catenin in endothelial cells leads to disruption of TJs 77 indicating the importance of AJs in assembly and maintenance of TJs. Disassembly of AJs compromised by the integrity of the VE-cadherin adhesion complex is the leading cause of tissue edema associated with a broad range of pathological conditions 84–87.
Another major cadherin present in endothelial cells, Neural (N)-cadherin 88, 89 has been shown to mediate the interaction between endothelial cells and the surrounding mural cells (smooth muscle cells and pericytes), and is critical for endothelial vessel maturation and stabilization 90–92. N-cadherin adhesions are excluded from AJs both in vitro and in vivo 87, 93, 94. Deletion of N-cadherin gene cdh2 in endothelial cells causes embryonic lethality due to severe vascular defects 95. Multiple lines of evidence indicate a specific role of pericytes in the formation of the BBB and iBRB 96–99. The study demonstrated that interaction between pericytes and endothelial cells was required for the formation of TJs in iBRB and BBB 100–102. An attractive hypothesis is that N-cadherin adhesion, which is involved in the recruitment of pericytes 90, 92, also contributes to the assembly of TJs (although the mechanisms of this unknown, this idea deserves scrutiny). Another study demonstrating that N-cadherin adhesion-induced signaling contributes to the resolution of lung vascular injury through an AMP kinase dependent mechanism 103 is consistent with this concept.
T-cadherin (cadherin 13) is also highly expressed in the vasculature 104. Unlike most cadherins, T-cadherin lacks a transmembrane as well as cytoplasmic region, and is not involved in cell-cell adhesion or anchorage to the actin cytoskeleton 105. T-cadherin is anchored to lipid raft regions via a glycosylphosphatidylinositol anchor, where it acts as a signaling molecule 106. T-cadherin has been suggested to act as a receptor for LDL, and may play a role in angiogenesis by a yet undefined mechanism 107. Furthermore, T-cadherin enhances endothelial barrier function in monolayers, but appears to negatively regulate the barrier when challenged with thrombin 108.
Retinal (R)-cadherin is critical for retinal vascular formation, and relies on a similar network pattering as found in neurons 109. It has also been reported that R-cadherin forms functional, heterotypic interactions with N-cadherin, suggesting a possible role for R-cadherin in endothelial-mural cell interactions 110.
VE-cadherin 2 (protocadherin 12, PCDH12) is also localized to endothelial cell-cell junctions, and while sharing a common extracellular cadherin sequence it has a cytosolic region with unknown homology to typical cadherins 111, 112. VE-cadherin 2 does not bind catenins and is only weakly associated with the cytoskeleton. VE-cadherin 2 does not seem to affect endothelial permeability, and seems to be only involved in cell-cell adhesion 112. Transgenic mice deficient in VE-cadherin 2 had no gross morphological defects 111. However recent studies showed that arteries lacking VE-cadherin2 had medial elastic lamellae, increased inner-diameter and circumferential mid-wall stress indicating it is required for both the structure and function of arteries 113.
3. Mechanisms of VE-cadherin cis- and trans-interaction
VE-cadherin homophilic dimerization
VE-cadherin is a member of the classical cadherin family that possess a modular structure of five ectodomains, a transmembrane domain, and a cytoplasmic tail 114. VE-cadherin displays characteristics of both type I and type II cadherins 115. Like type I cadherins, it lacks the hydrophobic non-swapped region that extends the hydrophobicity of the docking surface. Similar to type II cadherins, it contains two conserved tryptophans, Trp2 and Trp4, important for its adhesive property. Anchorage of these tryptophans to a hydrophobic pocket of the partner ectodomain 1 induces “strand-swap” binding mode, resulting in the so-called trans-dimerization of VE-cadherin 116–118 (Figure 1b–c). Trans-interaction reduces the flexibility of the extracellular domain, which enables a secondary adhesion event between ectodomains 1 and 2 of two cadherins on the same side of an endothelial cell (Figure 1d). This low-affinity cis-interaction is proposed to be responsible for lateral clustering of VE-cadherin, which may increase the strength of adhesive bonds 116, 119. Formation of both trans- and cis-interactions is an intrinsic property of the extracellular moiety of VE-cadherin that does not require the intracellular portion of the protein or assembly of the cadherin-catenin complex 116.
Tethering of VE-cadherin adhesion complex to actin cytoskeleton
The strength of adhesive bonds, defined specifically as the ability of VE-cadherin adhesion to sustain mechanical stresses from blood flow and pressure, is regulated through attachment of the adhesion complex to the actin cytoskeleton 114, 120–122. The actin cytoskeleton contributes to the strength of AJs) by several fundamental mechanisms. It generates intracellular tension and clustering of VE-cadherin at AJs 121–124, and facilitates assembly of the VE-cadherin mechanosensory complex 73,125, 126.
α-catenin is the only member of the cadherin-associated catenin proteins that contains an actin-binding domain 120, 127 enabling the direct association between VE-cadherin adhesion and the actin cytoskeleton 127–129. α-catenin can either tether pre-existing actin filaments to the VE-cadherin complex 130, or alternatively, induce de novo polymerization of actin filaments at sites of AJs 130. The latter mechanism involves recruitment of actin binding proteins such as α-actinin, Epithelial Protein Lost in Neoplasm (EPLIN), and vinculin to VE-cadherin adhesion in the presence of intracellular tension 123, 130 (for review, see 131).
α-catenin and vinculin are allosteric molecules that undergo a rapid and reversible switch between conformational states depending on the applied tension 128, 132–134. α-catenin-mediated recruitment of vinculin, along with N-WASP, VASP, and myosin II to AJs enhances the strength of VE-cadherin adhesion by promoting Arp2/3-dependent polymerization of de novo actin filaments 131, 135–137. Recent work from our group has shown that p120-catenin forms a complex with Arp2/3 and N-WASP 138. Knock down of Arp2 did not inhibit N-WASP interaction with p120 catenin, suggesting that N-WASP binds directly to p120 catenin and induces organization of cortical actin 138. Stabilization of F-actin filaments occurs through the binding of a variety of capping proteins including Capping Protein (CP, aka β-actinin), CapZ, FSGS3/CD2-associated protein (FSGS3/CD2AP) to the barbed (plus) end of F-actin, and is required for actin assembly 139. Hence, the strength of VE-cadherin adhesive bonds and therefore integrity of the endothelial barrier is regulated by a complex network involving the aforementioned regulators of actin-polymerization.
Role of intracellular tension in regulating VE-cadherin adhesion
Intracellular tension is a critical component regulating stable anchorage of VE-cadherin to the actin cytoskeleton (for review, see 140). Simultaneous binding of α-catenin to both β-catenin and F-actin filament occurs only in the presence of tension 129, 130, 141. Tension of up to 10 pN induces stable bond formation between the β-catenin/α-catenin complex and F-actin in vitro 129. VE-cadherin adhesion at AJs are formed in a tension-dependent manner 126 indicating an important role of the acto-myosin apparatus at AJs. Endothelial cell monolayers generate an intercellular tugging force of ~40 nN 126 with an average tension on a VE-cadherin molecule from the actin cytoskeleton ranging from 1.8 to 2.4 nN per molecule 142. Pro-inflammatory mediators such as α-thrombin increase traction forces and the resultant mechanical stress at AJs (up to ~ 8 nN/μm2) that uncouples the VE-cadherin complex from the actin cytoskeleton 126.
Intracellular tension is generated by the acto-myosin contractile apparatus 126, 143 (Figure 2a–b). The ubiquitously expressed non-muscular actin motor myosin-IIA and B 133, 144 are central to control of intracellular tension at endothelial AJs 126. Myosin II binds to F-actin filaments and generates tension by sliding these filaments along each other 132. The ability of myosin II to assemble antiparallel filaments consisting of 10–30 motors 135, 145 is the main determinant of the magnitude of intracellular tension (Figure 2a).
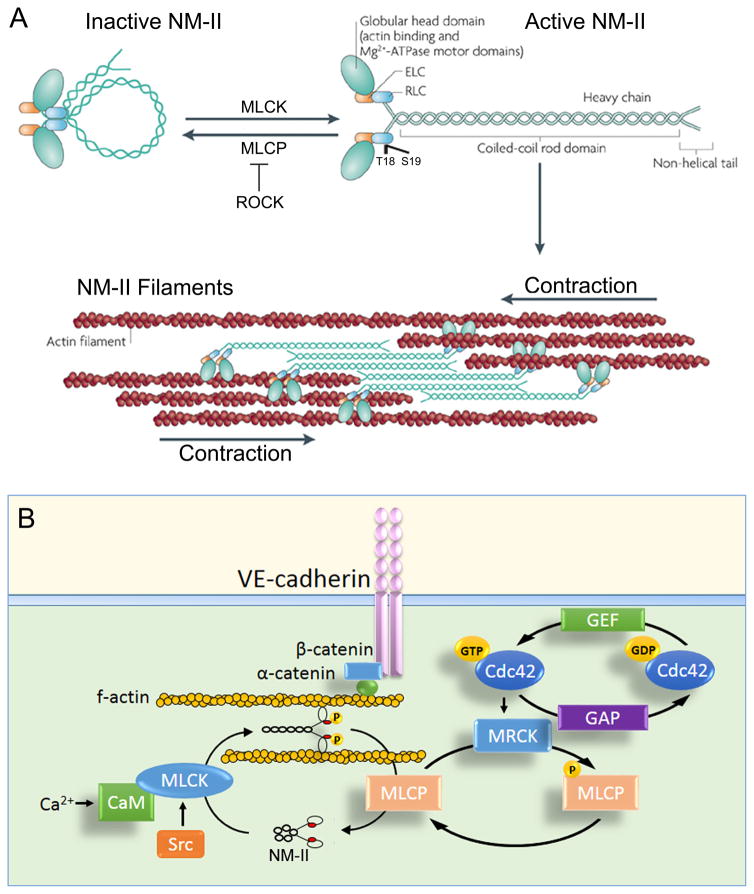
Figure 2. Role of acto-myosin apparatus in stabilizing AJs.
A) Domain structure of non-muscle myosin II (NM-II). The NM-II consists of a globular head domain containing both actin-binding and motor domains, essential light chains (ELCs), regulatory light chains (RLCs), and heavy chains. NM-II possesses a head to tail interaction in the absence of phosphorylation. Phosphorylation of regulatory light chain at Thr18/Ser19 by myosin light chain kinase (MLCK) unfolds the molecule, enabling assembly of anti-parallel filaments through interactions between their rod domains. Activation of Rho-associated kinase (ROCK), which inhibits phosphatase activity of myosin light chain phosphatase (MLCP) in a phosphorylation-dependent manner, also favors RLC phosphorylation. NM-II filaments bind to actin filaments, which slide along each other, and cause a cell contraction. Adapted with permission from (Vicente-Manzanares et al., Nature Reviews Molecular Cell Biology, 2009).
B) Proposed mechanism of regulation of NM-II activity at AJs in confluent endothelium. NM-II regulates attachment of the VE-cadherin adhesion complex to the actin cytoskeleton, thereby generating mechanical tension required for binding of α-catenin to both β-catenin and f-actin. NM-II phosphorylation is controlled by MLCK and MLCP activities. In the model, we propose that Src and Cdc42 pathways cooperate in regulating NM-II activity at AJs. Cdc42 facilitates activation of NM-II through myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK)-dependent phosphorylation of MLCP whereas Src phosphorylates MLCK at sites of VE-cadherin adhesion. CaM = calmodulin; GEF = guanine nucleotide exchange factor; GAP = GTPase activating protein; GTP = guanosine triphosphate; GDP = guanosine diphosphate.
Phosphorylation of regulatory myosin light chain (MLC) at Thr18 and Ser19 is a prerequisite for motor activity 132, 136 (Figure 2a). The activity of myosin-II in endothelial cells is finely regulated by a variety of intracellular signals 146. The canonical pathway involves phosphorylation of MLC by endothelial-specific myosin light-chain kinase (MLCK), which is commonly activated by Ca2+/calmodulin binding 147 or Src-dependent phosphorylation at Tyr464 and Tyr471 148. Myosin light chain phosphatase (MLCP) counteracts MLCK activity by dephosphorylating MLC 146. Therefore, a fine balance between MLCK and MLCP is essential for limiting myosin II phosphorylation and thereby magnitude of contractile forces at endothelial AJs.
Activity of MLCP (PP1, type 1 protein phosphatase), is downregulated by RhoA signaling 149. RhoA activates Rho-associated coiled-coil forming protein kinase (ROCK), which in turn, elicits its effect through phosphorylation of PP1 at Thr-695, Ser-894, and Thr-850 149, 150. The latter inhibits PP1 activity, allowing for myosin II phosphorylation by MLCK and assembly of the acto-myosin apparatus 149, 150.
In endothelial monolayers, myosin-II activity is finely tuned at VE-cadherin adhesions by a yet unknown mechanism. A basal level of ROCK activity appears to be essential for the maintenance of endothelial AJs 151. Recent studies utilizing the RhoA/B/C biosensors show that both RhoA and RhoB are constitutively activated at AJs 152, 153. It remains unclear, however, how the basal level of Rho activity is maintained at AJs. To date, we have a better understanding of the regulation of RhoA signaling at epithelial AJs. In epithelial cells, the RhoA zone at E-cadherin adhesion represents the main molecular mechanism for generation of apical-lateral patterns of junctional contractility 154–156. Both p120-catenin and myosin-IIA recruit ROCKI to nascent adhesions and provide positive feedback regulation of RhoA activity at E-cadherin adhesions 154, 156. ROCKI phosphorylates Rnd3 and prevents cortical recruitment of the GTPase-activating protein (GAP), p190RhoGAP to AJs, and hence preserves RhoA from inactivation 156.
It is unlikely that a similar mechanism operates in endothelial cells where p190RhoA accumulation and activity at AJs is required for maintenance of stable AJs 157–159. A possible mechanism of myosin-II might involve Cdc42 signaling as evident by the finding that Cdc42 also activates myosin II (Figure 2b) 160, 161. Cdc42 mediates the assembly of myosin-II filaments through its effectors Pak2, Pak4 (162), and myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) 160. MRCK phosphorylates and thereby inhibits MLCP, although it is less potent than ROCK in activating myosin-II 160. This makes MRCK the best candidate for induction of low-grade tension at endothelial AJs. Therefore, it is possible that Cdc42 coordinates N-WASP-mediated polymerization of actin filaments with that of p120-catenin 138, 163, such that activation of myosin II at VE-cadherin adhesion is able to strengthen AJs (Figure 2b).
Mechanosensing at the level of AJs
Cells experience external mechanical forces from neighboring cells and the extracellular matrix (ECM) as well as the internal force generated by the actomyosin contractile machinery. In the vascular system, endothelial cells are also exposed to hemodynamic forces resulting from hydrostatic pressure in vessels and blood flow 164. VE-cadherin forms a mechanosensory complex with platelet endothelial cell adhesion molecule 1 (PECAM-1) along with VEGFR2 and VEGFR3 enabling the endothelium to sense changes in hemodynamics and thus activate a variety of signaling pathways 165, 166 (Figure 3). These signaling pathways in turn orchestrate a coordinated cellular response resulting in reorganization of the actin cytoskeleton, redistribution of intracellular tension, and a shift in phosphorylation of VE-cadherin and associated catenins 142, 167–169. Using a recently developed biosensor that measures actomyosin-mediated tension across VE-cadherin adhesion and PECAM-1, it was shown that shear stress applied to an endothelial monolayer reduces tension across VE-cadherin adhesion concomitant with a decrease in total cell-cell force 142, 170. Thus, it appears that the distribution of intracellular tension is tightly regulated in response to external mechanical forces thus allowing AJs to align in the direction of flow.
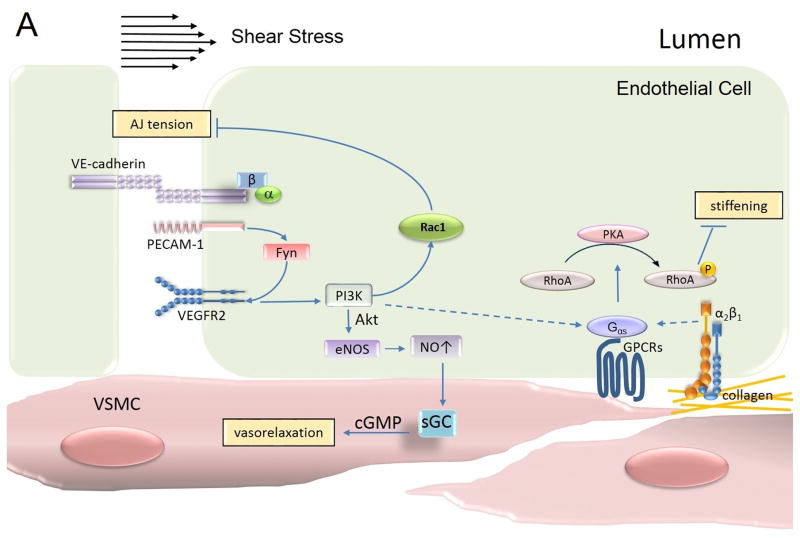
Figure 3. Mechanotransduction at AJs.
The mechanosensory complex in endothelial cells is comprised of vascular endothelial (VE)-cadherin, platelet endothelial cell adhesion molecule (PECAM)-1 and vascular endothelial growth factor receptor (VEGFR)2. Mechanosensing of shear stress occurs through PECAM-1-dependent activation of Fyn, which in turn facilitates VEGFR2-mediated signaling in a ligand-independent manner and activates PI3K. PI3K activates both Rac1 and eNOS signaling pathways. Rac1 relieves tension at AJs whereas NO concomitantly promotes vasorelaxation of smooth muscle cells. PECAM-1-dependent sensing of shear stress also promotes α2β1 integrin signaling and consequently activation of PKA in atheroresistant regions. PKA phosphorylates RhoA and decreases RhoA-dependent cellular stiffness allowing the endothelial cell to align in the direction of blood flow. PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; PKA = protein kinase A; VSMC = vascular smooth muscle cell; NO = nitric oxide; eNOS = endothelial nitric oxide synthase; cGMP = cyclic guanosine monophosphate; sGC = soluble guanylyl cyclase.
The current concept of mechanosensing at AJs involves a series of distinct (and perhaps linear) sequences of signal transduction events (Figure 3). Signaling is initiated with conformational changes in PECAM-1 followed by activation of Src family kinase Fyn at AJs 171. Fyn in turn phosphorylates PECAM-1 and activates the receptor tyrosine kinase VEGFR2 in a ligand independent manner 171. VEGFR2, in turn, induces phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), Akt, and endothelial nitric oxide synthase (eNOS) 120, 165, 172. PI3K subsequently activates Rac1, relieving the tension at AJs 173, 174 whereas eNOS mediates vasorelaxation through the effect of nitric oxide on vascular smooth muscle cells 120, 172.
Interestingly, the response to shear stress varies depending on the composition of the ECM and corresponding integrin signaling 175–177. Shear stress induces stiffening of endothelial cells adherent to fibronectin (through α5/β1 integrin signaling) but not on collagen (through α2/β1 integrin singling) 175, 178 signifying the importance of cross-talk between AJs and focal adhesions (FAs) in the mechanosensory response. In cells adherent to collagen, mechanosensing at AJs resulted in Gαs-dependent protein kinase A (PKA) signaling and subsequent phosphorylation of RhoA 175, 179. PKA phosphorylates RhoA at serine 188 and promotes the association of RhoA with Rho-guanine dissociation inhibitor, which sequesters RhoA in the cytosol 180, 181. PKA-mediated inhibition of RhoA signaling is responsible for blunted stiffening of endothelial cells in response to hemodynamic shear stress 175. Hence, specifics of ECM composition might permit a differential response of endothelial cells to shear stress 175. In the fibronectin-rich aortic arch, which is prone to atherosclerosis, endothelial cells are stiffer and more permeable to protein-rich fluids and leukocytes. Failure to activate PKA and reduce stiffness of endothelial cells in these regions of the aorta might contribute to the development of atherosclerosis. In this context, remodeling of the ECM, itself a function of endothelial cell activation is likely a key determinant of change in endothelial barrier integrity at the level of AJs.
4. Signaling mechanisms mediating stability and remodeling of VE-cadherin adhesion
VE-cadherin adhesion as a “gatekeeper” of endothelial barrier
The steady-state dynamics of VE-cadherin at AJs is a critical determinant of AJ integrity. This includes several interdependent events concerning both biophysical properties of VE-cadherin adhesive bonds and the integration of intracellular proteins within VE-cadherin. VE-cadherin adhesive bonds undergo continuous assembling, disassembling, and remodeling at AJs; the kinetics of these events are defined by the affinity of trans-dimerization 114, 115. This primary adhesion event requires neither energy nor attachment of the VE-cadherin complex to the actin cytoskeleton114, 115.
In contrast, turnover of VE-cadherin molecules at AJs, specifically the exchange between junctional and intracellular pools, is tightly regulated by the interaction of VE-cadherin with associated catenin proteins and the actin cytoskeleton 123, 126, 182. The steady-state kinetics of VE-cadherin at AJs is controlled through the stability of the cadherin-catenin complex, intracellular tension, and organization of the actin cytoskeleton 126, 182. The disassembly of VE-cadherin adhesion in response to extracellular stimuli is triggered by phosphorylation of VE-cadherin and associated catenins and the re-distribution of the actin cytoskeleton to the sites of FAs. Depending on the duration and magnitude of the intracellular response, changes in VE-cadherin dynamics at AJs can lead to weakening or disassembly of AJs, causing either transient or prolonged increase in junctional permeability. For example, tumor vessels represent a case of chronic vascular leakage that is associated with downregulation of VE-cadherin expression 183.
Multiple lines of evidence suggest that the hyper-permeability response to pro-inflammatory mediators can be mitigated if the integrity of VE-cadherin internalization is preserved. Various strategies have been developed to stabilize VE-cadherin adhesion. They include overexpression of p120-catenin, which blocks clathrin-mediated VE-cadherin internalization 184–186; expression of a VE-cadherin-α-catenin chimera 166, which directly tethers adhesion to the actin cytoskeleton; and artificial bridging of opposing VE-cadherin molecules at AJs with a cyclic peptide 187. This evidence suggests that it is possible to manipulate the integrity of VE-cadherin adhesion, the main gatekeeper of the endothelial barrier.
Kinase-mediated regulation of VE-cadherin turnover at AJs
The spatio-temporal control of VE-cadherin turnover at AJs is an integral part of the intracellular response to environmental cues. Destabilization of VE-cadherin adhesion occurs during trans-endothelial migration of leukocytes 161, 164–166 and in response to extracellular stimuli associated with opening AJs and increased barrier permeability 165, 166, 188–190. Intracellular signaling such as phosphorylation of VE-cadherin and associated catenin proteins (summarized in Table 1) induce disassembly of the VE-cadherin-catenin complex. In particular, dissociation of p120-catenin from the juxtamembrane region of VE-cadherin unmasks the binding site for AP2, an adaptor protein complex of the endocytic machinery, and primes VE-cadherin for internalization 159, 184, 185, 189 (Figure 4). VEGF promotes VE-cadherin internalization via β-arrestin2-mediated endocytosis 188. In this context, VEGF induces cSrc-dependent phosphorylation of the guanine nucleotide exchange factor Vav2, which in turn activates Rac1 and p21-activated kinase PAK 188. PAK phosphorylates VE-cadherin at S665 and targets VE-cadherin for β-arrestin2-mediated internalization 188. Moreover, VEGF signaling decreases VE-cadherin/p120-catenin association, promoting clathrin-dependent VE-cadherin endocytosis 184. Multiple pro-inflammatory mediators including thrombin, histamine, platelet-activating factor, Vascular Endothelial Growth Factor (VEGF), and tumor necrosis factor (TNF)-α facilitate disassembly of VE-cadherin adhesion, although they do not seem to function by inducing a similar pathway. This is evident by the finding that they induce differential phosphorylation of VE-cadherin and p120-catenin 165, 166, 188–190. Some mediators, such as thrombin, function through phosphorylating VE-cadherin at Tyr 658 by c-Src 170, 172 or p120 catenin at Ser879 by protein kinase C (PKC)α resulting in decreased binding of VE-cadherin to p120-catenin 185, 189. Indeed, leakage of tumor vessels is associated with c-Src-dependent phosphorylation of VE-cadherin and β- and p120-catenin proteins 191. Other studies showed that phosphorylation of β-catenin at Tyr654 and Tyr489 by c-Src and Abelson, respectively, reduced β-catenin affinity to VE-cadherin allowing dissociation of β-catenin from VE-cadherin 192, 193. The latter events uncouple VE-cadherin from the actin cytoskeleton, and thereby reduce VE-cadherin adhesion strength 120. In addition, phosphorylation of β-catenin at Tyr142 by Fer or Fyn interferes with the formation of the β-catenin/α-catenin complex and detaches VE-cadherin adhesion from the actin cytoskeleton 193. An explanation for the complexity of regulation of VE-cadherin adhesion is that endothelial permeability is a fundamental evolutionarily conserved process requiring activation of multiple “phospho-switches”.
Table 1.
The role of kinases at AJs.
| Kinases | Activity within VE-cadherin complex |
|---|---|
| c-Src (p60 Src, tyrosine kinase) | Phosphorylates VE-cadherin at Y658 and reduces binding to p120-cat; Phosphorylates VE-cadherin Y685 and increases binding to CSK Phosphorylates β-catenin Y654 and reduces binding to VE-cadherin |
| CSK (cytosolic C-terminal Src kinase, tyrosine kinase) | Phosphorylates c-SRC Y530 and inhibits c-Src activity at AJs |
| Fer (tyrosine kinase) | Phosphorylates PTP1B Y152 and induces binding to VE-cadherin |
| Fyn (SFK, tyrosine kinase) | Phosphorylates β-catenin Y142 and reduces binding to VE-cadherin |
| Yes (SFK) | Phosphorylates β-catenin Y142 and reduces binding to VE-cadherin |
| Abelson (tyrosine kinase) | Phosphorylates β-catenin Y489 and reduces binding to VE-cadherin |
| PYK2 (proline-rich tyrosine kinase) | Phosphorylates VE-cadherin Y731 and reduces binding to β-catenin |
| PAK (Ser/Thr kinase) | Phosphorylates VE-cadherin S665 and targets VE-cadherin for β-arrestin-mediated internalization |
Figure 4. Role of specialized kinases and phosphatases in stabilizing AJs.
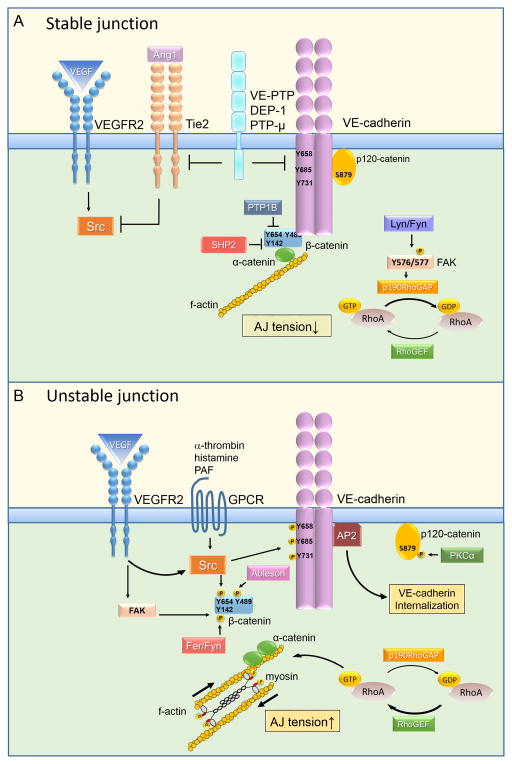
A) Stable adherens junctions are characterized by low phosphorylation of VE-cadherin and associated catenin proteins. Protein tyrosine phosphatases DEP1, VE-PTP, PTPμ, SHP2, and PTP1B at AJs counteract the effect of tyrosine kinases (Src, Fen, Fyn, and Ableson) to stabilize the VE-cadherin-catenin complex. FAK also stabilizes VE-cadherin adhesion by inhibiting RhoA signaling through phosphorylation-dependent activation of p190RhoGAP. Ang1 = angiopoietin 1; VEGF = vascular endothelial growth factor ; VEGFR2 = vascular endothelial growth factor receptor 2; DEP1 = density enhanced phosphatase 1; VE-PTP = vascular endothelial protein tyrosine phosphatase; PTPμ = protein tyrosine phosphatase μ; SHP2 = Src homology phosphatase; PTP1B = protein tyrosine phosphatase 1 B; FAK = focal adhesion kinase; RhoGAP = Rho GTPase activating protein; RhoGEF = Rho guanine nucleotide exchange factor; GDP = guanosine diphosphate; GTP = guanosine triphosphate.
B) Phosphorylation-dependent activation of kinases by VEGF, histamine, thrombin, PAF, and TNF-α leads to phosphorylation of VE-cadherin, β-catenin, and p120-catenin (residues are indicated) by distinct kinases. This results in destabilization of the VE-cadherin complex. Dissociation of p120-catenin due to phosphorylation of VE-cadherin at Y658 or p120-catenin at S879 exposes a VE-cadherin binding site for AP2 to facilitate VE-cadherin endocytosis via clathrin coated pits. Phosphorylation of β-catenin induces the uncoupling of VE-cadherin adhesion from the actin cytoskeleton. Activation of RhoA leads to phosphorylation of MLC, formation of stress fibers, and increased tension across VE-cadherin adhesion. TNF-α = tumor necrosis factor alpha; PAF = platelet-activating factor; AP2 = adaptor protein 2; MLC = myosin light chain; PKCα = protein kinase C alpha.
In contrast to c-Src and Yes, intriguingly, the other Src family kinases Lyn and Fyn stabilize VE-cadherin adhesion by inducing phosphorylation of focal adhesion kinase (FAK) at Tyr 576/577 194, 195. FAK, in turn, inhibits RhoA activity at AJs basally and during reannealing of AJs after challenge with α-thrombin through phosphorylation-dependent activation of p190RhoGAP 196. Inducible deletion of FAK in endothelial cells impairs the balance between RhoA and Rac1 activities leading to hyper activation of RhoA signaling, disruption of AJs, and endothelial barrier leakage 196, 197.
Interestingly, FAK can also contribute to destabilization of VE-cadherin adhesion during angiogenesis when activation of FAK converges with VEGF-activated signaling pathway 198. FAK contributes to increased endothelial permeability of glioma tumor vessels 199. FAK appears to facilitate translocation of c-Src to AJs where Src induces the phosphorylation of VE-cadherin at Tyr658 200. In addition, activation of FAK downstream of VEGF induces the phosphorylation of β-catenin at Y142 and its subsequent dissociation from the VE-cadherin complex 198. Deficiency of FAK in endothelial cells decreases extravasation of tumor cells and prevents spontaneous orthotopic melanoma metastasis 198. Together these studies suggest a critical role of FAK in regulating endothelial barrier integrity; however, this function of FAK may either be through direct interaction or upstream of another kinase such as Src.
It is also important to note that many of these kinases are themselves constituents of the VE-cadherin adhesion complex in the resting endothelium 201, 202. Many of them are basally inactive because their activity is suppressed by phosphatases and other kinases at AJs 202. This negative feed-back regulation is disrupted in response to pro-inflammatory mediators allowing fast phosphorylation of proteins within the junctional complex 190, 203–205. Activation of c-Src by Gα13 in response to oxidative stress, a common signal activated by multiple pro-inflammatory stimuli 190, is one such example. Whereas c-Src activity is basally suppressed by Csk at AJs, this suppression is relieved by Gα13 interaction with VE-cadherin downstream of oxidative redox signaling 190.
Role of AJ localized phosphatases in regulating junctional integrity
Protein tyrosine phosphatases (PTP) such as PTP1B, PTPμ, PTPβ (also known as Vascular Endothelial [VE]-PTP), Src homology 2-domain containing tyrosine phosphatase (SHP2), and density-enhanced phosphatase-1 (DEP1), are also constituents of the VE-cadherin adhesion complex 203, 204, 206, 207 (Figure 4). They stabilize the cadherin-catenin complex by opposing the barrier-disruptive action of kinases (summarized in Table 2) 204, 207. PTP1B is required for continuous dephosphorylation of β-catenin at Tyr654, thus preventing the dissociation of β-catenin from AJs 206. SHP2 also induces dephosphorylation of β-catenin and promotes re-assembly of AJs after inflammatory insult 207.
Table 2.
The role of phosphatases at AJs.
| Phosphatases | Activity within VE-cadherin complex |
|---|---|
| PTP1B | Dephosphorylates β-catenin Y654 and increases binding to VE-cadherin |
| SHP2 (tyrosine phosphatase) | Dephosphorylates β-, γ- and p120-catenins |
| PTPμ (tyrosine phosphatase) | Dephosphorylates VE-cadherin |
| DEP1 (tyrosine phosphatase) | scaffold function |
| VE-PTP (tyrosine phosphatase) | Dephosphorylates VE-cadherin and γ-catenin |
VE-PTP, the most studied of the AJ-associated phosphatases in endothelial cells, interacts with VE-cadherin through the membrane proximal fibronectin (FN)-like extracellular domain 203. It stabilizes basal VE-cadherin adhesion by decreasing the rate of VE-cadherin internalization 190, 165. Phosphatase activity per se may not be required for this effect since inhibition of VE-PTP activity with a small molecule inhibitor stabilizes AJs and restores tissue-fluid balance in eye and lung vascular inflammation models 177, 208. The therapeutic effect of VE-PTP inhibitor has been explained by angiopoietin-1 signaling, which is suppressed by VE-PTP-dependent dephosphorylation of Tie-2 177, 208 (Figure 4). Angiopoietin-1 elicits a barrier protective effect in the endothelium by activating Tie-2 receptor signaling that uncouples Src kinase from VEGFR2 and inactivates VEGFR2 signaling 179. The angiopoietin-1/Tie-2 axis also triggers sequential activation of Rap1 and Rac1 in the endothelium 177, 208. Rac1, in turn, causes dissolution of actin stress fibers and stabilizes VE-cadherin trans-interaction by preventing RhoA-mediated intracellular tension at AJs 182. As discussed below, this “tug of war” between RhoA and Rac1 at AJs is a major determinant of the stability and plasticity of VE-cadherin adhesion both basally and in response to permeability-increasing stimuli.
Role of nitric oxide synthases
Endothelial nitric oxide synthase (eNOS) is responsible for constitutive synthesis of nitric oxide (NO) in the resting endothelium 210–212. Basal production of NO controls vascular tone and vasorelaxation in response to increased blood flow, whereas hyper-activation of eNOS in response to VEGF or pro-inflammatory stimuli such as Platelet-Activating factor (PAF) triggers S-nitrosylation of VE-cadherin, β-catenin, and p120 catenin 213–215. S-nitrosylation, the covalent attachment of S-nitrosothiol to a cysteine thiol 216, represents another regulatory pathway of AJ stability. Similar to phosphorylation, S-nitrosylation reversibly modulates affinity of β- and p120- catenin proteins to VE-cadherin 213–215, 217. S-nitrosylation of β-catenin on the Cys619 residue promotes dissociation of β-catenin from VE-cadherin causing destabilization of AJs and resultant hyper-permeability of the endothelial barrier 213–215, 217. Deletion of eNOS causes a blunted VEGF-mediated permeability response 217, further supporting the role of NO redox signaling in regulating endothelial barrier function.
PAF induces S-nitrosylation of p120-catenin on multiple cysteine residues, Cys579, Cys429, Cys450, Cys618, and Cys692 214. S-nitrosylation of Cys579, located within the VE-cadherin-interacting domain, might represent a critical event associated with NO redox signaling in regulating endothelial hyper-permeability 214. PAF can also induce S-nitrosylation of VE-cadherin and consequent disruption of AJs 215. In this context, S-nitrosylation of VE-cadherin is required for tyrosine phosphorylation and internalization of VE-cadherin 215. These data indicate that S-nitrosylation of junctional proteins is an important mechanism for destabilization of AJs.
Another post-translational modification induced by reactive nitrogen species such as anion (ONOO-) is nitraton of tyrosine residues 218. Nitration of junctional proteins, such as p190RhoGAP, which is associated with p120-catenin 157, and β-catenin itself 219, facilitates disassembly of the VE-cadherin adhesion complex. The pro-inflammatory mediator serine protease α-thrombin triggers nitration of p190RhoGAP on Tyr1105 downstream of eNOS-mediated NO redox signaling 157. This inhibits GAP activity, consequently activating RhoA signaling at AJs and corresponding acto-myosin cell contraction 157. Hence, nitration of p190RhoGAP represents a crucial mechanism in the activation of RhoA signaling implicated in hyper-permeability of the endothelial barrier during inflammation.
Nitration of β-catenin occurs in the context of chronic or acute inflammation associated with the expression of inducible iNOS. Many inflammatory processes including diabetes, atherosclerosis, and systemic inflammation are associated with protein nitration due to activation of iNOS in the endothelium 220–225. Induction of iNOS in macrophages triggers nitration of β-catenin in endothelial cells and the resultant dissociation of VE-cadherin adhesion-mediated complex 219. Nitration of β-catenin also promotes its translocation to the nucleus where it is associated with T-cell factor (TCF)/Lef transcription factors 219. This ultimately leads to vascular remodeling after injury 226. In this context, nitration of β-catenin induces vascular leakage, but at the same time limits endothelial injury by promoting pro-survival pathways 219, 226.
Role of acetyltransferases
A growing body of evidence suggests that lysine acetylation of β-catenin might also provide an important mechanism for regulating endothelial barrier permeability 227. Acetylation is a reversible process controlled by acetyltransferases and deacetylases. Acetylation of β-catenin induces its association with the plasma membrane 228 and modulates β-catenin activity towards specific genes 229, 230. Acetylation of β-catenin at Lys49 is mediated by CREB-binding protein (CBP) acetyltransferase 229 and is known to modulate Wnt signaling in a promoter-specific fashion. In contrast, acetylation of β-catenin at Lys345 by the transcriptional coactivator p300 increases its affinity for TCF4 230, suggesting that acetylation might differentially modulate β-catenin transcriptional activity.
In contrast, deacetylation of β-catenin at Lys49 is controlled by a member of class II histone deacetylase HDAC6 228. Knockout of HDAC6 or treatment of animals with the specific HDAC6 inhibitor tubastatin A protects against endotoxin-induced pulmonary edema and acute lung injury and improves survival of mice in septic shock 227, 231.
5. Role of endothelial cells expressed transient receptor potential (trp) channels
Multiple pathological conditions are associated with calcium signaling, which represent a crucial pathway in mediating hyper-permeability of endothelial barrier 232–236. A superfamily of transient receptor potential (TRP) channel that are responsible for regulation of Ca2+ entry in endothelial cells presented by TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin) 234–236, and therefore, have been extensively studied for their role in mediating hyper-permeability response.
TRPC
Endothelial cells express 5 non-selectively permeable transient receptor potential cation channels (canonical), TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, and TRPC7 234–241. These channels mediate store- and receptor-operated Ca2+ entry from extracellular spaces in response to edemagenic and pro-angiogenic mediators.
Store-operated calcium entry (SOCE) involves TRPC1 and TRPC4. These channels are activated by G protein-coupled receptors (GPCR), and receptor tyrosine kinases coupled to activation of phospholipase C (PLC). Multiple pro-inflammatory mediators such as serine protease α-thrombin, histamine and PAF induce disruption of the endothelial barrier through binding to GPCRs on the surface of endothelial cells. This triggers activation of PLC, which catalyzes phosphoinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (for review, see 244, 245). IP3 activates Ca2+ release from intracellular stores and consequent Ca2+ entry through SOCE.
The critical role of SOCE in the hyper-permeability response is evident from genetic deletion of murine trpc1 and trpc4 genes. These animals demonstrate markedly reduced hyper-permeability responses in lungs and reduced mortality induced by endotoxins 240, 246. Intriguingly, full activation of TRPC1 is required for RhoA-mediated reorganization of the actin cytoskeleton, enabling the interaction of TRPC1 with IP3Rs, calcium channels on the endoplasmic reticulum membrane responsible for Ca2+ release from stores 236, 238, indicative of a positive amplification loop. Other studies demonstrate that TRPC1 downregulates both the expression and activity of sphingosine kinase 1 (SPHK1), the kinase responsible for production of the barrier enhancing mediator Sphingosine-1-phosphate (S1P), thereby weakening AJs in both resting and inflammatory (activated) endothelium 246. This function of TRPC1 appears to be independent of Ca2+ entry since expression of a TRPC1 pore-defective mutant is sufficient to limit expression of SPHK1 and restore the permeability response of the endothelial barrier 246.
Receptor-activated calcium entry (ROCE) is mediated through TRPC6 and TRPC7 in endothelial cells (234–236). These channels are activated by a diacylglycerol (DAG) dependent mechanism and are independent of intracellular store depletion 247, 248. Multiple lines of evidence show that TRPC6 promotes both histamine- and lipopolysaccharide (LPS)-mediated increases in endothelial permeability 236, 235, 249. Importantly, TRPC6 co-localizes with PECAM-1 at AJs during trans-endothelial migration of leukocytes 250. In this context, activation of TRPC6 is mediated through homophilic PECAM-1 adhesion between endothelial cells and leukocytes 250. Furthermore, TRPC6-mediated ROCE is required for neutrophil transendothelial migration (TEM), since expression of a pore-defective channel or knockdown of TRPC6 in endothelial cells arrests neutrophils within AJs 250.
TRPV
The subfamily members of TRPV induce Ca2+ entry in response to osmolar, thermal, mechanical, and chemical stimuli 251, 252. In endothelial cells, TRPV4 is activated by heat and endogenous lipid mediators such as epoxyeicosatrienoic acids (14,15-EET) and phorbol ester 4a-phorbol 12,13-didecanoate (4αPDD) 252–255. In mice, knockout of trpv4 gene inhibits permeability responses of lung microvasculature to both 4αPDD and 14,15-EET without affecting SOCE 253. Other studies indicate that TRPV4 might also be involved in the mechanism of Ca2+ entry in response to shear stress 256.
TRPM
The TRPM family is presented by TRPM2 and TRPM4 in endothelial cells 257–260. TRPM2 is activated by intracellular ADP-ribose, hydrogen peroxide, and Nicotinamide adenine dinucleotide 257, 261. TRPM2 has been shown to induce Ca2+entry in response to H2O2 in a dose-dependent manner 258. It is plausible that TRPM2 may serve as a cellular redox sensor in endothelial cells.
6. Role of RhoGTPases in Regulating Integrity of AJs
The subfamily of Rho (Ras homologous) RhoGTPases belongs to the Ras-sarcoma (Ras)-related superfamily of low molecular weight monomeric G proteins with highly conserved sequence homology 262, 263. RhoA, Rac1, and Cdc42 are the best-studied members of the RhoGTPases sub-family due to their critical role in organization of the actin cytoskeleton as well as profoundly affecting the integrity of AJs 264–268.
A fine balance among RhoA, Rac1, and Cdc42 at AJs is regulated by VE-cadherin “outside-in” signaling 133, 269. Formation of nascent VE-cadherin adhesions activates Rac1 270. Rac1, in turn, induces polymerization of actin filaments specifically at sites of VE-cadherin adhesion and contributes to the stabilization of AJs 270. Rac1 also stabilizes VE-cadherin trans-interaction by counteracting RhoA activity and suppressing acto-myosin tension 182. Hence, a subtle balance between RhoA and Rac1 activities is a critical control point of VE-cadherin turnover at AJs 182.
RhoGTPases are also involved in destabilization and reannealing of AJs in response to mechanical and humoral stimuli. The net effect of RhoGTPases on barrier integrity depends on the nature of extracellular stimuli and activation of convergent signaling pathways that are able to re-wire RhoGTPase signaling to specific intracellular locations and establish their interactions with particular downstream effectors. As described below, the complexity of these biological outcomes can be explained by the combinatorial effects of activation of multiple RhoGTPases.
Sub-family of RhoGTPases
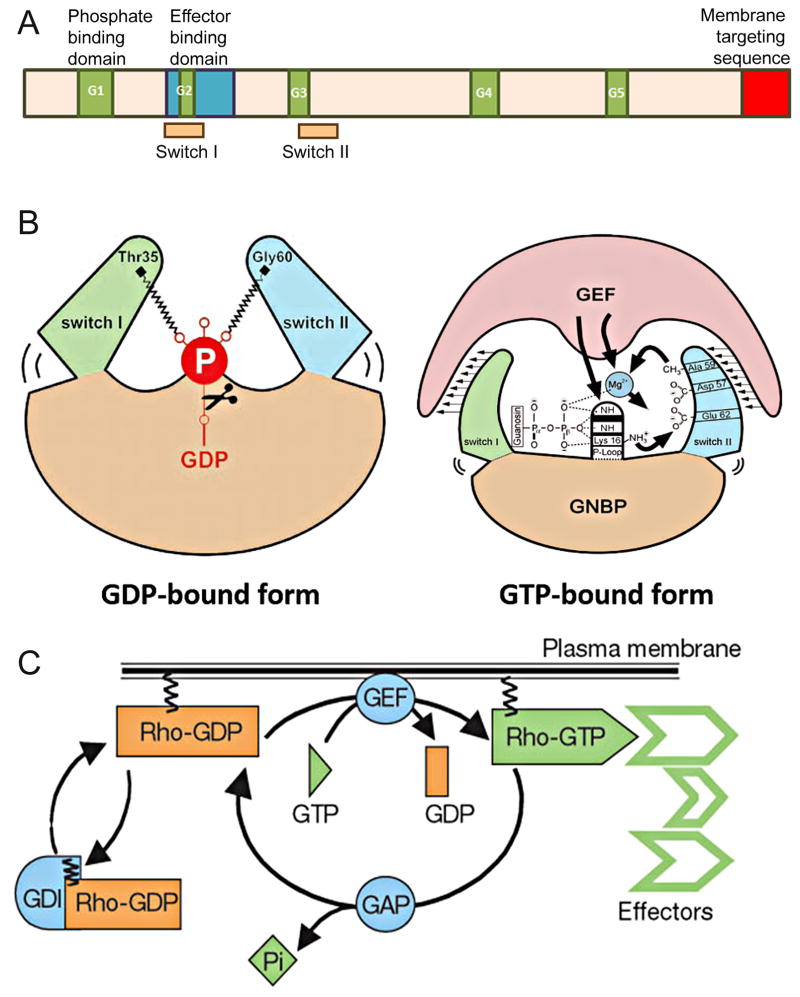
Monomeric RhoGTPases cycle between active (GTP-bound) and inactive (GDP-bound) states and thus act as binary molecular switches 263, 271, 272. In the GTP-bound state, they interact with the downstream effectors to elicit a physiological response 181, 184, 186. RhoGTPases interact with a wide spectrum of downstream effectors that are structurally different from each other 186, 205, and yet the RhoGTPase domain structure itself is highly conserved. All members of the RhoGTPase sub-family contain a G domain structure at the N-terminal, which is comprised of 5 sets of G box binding motifs (for review, see 273) (Figure 5A). The G domain consists of the nucleotide binding site (also called the p-loop), core effector domain, and switch regions (I and II) forming the interface for interaction with GEFs (Figure 5B). The p-loop motif inside the switch I and switch II regions represents the site of GDP to GTP exchange as well as the interface for interaction with downstream effectors upon binding to GTP 274–276. This ability to interact with effectors is lost when the switch region possesses a conformational change due the release of the hydrolyzed phosphate 273.
Figure 5. Regulation of RhoGTPase activity.
A) Schematic representation of general domain structure for RhoGTPases. The 5 G-box motifs (green) represent nucleotide binding motifs whereas the switch I and Switch II are the region of GDP/GTP exchange. C-terminus (red) undergoes post translational modification required for modulating the membrane-targeting of RhoGTPases.
B) Conformational changes within Switch I and II regions upon GTP hydrolysis and exchange. The closed GTP-bound conformation has a higher affinity for GAP binding. Cleavage of hydrolyzed phosphate by GAPs put the switch regions into a relaxed, open conformation. The open GDP-bound conformation has a high affinity for GEF binding. GAP = GTPase activating protein; GEF = guanine nucleotide exchange factor; GDP = guanosine diphosphate; GTP = guanosine triphosphate; GNBP = guanine nucleotide binding protein. Adapted with permission from Vetter and Wittinghofer, Science, 2001.
C) Regulation of RhoGTPase cycle. In the GDP-bound state, RhoGTPases are prevented from interacting with downstream effectors. Release of GDP is facilitated by GEFs allowing exchange for GTP. GAPs catalyze the hydrolysis of GTP resulting in inactivation of the GTPase. GDIs prevent GTP exchange by binding to the GDP bound state. GDI = guanosine nucleotide dissociation inhibitor. Adapted with permission from Etienne-Manneville and Hall; Nature, 2002.
Because of the high binding affinity of GTPases for both GDP and GTP and slow rate of intrinsic GTP hydrolysis, the GTPase cycle is controlled by upstream regulators; specifically GTPase Activating Proteins (GAPs), Guanine Nucleotide Exchange Factors (GEFs), and Guanine Nucleotide Dissociation Inhibitors (GDIs) (Figure 5C). GAPs accelerate the rate of GTP hydrolysis and switch “off” RhoGTPase activity, whereas GEFs promote GDP to GTP exchange, thus turning RhoGTPases “on” 277–281. The latter is a multi-step process involving formation of a ternary complex between the GTPase, GEF, and nucleotide followed by nucleotide release (Figure 5B). Rebinding of GTP, predominantly due to higher concentration in the cell, restores GTPase activity. GEFs promote GTP exchange by increasing the rate of GDP release 282, 283. Another regulator, GDI interacts with the GDP-bound form and prevents GTP exchange 280, 281. GDIs shield the hydrophobic tail by binding to a prenylated COOH-terminus, and hence sequesters GTPase from the membrane compartment 284, 285.
The interaction between RhoGTPases and downstream effectors requires translocation of GTPases from the cytosol to the plasma membrane 284, 285. This is controlled by post-translational modifications (PTMs) by the lipids farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP). CAAX sequence at the C-terminus serves as both membrane targeting signal and a recognition motif for farnesylation and geranylgeranylation 286–288. Some members of the RhoGTPase family such as RhoA and RhoC are only geranylgeranylated and are localized in the cytoplasm, whereas others such as RhoB possess geranylgeranylated, farnesylated, or palmitoylated sites and can be localized at the plasma membrane or in the cytoplasm (localized to endosomes) 288–290.
The recruitment of RhoGTPases to membranes, as demonstrated for Rac1, occurs preferentially at the boundaries between the cholesterol-rich, ordered domains (i.e., lipid rafts) and the liquid disordered phase 291. Rac1 then diffuses into both raft and non-raft domains, where it interacts with either downstream effectors inside of ordered domains or can be selectively inactivated by GAPs that prefer non-raft regions 291. Hence, on one hand, PTMs target small RhoGTPases to distinct sub-cellular localizations, allowing them to interact with a specific set of downstream effectors and thus elicit distinct biological outputs through spatially-regulated signaling networks. On the other hand, the organization of plasma membrane domains modulates RhoGTPase signaling by limiting their activities in the non-raft regions.
Rac1 and Cdc42 signaling pathways regulate stability of VE-cadherin adhesion
The role of Rac1 and Cdc42 on assembly and maturation of VE-cadherin adhesion is predominantly associated with their ability to induce nucleation, polymerization, and organization of the actin cytoskeleton through interactions with actin binding proteins 186, 292, 293. Whereas Rac1 promotes polymerization of branched actin network within lamellipodia protrusions 187, 189, 294, 270, Cdc42 facilitates polymerization of linear F-actin filaments into filopodia 295, 296. Upon activation, Rac1 interacts with several downstream effectors including the WASP-family verprolin-homologous protein (WAVE), IQRas GTPase-activating proteins (IQGAPs), partitioning-defective polarity protein PAR6, and members of p21 Activated Kinase (Pak) family 186 (Figure 6). Among the members of Pak family, Pak1 facilitates actin polymerization through activation of Lin1, Isl-1, and Mec-3 Kinase (LIMK) 297. The latter phosphorylates the actin binding protein cofilin at Ser3 and consequently blocks actin monomer de-polymerization 298.
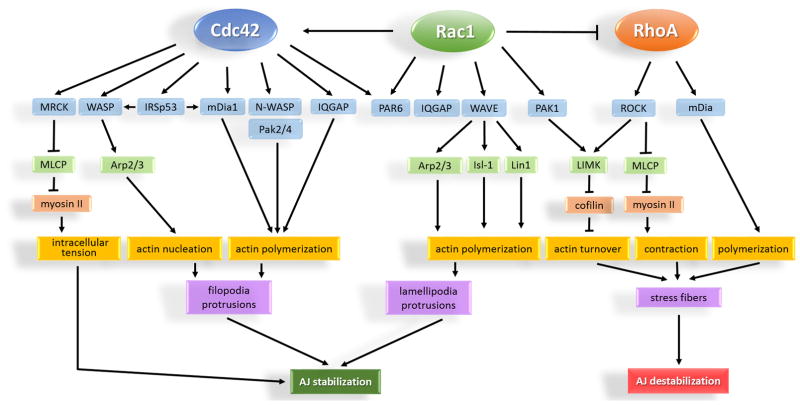
Figure 6. RhoA, Rac1 and Cdc42 regulation of endothelial AJs.
Rac1 and Cdc42 promote organization of the actin cytoskeleton into lamellipodia and filopodia protrusions resulting in re-annealing and stabilization of AJs. Regardless of differential effect on actin organization, Rac1 and Cdc42 share common downstream effectors such as PAR6 and IQGAP1. These effectors serve as scaffolds by recruiting active Rac1 and Cdc42 to AJs. Cdc42 can also generate low grade tension at AJs through activation of non-muscle myosin II. In contrast to Rac1 and Cdc42, RhoA activity is basally suppressed at AJs. Activation of RhoA is associated with formation of stress fibers, increased intracellular tension, and destabilization of AJs. MRCK = myotonic dystrophy kinase-related Cdc42-binding kinase; WASP = Wiskott-Aldrich Syndrome protein; IRSp53 = insulin receptor tyrosine kinase substrate p53; mDia = mammalian Diaphanous; Pak = p21 activated kinase; IQGAP = IQ motif containing GTPase activating protein; PAR6 = partitioning defective protein 6; WAVE = Wasp family verproline-homologue; MLCP = myosin light chain phosphatase; Arp2/3 = Actin-related proteins 2 and 3; LIMK = LIM (Lin1, Isl-1, & Mec-3) kinase; Isl-1 = Insulin gene enhancer protein; Lin1 = CD2 cytoplasmic tail binding protein 2.
The Cdc42 downstream effectors include Wiskott–Aldrich Syndrome protein (WASP), neuronal (N)-WASP, Diaphanous-related formin-1 (mDia1), IQGAPs, PAR6, and MRCK (Figure 6) 186. Cdc42 induces nucleation and polymerization of actin filaments through WASP and mDia pathways 163. It can also bind to the insulin receptor substrate p53 (IRSp53) that coordinates actin nucleation and polymerization through binding to both WASP and mDia at the plasma membrane 299, 300. The Cdc42-MRCK pathway activates myosin II and strengthens AJs by generating low magnitude intracellular tension 160. Hence, in addition to nucleation, polymerization, and stabilization of the actin cytoskeleton at AJs, the Cdc42 signaling pathway is also capable of generating intracellular tension independent of RhoA signaling.
Cdc42 plays a crucial role in assembly and maintenance of AJs 301. Deletion of Cdc42 in endothelial cells results in loss of apical-basal polarity and disrupted AJs (163). Consistent with the proposed role of Cdc42 in activating both actin polymerization and stabilization, these defects are associated with formation of aberrant filopodia as well as impaired assembly of the acto-myosin apparatus 163. The current model suggests a critical role of Cdc42 signaling in the assembly and maturation of AJs via effectors Pak2, Pak4, and N-WASP (Figure 2) 163. Cdc42 signaling thus elicits an endothelial barrier protective effect in inflammatory lung injury 302 and also promotes re-annealing of the barrier in inflammatory endothelium through N-WASP-mediated actin polymerization 138, 301. Moreover, Cdc42 can also act as a competitive inhibitor of Rac1 and thereby counteract the barrier-disruptive effect of p67phox signaling and ROS production 303, 304.
In contrast to Cdc42 that promotes AJ assembly, the outcome of Rac1 signaling on endothelial barrier integrity highly depends on intracellular context 305. In some cases, in response to shear stress or the bioactive lipid mediator Sphingosine-1-phosphate (S1P), the activation of Rac1 signaling enhanced endothelial barrier function 306–309. In other cases, such as stimulation of endothelial cells with TNFα, Platelet-activating factor (PAF), or VEGF, activation of Rac1 caused disruption of the endothelial barrier 296, 310–312. Recent work utilizing a photo-activatable Rac1 probe sheds light on the biological outcome of Rac1 signaling at AJs independent of convergent signaling events 182. Rac1 counterbalanced RhoA activity at mature AJs and promoted stabilization of VE-cadherin trans-interactions 182. This mechanism of RhoA inhibition appears to rely on junctional localization and activity of p190RhoGAP 313. Recruitment of p190RhoGAP to AJs is mediated through its direct interaction with p120-catenin, whereas p190RhoGAP activity is regulated by binding to Rac1 as well as Src- and FAK-mediated phosphorylation 159. Rac1 signaling through the effector Pak1 also suppresses MLCK-dependent phosphorylation of myosin II 292. Hence, activation of Rac1 at mature AJs is a pivotal mechanism for balancing the opposing RhoA signaling and suppressing intracellular tension at AJs 182.
Rac1 signaling may also cause disassembly of VE-cadherin adhesion and disruption of the endothelial barrier 188. This is evident by the finding that the pro-inflammatory mediator TNFα leads to a transient and robust increase in Rac1 activity 310 through phosphatidylinositol (3,4,5)-trisphosphate – dependent Rac exchanger 1 (P-Rex1) 296. In this case, Rac1 signals through p67phox effector leading to production of ROS, and subsequent activation of Src and VE-cadherin phosphorylation 174. Another pro-inflammatory mediator PAF also induces Rac1 signaling through T-lymphoma invasion and metastasis-inducing protein 1 (Tiam-1) 311. PAF-induced activation of Rac1 is associated with profound reorganization of the actin cytoskeleton and vascular leakage 314–315. Furthermore, VEGF activates Rac1 through Src-dependent phosphorylation of Vav2 and causes Pak-mediated phosphorylation of VE-cadherin at Serine 665 and subsequent VE-cadherin internalization by β-arrestin 188, 316. In conclusion, it appears that Rac1 signaling can have divergent effects on AJs ranging from stabilization to disassembly of VE-cadherin adhesions. These responses exemplify the central importance of intracellular environment, localized signaling, and interaction with specific partners in the net biological outcome of Rac1 signaling.
RhoA signaling pathway
In contrast to Rac1 and Cdc42 that mediate the assembly, stabilization, and maturation of AJs 182, 317–319, RhoA signaling mainly contributes to destabilizing AJs and increasing endothelial permeability 320–323. RhoA promotes the formation of actin stress fibers and acto-myosin contraction through activation of downstream effectors such as ROCK and mDia (Figure 6). The reorganization of the actin cytoskeleton via the mDia pathway and concurrent assembly of the contractile apparatus through activation of ROCK signaling leads to the generation of intracellular tension at junctions that disassembles AJs 151.
The mDia and ROCK pathways demonstrate a cooperative behavior downstream of RhoA activation 300, 324. mDia promotes the assembly of actin stress fibers, which are re-enforced by ROCK-mediated activation of myosin II 324. ROCKI and ROCKII are differentially regulated in endothelial cells 325, 326. ROCKI is basally active 325 and contributes to early responses of endothelial cells to pro-inflammatory mediators such as TNFα and Lipopolysaccharide (LPS) 194, 327. In contrast, activation of ROCKII in response to pro-inflammatory stimuli is required for the long-term effects of LPS and TNFα in disrupting endothelial barrier integrity 325, 328. Evidence also indicates that ROCKII maintains baseline junctional tension and primes the endothelium for hyperpermeability responses such as during thrombin challenge, independent from subsequent ROCKI-mediated contractile stress-fiber formation 326. Both ROCKs maintain MLC in a phosphorylated state through interaction with the PI3K/AKT pathway 329. ROCKs also block PI3K/AKT signaling, and thus limit the activation of Rac1 at AJs 173. Protracted RhoA signaling leads to persistent disruption of AJs and promotes sustained endothelial leakage 330, which may be important in the initiation and progression of chronic inflammatory diseases.
7. Spatial control of RhoGTPases at inter-endothelial junctions
Spatial control of RhoGTPases at AJs
VE-cadherin adhesion modulates the organization of the actin cytoskeleton at AJs through the recruitment of signaling and scaffolding proteins such as upstream regulators and downstream effectors of RhoGTPases 304, 331, 332. Engagement of VE-cadherin at cell-cell contacts initiates spatial activation of Rac1 and Cdc42 signaling 302, 304, 333. Rac1 signaling is induced through the activation of phosphatidylinositol 3-kinases (PI3K) 334 as well as recruitment of the RhoGEFs Tiam1, Vav2, and Triple functional domain protein (TRIO) to AJs 174, 270. Tiam1 serves as the scaffold for Rac1 at AJs 335 whereas Vav2, a common GEF for RhoA, Rac1, and Cdc42 336 promotes Rac1 GTP loading and hence facilitates activation of Rac1 signaling 174, 337. Some evidence suggests that triple functional domain protein TRIO, a GEF for both RhoA and Rac1, is also recruited to nascent VE-cadherin adhesion where it activates Rac1 signaling and promotes the formation of AJs 338. IQGAP1, which stabilizes both Cdc42 and Rac1 in the GTP-bound state and protracts the activity of these GTPases 308, 339, is also recruited to AJs through binding to β-catenin 308, 340. Recent data suggest that IQGAP1 is responsible for Rac1 activity at the sites of AJs and hence is an important regulator of AJ integrity and vascular leakage in acute lung injury 341, 342.
In contrast to Rac1 and Cdc42, RhoA activity is suppressed at endothelial AJs by multiple convergent pathways 180, 343. RhoA activity is finely counterbalanced by Rac1 signaling 182, 323. Rac1-mediated activation of p190RhoGAP, a RhoA specific GAP, as well as phosphorylation of p190RhoGAP by Src and FAK 157, 196, 344 play a central role in inhibiting RhoA signaling at endothelial AJs. Whether Cdc42 can also counteract RhoA signaling remains unclear. One tenable mechanism involves Cdc42/MRCK-dependent assembly of myosin-IIB filaments, which can then bind to and suppress activities of Dbl family GEFs containing a DH-PH module at AJs 345. It is an attractive possibility that the interaction between myosin-IIB and the RhoGEFs expressed in endothelial cells (TRIO, GEF-H1, Dbl, LARG, Tiam1 and Vav2) might provide a mechanism for switching small RhoGTPases ‘on’ and ‘off’ at AJs.
Spatial control of RhoGTPases at TJs
In contrast to endothelial AJs, which are characterized by low RhoA activity, TJs are shown to be sites of RhoA activation 73, 346, however there are important differences. RhoA activity is induced by p114GEF at endothelial TJs 347. ZO-1, an adaptor protein of TJs, scaffolds a complex consisting of junction-associated coiled-coil protein (JACOP) and p114GEF to provide spatial activation of RhoA 73. Conversely, both Rac1 and Cdc42 activities are suppressed at TJs. Rich1, a GAP for both Cdc42 and Rac1, is associated with angiomotin, a scaffolding protein of TJs, where it controls cell polarity and endothelial junction integrity through inhibition of Rac1 and Cdc42 348. High activity of RhoA and low activities of Rac1 and Cdc42 are required for generation of intracellular forces at the level of TJs that are transmitted to VE-cadherin adhesion allowing formation of stable AJs 73. This finely compartmentalized regulation of RhoGTPase signaling in endothelial cells might be critical for the stability of VE-cadherin adhesion. Activation of RhoA at the level of TJs rather than AJs might be beneficial for achievement of a proper balance, magnitude and directionality of mechanical forces across VE-cadherin adhesion, the main gatekeeper of junctional permeability in endothelial cells.
8. Role of RhoGTPases in response of endothelium to mechanical and humoral stimuli
Endothelial cells express at least 17 different RhoGAPs and 20 RhoGEFs at high levels (for review, see 349). This broad spectrum of upstream regulators of RhoGTPases might be important for spatio-temporal control of intracellular tension at endothelial AJs exposed to pulsatile blood pressure and blood flow 350. Both RhoA and Rac1 contribute to cell responses induced by mechanical forces 351–355. At least 11 different GEFs including Abr, alsin, ARHGEF10, Bcr, GEF-H1, LARG, p190RhoGEF, PLEKHG1, P-REX2, Solo, and a-PIX mediate endothelial cell adaptation to the cyclic stretch response to pulsatile blood pressure and flow 356. In particular, a GEF for RhoA, Solo, transduces mechanical force at cell–cell adhesion sites 356 whereas Leukemia-associated Rho GEF (LARG) and GEF-H1 are involved in integrin-dependent mechotransduction 176. Both responses contribute to cell alignment and stress fiber reorientation in the endothelium exposed to cyclic stretch.
Conversely, mechanosensing of laminar shear stress and resultant stabilization of VE-cadherin adhesion occurs through activation of Rac1 at AJs 174, 357, 358. Mechanotransduction emanates at the level of the sensory complex comprised of VE-cadherin/PECAM-1/VEGFR2. PECAM-1 induces activation of Src, which in turn promotes phosphorylation of Vav2 and hence Vav2-mediated activation of Rac1 signaling at AJs 174. Rac1 functions as a reversible modulator of intracellular tension at mature AJs and induces stabilization of VE-cadherin adhesion without notable reorganization of the actin cytoskeleton 182.
It is important to note that the vast majority of responses to humoral stimuli involve a broad spectrum of RhoGTPases expressed in endothelial cells. Activation of RhoA signaling in response to pro-inflammatory stimuli is induced by p115RhoGEF 197, GEF-H1 359, and TRIO 360, 361. In contrast, secondary messengers such as cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) elicit a barrier protective effect by inhibiting RhoA signaling. cGMP blocks RhoA signaling through PKG-mediated phosphorylation of RhoA 362, 363 whereas cAMP inhibits RhoA activity by both activating p190RhoGAP 158 and preventing RhoA dissociation from RhoGDI 180. Hence, modulation of RhoA activity might represent an attractive strategy for preventing or treating vascular leakage in disease states.
9. RhoGTPases as therapeutic targets in vascular inflammation
As discussed above, RhoGTPases are fundamental to the biology of endothelial AJs. They serve as a control point for many signaling pathways, making them ideal targets for ameliorating inflammatory disease. Thus, a possible therapeutic approach for treating vascular inflammation may depend on “rewiring” of signaling pathways to restore AJs quickly by shifting the balance from RhoA towards Rac1 and Cdc42 activities. In the following sections, we provide an overview of agents and describe newer targets preventing or resolving inflammation due to leaky AJs.
Small molecule inhibitors
Rho kinase inhibitors
While a number of targets downstream of RhoA have been identified, Rho kinase, a serine/threonine protein kinase of ~160 kDa (also referred to as ROCK) is the major RhoA downstream effector 364. Two isoforms of ROCK, namely ROCKI (also known as ROCKβ) and ROCKII (ROCKα), are encoded by two independent genes 365. Kinase activities of both ROCKs are autoregulated by the COOH-terminal domain, which folds into the active site and inhibits kinase activity. Despite their structural similarity, the two proteins have distinct functions in endothelial cells. ROCKII but not ROCKI regulates basal tension across AJs and shifts the endothelium towards hyper-permeability 326. The deletion of either ROCKI or ROCKII genes in mice has no apparent phenotype except for a defective placenta–embryo interaction 366, 367. The above results suggest that ROCK potentially can be used as a drug target.
The small-molecule inhibitors targeting ROCK kinase activity can be grouped into four classes: isoquinolines, 4-aminopyridines, indazoles, and amide and urea derivatives. Fasudil, dimethyl-fasudil (H-1152), and compound 4 belong to the isoquinoline series of Rho kinase inhibitors. Fasudil shows higher potency towards ROCKI (Ki of 330 nM) 368 whereas H-1152 and compound 4 inhibited both ROCKs with Ki values of 1.6 to 23 nM, respectively 369, 370. Fasudil has demonstrated high efficacy in pre-clinical models of pulmonary hypertension, pulmonary fibrosis, and vascular leakage 371–373. Fasudil is approved in Japan for the treatment of cerebral vasospasm after aneurysm rupture 374, and has been tested in US clinical trials of angina and pulmonary hypertension 375. Y-27632, a member of the 4-aminopyridine series, binds the ATP binding pocket of ROCK and has a Ki of 220–300 nM 376. Like fasudil, Y-27632 has been widely used in animal models of hypoxia-induced pulmonary hypertension, lung injury, and cerebral vasospasm 197, 377. However, Y-27632 has been much less investigated clinically.
Statins
Statins can influence endothelial function by virtue of their direct effect on HMG-CoA reductase, a rate limiting enzyme which generates cholesterol by converting HMG-CoA to mevalonic acid. However, statins can block the synthesis of isoprenoid intermediates such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which are important lipid attachments for post-translational modification of a variety of proteins including attachment of small GTP-binding proteins at the plasma membrane. Statins are shown to prevent thrombin- and LPS-induced loss of endothelial barrier function using in vitro and in vivo models of vascular injury through their “pleiotrophic” effects on RhoA- and Rac1-mediated cytoskeletal arrangements and ROS generation 378. Interestingly, simvastatin reduces pulmonary and systemic inflammatory responses in healthy human patients following LPS inhalation 379 and improves organ function in a small, single-center, randomized study on ALI patients 380. However, a recent large clinical trial failed to demonstrate such a protective effect of statins in ARDS patients 381.
Sphingosine-1-phosphate receptor agonists and antagonists
S1P acts as a ligand in an autocrine or paracrine manner for the G protein coupled receptor (GPCR) S1P receptors (S1PR1–5; formerly termed the endothelial differentiation gene [Edg] receptors). In several studies, S1P enhances endothelial barrier function 382–384 through ligating its high affinity receptor, S1PR1 385. S1PR1 functions in endothelial cells through interaction with heterotrimeric Gi proteins and downstream activation of Rac1 382–384. FTY720 has a barrier enhancing effect both in vitro and in vivo 386–388. However, FTY720P induces vascular leak in a mouse model through promoting phosphorylation of S1PR1 at several serine residues, which trigger receptor ubiquitination and degradation 385, 389. In contrast, newly developed and modified FTY720, (R)-methoxy-FTY720 ((R)-OMe-FTY), (R)/(S)-fluoro-FTY720 (FTY-F), and β-glucuronide-FTY720 (FTY-G) compounds were shown to display in vitro barrier-enhancing properties 390. Tyrosine phosphorylation of S1PR1 at Y143 in endothelial cells also regulates receptor expression at the cell surface and hence the responsiveness to S1P 384. Thus, newer S1P analogs may be efficacious in repairing endothelial AJ integrity.
In contrast to S1PR1, S1PR3 has a barrier disruptive role 391. Lung endothelial cells shed S1PR3 in microparticles following activation with LPS or low-molecular-weight hyaluronan 392. Exposure of normal endothelial cells to S1PR3-containing microparticles significantly reduces AJ integrity, consistent with increased permeability response 392. These changes are attenuated by RNAi-mediated depletion of S1PR3. Intriguingly, elevated S1PR3 plasma concentration has been linked to sepsis and ALI mortality 391, indicating S1PR3 antagonists as novel therapeutics targeting AJ integrity 391.
Growth factors
Angiopoietin (Ang1 and 2) stabilize developing vessels by ligating the endothelial Tie2 receptor (393). However, both have different effects on mature endothelium. While Ang1 promotes endothelial survival, migration, and barrier formation, Ang2 induces vascular leak by disrupting AJs through activation of actin-myosin induced stress fiber formation 394–396. Ang2 has emerged as a predictor of patient mortality from ARDS and sepsis, as circulating levels of Ang2 are consistently greater in patients who died of ARDS or sepsis as compared to control groups 397– 401. Ang1 infusion has been thought to be a therapeutic approach to counteract Ang2 disruption of the endothelial barrier 402 but its use has been limited due to its side effect of inducing pulmonary hypertension 403– 405.
microRNAs
miRNAs are small (19–23 nucleotides) non-coding RNAs which can suppress or augment cellular signaling in several cell types, including endothelial cells, based on their ability to target mRNA 406. miR-27a targets VE-cadherin and induces vascular leak during ischemia and reperfusion injury 406, indicating inhibition of miR-27a may be a useful approach for preventing vascular barrier disruption. The expression of mature miR-150-5p but not miR-150-3p is induced during recovery from endothelial injury post LPS challenge 407. Loss of miR-150 does not alter AJ organization or barrier function under basal conditions but markedly impairs AJ re-annealing after LPS challenge leading to persistent vascular injury 408. miR-150 restores endothelial barrier function post injury by suppressing Ang2 generation through targeting the transcription factor EGR2 409. Conversely, depletion of Ang2 in miR-150 null endothelial cells rescues AJ re-annealing and barrier function 409 demonstrating that miR-150 functions by suppressing Ang2 generation.
Cellular therapy
Recent evidence suggests that trafficking and differentiation of non-resident and resident stem cells facilitates the repair of injured vessels 309, 410. Stem cells can be mobilized from the bone-marrow to the damaged tissue where they proliferate and function in the same manner as the original cell type 411. Hematopoietic and non-hematopoietic stem cells are located in the bone marrow and are critical in tissue repair 309, 410. Mesenchymal stem cells have been shown to prevent lung vascular injury by secreting growth factors such as keratinocyte growth factor (KGF) or S1P which may alter Rho-Rac1 signaling 412. Studies also demonstrated that transplanted bone-marrow derived hematopoietic stem cells are detected in several organs including lungs 413. A number of studies have shown the so called endothelial progenitor cells (EPCs) to be pro-angiogenic 414. Wary et al 410 further showed that VE-cadherin+/Flk1+ EPCs promote endothelial barrier function and that loss of the barrier is prevented by activation of α4 and α5 integrins in an LPS mouse lung vascular injury model. Additionally, Zhao et al 309 have demonstrated that bone marrow derived MSCs can be directed to AJs where they restore normal endothelial permeability through the generation of S1P and thereby prevent LPS-induced lung injury.
10. Concluding remarks and future directions
Here we have discussed the current view on the organization and dynamics of VE-cadherin adhesion, the main gatekeeper of AJs, in resting and activated (or inflamed) endothelium. Although the list of constituents of the VE-cadherin complex is incomplete, it is apparent that it forms an extraordinary well-organized network of signaling molecules at AJs. VE-cadherin assembles a mechanosensory complex with another adhesion molecule PECAM-1 and receptor tyrosine kinases VEGFR2 and VEGFR3 at AJs enabling sensing and adaptation of endothelial cells to rapid shifts in local perfusion and pressure. The signals sensed by this complex are tuned and then transmitted to integrins at the sites of FAs to elicit coordinated responses to modify barrier properties of the junctions.
Small RhoGTPases at the level of AJs are key molecular switches that play a fundamental role in regulating the plasticity of VE-cadherin adhesion, and hence endothelial permeability. They are essential for signaling endothelial responses to both humoral and mechanical stimuli making. They are therefore potential drug targets in a variety of inflammatory disorders. The endothelium expresses numerous upstream regulators of RhoGTPases that regulate GTPase activation in space and time. These are also drug targets that might be exploited.
Several fundamental questions remain unanswered, such as how PECAM1, VE-cadherin, and VEGFR2 organize themselves to serve as a unified mechanosensor, and whether the mechanosensing function is different in the quiescent endothelium as opposed to the activated endothelium. Perturbation of the mechanosensory complex has consequences in disease pathogenesis. Another question is what dictates the balance between RhoA, Rac1, and Cdc42 activities. It is clear that Rac1 and Cdc42 activities (as opposed to RhoA activity) need to be exquisitely balanced, but how this is achieved remains unknown. Another question is how inflammatory mediators in disease states disrupt the sensing function of AJs and how this leads to short- or long-term disruption of AJs. Hence, better understanding of the formation of the mechanosensory complex in the endothelium and the function of RhoGTPases at AJs will be a critical for development of novel therapeutic targets for treating inflammatory diseases.
VE-cadherin also assembles a complex with signaling molecules comprising kinases (e.g., Src kinases), phosphatases (e.g., VE-PTP), and RhoGTPases, which in turn, provide spatial control of VE-cadherin adhesion under physiological and pathological conditions. An increasing body of evidence suggests that VE-cadherin adhesion undergoes continuous reorganization resulting in on-demand remodeling at AJs. Exchange of VE-cadherin molecules between junctional and cytosolic pools is a constitutive process accounting from the permeable nature of the endothelial barrier. VE-cadherin adhesion is finely regulated by specific intracellular signaling pathways that assemble and disassemble AJs. Depending on the stimuli, these signals can also enhance or weaken the endothelial barrier. Disruption of VE-cadherin adhesion triggers increased endothelial permeability and tissue edema, a central feature of human diseases ranging from cancer to acute inflammatory disorders such as ARDS. Thus, an important concept evolving from these studies is whether a cross-talk mechanism exists between phosphatases such as VE-PTP and myosin phosphatase (PP1), which stabilize AJs.
It is also apparent that the tyrosine kinases Fyn, Src, and FAK differentially regulate AJs depending on the context and their mode of activation. For example, as described above, FAK is involved in both formation and disruption of AJs. FAK also influences RhoGTPases. What determines whether FAK functions one way or the other? It will be important in future studies to understand how the interplay between the underlying extracellular matrix and AJs dictates the function of FAK, Fyn, or Src and how the function of these kinases influences the endothelial barrier. It is likely that further clarification of how mechanical stimuli sensed by the AJ mechanosensor influence endothelial permeability in response to inflammatory mediators will advance our understanding of mechanisms regulating endothelial permeability.
Acknowledgments
Sources of Funding
Supported by NIH grants R01 HL103922 to Y.A.K.; R01 HL84153 to D.M.; AHA AWARD 16PRE27260230 to K.K.; R01 HL 45638 and PO1 HL60678 to A.B.M.
Non-standard Abbreviations and Acronyms
- AJ
adherens junction
- ARDS
acute respiratory distress syndrome
- BBB
blood brain barrier
- cAMP
cyclic adenosine monophosphate
- CBP
CREB-binding protein
- cGMP
cyclic guanosine monophosphate
- CNS
central nervous system
- CP
capping protein
- DAG
diacylglycerol
- DEP1
density-enhanced phosphatase-1
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- EPLIN
epithelial protein lost in neoplasm
- FA
focal adhesion
- FAK
focal adhesion kinase
- FN
fibronectin
- FPP
farnesylpyrophosphate
- GAP
GTPase-activating protein
- GDI
guanine nucleotide dissociation inhibitors
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- GGPP
geranylgeranylpyrophosphate
- GJ
gap junction
- GPCR
G protein-coupled receptors
- GTP
guanosine triphosphate
- HDA
histone deacetylase
- iBRB
inner blood retinal barrier
- IQGAP
IQRas GTPase-activating protein
- IRSp53
insulin receptor substrate p53
- JACOP
junction-associated coiled-coil protein
- JAM
junctional adhesion molecules
- KGF
keratinocyte growth factor
- LDL
low density lipoprotein
- LIMK
Lin1, Isl-1, and Mec-3 Kinase
- LPS
lipopolysaccharide
- MLC
myosin light chain
- MLCK
myosin light-chain kinase
- MLCP
myosin light-chain phosphatase
- MRCK
myotonic dystrophy kinase-related Cdc42-binding kinase
- MSC
mesenchymal stem cell
- NO
nitric oxide
- N-WASP
Neural Wiskott-Aldrich syndrome protein
- PAF
platelet-activating factor
- PAK
p21-activated kinase
- PAR6
partitioning-defective polarity protein
- PECAM-1
platelet endothelial cell adhesion molecule 1
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PTM
post-translational modification
- PTP
protein tyrosine phosphatase
- ROCE
receptor-activated calcium entry
- ROCK
Rho-associated coiled-coil forming protein kinase
- ROS
reactive oxygen species
- S1P
sphingosine-1-phosphate
- SHP2
Src homology 2-domain containing tyrosine phosphatase
- SOCE
store-operated calcium entry
- SPHK1
sphingosine kinase 1
- TCF
T-cell factor
- TEM
transendothelial migration
- TJ
tight junction
- TNF
tumor necrosis factor
- TRIO
triple functional domain protein
- TRP
transient receptor potential
- VASP
vasodilator-stimulated phosphoprotein
- VE
vascular endothelial
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- WASP
Wiskott-Aldrich syndrome protein
- WAVE
WASP-family verprolin-homologous protein
- ZO
zonula occludens
Footnotes
Disclosures
None
References
- 1.Pappenheimer JR, Renkin EM, Borrero LM. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. The American journal of physiology. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Del Vecchio PJ, Siflinger-Birnboim A, Shepard JM, Bizios R, Cooper JA, Malik AB. Endothelial monolayer permeability to macromolecules. Federation proceedings. 1987;46:2511–2515. [PubMed] [Google Scholar]
- 3.Siflinger-Birnboim A, Del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132:111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- 4.Schneeberger EE. Circulating proteins and macromolecular transport across continuous, nonfenestrated endothelium. Annals of the New York Academy of Sciences. 1982;401:25–37. doi: 10.1111/j.1749-6632.1982.tb25704.x. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg HF. Osmotic pressure of the serum proteins. Annals of clinical and laboratory science. 1978;8:155–164. [PubMed] [Google Scholar]
- 6.Xie Z, Ghosh CC, Patel R, Iwaki S, Gaskins D, Nelson C, Jones N, Greipp PR, Parikh SM, Druey KM. Vascular endothelial hyperpermeability induces the clinical symptoms of clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119:4321–4332. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouillet L, Mannic T, Arboleas M, Subileau M, Massot C, Drouet C, Huber P, Vilgrain I. Hereditary angioedema: Key role for kallikrein and bradykinin in vascular endothelial-cadherin cleavage and edema formation. J Allergy Clin Immunol. 2011;128:232–234. doi: 10.1016/j.jaci.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175–179. doi: 10.1056/NEJMcp011186. [DOI] [PubMed] [Google Scholar]
- 9.Herwig MC, Tsokos M, Hermanns MI, Kirkpatrick CJ, Muller AM. Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures. Pathobiology. 2013;80:245–251. doi: 10.1159/000347062. [DOI] [PubMed] [Google Scholar]
- 10.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 11.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Tikellis G, Wang JJ. Diabetic retinopathy. Ophthalmology. 2007;114:2098–2099. doi: 10.1016/j.ophtha.2007.07.010. author reply 2099. [DOI] [PubMed] [Google Scholar]
- 13.Aroca PR, Salvat M, Fernandez J, Mendez I. Risk factors for diffuse and focal macular edema. Journal of diabetes and its complications. 2004;18:211–215. doi: 10.1016/S1056-8727(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 14.Zlokovic BV. Remodeling after stroke. Nat Med. 2006;12:390–391. doi: 10.1038/nm0406-390. [DOI] [PubMed] [Google Scholar]
- 15.Ilzecka J. The structure and function of blood-brain barrier in ischaemic brain stroke process. Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina. 1996;51:123–127. [PubMed] [Google Scholar]
- 16.Ilzecka J, Mitosek-Szewczyk K. Cerebral strokes immunopathology. Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina. 1996;51:103–107. [PubMed] [Google Scholar]
- 17.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Current opinion in critical care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huttner I, Boutet M, More RH. Gap junctions in arterial endothelium. J Cell Biol. 1973;57:247–252. doi: 10.1083/jcb.57.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firth JA, Bauman KF, Sibley CP. The intercellular junctions of guinea-pig placental capillaries: A possible structural basis for endothelial solute permeability. Journal of ultrastructure research. 1983;85:45–57. doi: 10.1016/s0022-5320(83)90115-6. [DOI] [PubMed] [Google Scholar]
- 22.Qu Y, Dahl G. Accessibility of cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophysical journal. 2004;86:1502–1509. doi: 10.1016/S0006-3495(04)74218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoh JH, Sosinsky GE, Revel JP, Hansma PK. Structure of the extracellular surface of the gap junction by atomic force microscopy. Biophysical journal. 1993;65:149–163. doi: 10.1016/S0006-3495(93)81074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das Sarma J, Meyer RA, Wang F, Abraham V, Lo CW, Koval M. Multimeric connexin interactions prior to the trans-golgi network. J Cell Sci. 2001;114:4013–4024. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 25.Vanslyke JK, Naus CC, Musil LS. Conformational maturation and post-er multisubunit assembly of gap junction proteins. Mol Biol Cell. 2009;20:2451–2463. doi: 10.1091/mbc.E09-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith TD, Mohankumar A, Minogue PJ, Beyer EC, Berthoud VM, Koval M. Cytoplasmic amino acids within the membrane interface region influence connexin oligomerization. The Journal of membrane biology. 2012;245:221–230. doi: 10.1007/s00232-012-9443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MV, Nicholson BJ. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol. 2001;281:H1675–1689. doi: 10.1152/ajpheart.2001.281.4.H1675. [DOI] [PubMed] [Google Scholar]
- 29.Beyer EC, Gemel J, Martinez A, Berthoud VM, Valiunas V, Moreno AP, Brink PR. Heteromeric mixing of connexins: Compatibility of partners and functional consequences. Cell Commun Adhes. 2001;8:199–204. doi: 10.3109/15419060109080723. [DOI] [PubMed] [Google Scholar]
- 30.Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric cx40 and cx43 connexons. American journal of physiology. Cell physiology. 2001;281:C1559–1567. doi: 10.1152/ajpcell.2001.281.5.C1559. [DOI] [PubMed] [Google Scholar]
- 31.Johnson TL, Nerem RM. Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium : journal of endothelial cell research. 2007;14:215–226. doi: 10.1080/10623320701617233. [DOI] [PubMed] [Google Scholar]
- 32.Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol (1985) 2004;97:1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 34.Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285:H119–126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney international. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 37.Wagner C, de Wit C, Gerl M, Kurtz A, Hocherl K. Increased expression of cyclooxygenase 2 contributes to aberrant renin production in connexin 40-deficient kidneys. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R1781–1786. doi: 10.1152/ajpregu.00439.2007. [DOI] [PubMed] [Google Scholar]
- 38.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 39.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest. 2006;116:2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976;68:705–723. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneeberger EE. Structure of intercellular junctions in different segments of the intrapulmonary vasculature. Annals of the New York Academy of Sciences. 1982;384:54–63. doi: 10.1111/j.1749-6632.1982.tb21361.x. [DOI] [PubMed] [Google Scholar]
- 42.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebner S, Gerhardt H, Wolburg H. Differential expression of endothelial beta-catenin and plakoglobin during development and maturation of the blood-brain and blood-retina barrier in the chicken. Dev Dyn. 2000;217:86–98. doi: 10.1002/(SICI)1097-0177(200001)217:1<86::AID-DVDY8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 44.Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. European journal of cell biology. 2000;79:707–717. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- 45.Lippoldt A, Liebner S, Andbjer B, Kalbacher H, Wolburg H, Haller H, Fuxe K. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2 and -5 expression by protein kinase c. Neuroreport. 2000;11:1427–1431. doi: 10.1097/00001756-200005150-00015. [DOI] [PubMed] [Google Scholar]
- 46.Rascher G, Wolburg H. The tight junctions of the leptomeningeal blood-cerebrospinal fluid barrier during development. Journal fur Hirnforschung. 1997;38:525–540. [PubMed] [Google Scholar]
- 47.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 48.Luo Y, Xiao W, Zhu X, Mao Y, Liu X, Chen X, Huang J, Tang S, Rizzolo LJ. Differential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011;52:7556–7564. doi: 10.1167/iovs.11-7185. [DOI] [PubMed] [Google Scholar]
- 49.Petito CK, Pulsinelli WA, Jacobson G, Plum F. Edema and vascular permeability in cerebral ischemia: Comparison between ischemic neuronal damage and infarction. Journal of neuropathology and experimental neurology. 1982;41:423–436. doi: 10.1097/00005072-198207000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Bralet AM, Beley A, Beley P, Bralet J. Brain edema and blood-brain barrier permeability following quantitative cerebral microembolism. Stroke; a journal of cerebral circulation. 1979;10:34–38. doi: 10.1161/01.str.10.1.34. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 52.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 54.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamazaki Y, Okawa K, Yano T, Tsukita S, Tsukita S. Optimized proteomic analysis on gels of cell-cell adhering junctional membrane proteins. Biochemistry. 2008;47:5378–5386. doi: 10.1021/bi8002567. [DOI] [PubMed] [Google Scholar]
- 58.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. American journal of physiology. Cell physiology. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 59.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta neuropathologica. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 61.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta neuropathologica. 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 62.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated maguks, zo-1, zo-2, and zo-3, with the cooh termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Itoh M, Morita K, Tsukita S. Characterization of zo-2 as a maguk family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- 64.Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738–743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and alzheimer amyloid beta-peptide. Brain pathology. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 67.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The penn state retina research group. Invest Ophthalmol Vis Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 69.Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase czeta phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J. 2011;437:289–299. doi: 10.1042/BJ20110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia L, Wang H, Munk S, Kwan J, Goldberg HJ, Fantus IG, Whiteside CI. High glucose activates pkc-zeta and nadph oxidase through autocrine tgf-beta1 signaling in mesangial cells. Am J Physiol Renal Physiol. 2008;295:F1705–1714. doi: 10.1152/ajprenal.00043.2008. [DOI] [PubMed] [Google Scholar]
- 71.Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. Tnf-alpha signals through pkczeta/nf-kappab to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S, Tsukita S. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. Zo-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208:821–838. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. The Journal of pathology. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- 75.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: Contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 76.Hawkins BT, Ocheltree SM, Norwood KM, Egleton RD. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neuroscience letters. 2007;411:1–5. doi: 10.1016/j.neulet.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Gothert JR, Malik AB, Valyi-Nagy T, Zhao YY. Endothelial beta-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation. 2016;133:177–186. doi: 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leach L, Clark P, Lampugnani MG, Arroyo AG, Dejana E, Firth JA. Immunoelectron characterisation of the inter-endothelial junctions of human term placenta. J Cell Sci. 1993;104(Pt 4):1073–1081. doi: 10.1242/jcs.104.4.1073. [DOI] [PubMed] [Google Scholar]
- 80.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (n-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 81.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (ve-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. [PubMed] [Google Scholar]
- 83.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by ve-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 84.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 85.Crosby CV, Fleming PA, Argraves WS, Corada M, Zanetta L, Dejana E, Drake CJ. Ve-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood. 2005;105:2771–2776. doi: 10.1182/blood-2004-06-2244. [DOI] [PubMed] [Google Scholar]
- 86.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frye M, Dierkes M, Kuppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D. Interfering with ve-ptp stabilizes endothelial junctions in vivo via tie-2 in the absence of ve-cadherin. J Exp Med. 2015;212:2267–2287. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liaw CW, Cannon C, Power MD, Kiboneka PK, Rubin LL. Identification and cloning of two species of cadherins in bovine endothelial cells. EMBO J. 1990;9:2701–2708. doi: 10.1002/j.1460-2075.1990.tb07456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of n-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102(Pt 1):7–17. doi: 10.1242/jcs.102.1.7. [DOI] [PubMed] [Google Scholar]
- 90.Gerhardt H, Liebner S, Redies C, Wolburg H. N-cadherin expression in endothelial cells during early angiogenesis in the eye and brain of the chicken: Relation to blood-retina and blood-brain barrier development. Eur J Neurosci. 1999;11:1191–1201. doi: 10.1046/j.1460-9568.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 91.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 92.Tillet E, Vittet D, Feraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400. doi: 10.1016/j.yexcr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 93.Navarro P, Ruco L, Dejana E. Differential localization of ve- and n-cadherins in human endothelial cells: Ve-cadherin competes with n-cadherin for junctional localization. J Cell Biol. 1998;140:1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gentil-dit-Maurin A, Oun S, Almagro S, Bouillot S, Courcon M, Linnepe R, Vestweber D, Huber P, Tillet E. Unraveling the distinct distributions of ve- and n-cadherins in endothelial cells: A key role for p120-catenin. Exp Cell Res. 2010;316:2587–2599. doi: 10.1016/j.yexcr.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Luo Y, Radice GL. N-cadherin acts upstream of ve-cadherin in controlling vascular morphogenesis. J Cell Biol. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in pdgf-b-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 97.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 98.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Wang YL, Hui YN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye. 2007;21:1501–1510. doi: 10.1038/sj.eye.6702716. [DOI] [PubMed] [Google Scholar]
- 101.Kim JH, Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. Journal of neuroscience research. 2009;87:653–659. doi: 10.1002/jnr.21884. [DOI] [PubMed] [Google Scholar]
- 102.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jian MY, Liu Y, Li Q, Wolkowicz P, Alexeyev M, Zmijewski J, Creighton J. N-cadherin coordinates amp kinase-mediated lung vascular repair. Am J Physiol Lung Cell Mol Physiol. 2016;310:L71–85. doi: 10.1152/ajplung.00227.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ivanov D, Philippova M, Antropova J, Gubaeva F, Iljinskaya O, Tararak E, Bochkov V, Erne P, Resink T, Tkachuk V. Expression of cell adhesion molecule t-cadherin in the human vasculature. Histochem Cell Biol. 2001;115:231–242. doi: 10.1007/s004180100252. [DOI] [PubMed] [Google Scholar]
- 105.Dames SA, Bang E, Haussinger D, Ahrens T, Engel J, Grzesiek S. Insights into the low adhesive capacity of human t-cadherin from the nmr structure of its n-terminal extracellular domain. J Biol Chem. 2008;283:23485–23495. doi: 10.1074/jbc.M708335200. [DOI] [PubMed] [Google Scholar]
- 106.Ivanov D, Philippova M, Tkachuk V, Erne P, Resink T. Cell adhesion molecule t-cadherin regulates vascular cell adhesion, phenotype and motility. Exp Cell Res. 2004;293:207–218. doi: 10.1016/j.yexcr.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 107.Philippova M, Banfi A, Ivanov D, Gianni-Barrera R, Allenspach R, Erne P, Resink T. Atypical gpi-anchored t-cadherin stimulates angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2222–2230. doi: 10.1161/01.ATV.0000238356.20565.92. [DOI] [PubMed] [Google Scholar]
- 108.Andreeva AV, Han J, Kutuzov MA, Profirovic J, Tkachuk VA, Voyno-Yasenetskaya TA. T-cadherin modulates endothelial barrier function. J Cell Physiol. 2010;223:94–102. doi: 10.1002/jcp.22014. [DOI] [PubMed] [Google Scholar]
- 109.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific r-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- 110.Shan WS, Tanaka H, Phillips GR, Arndt K, Yoshida M, Colman DR, Shapiro L. Functional cis-heterodimers of n- and r-cadherins. J Cell Biol. 2000;148:579–590. doi: 10.1083/jcb.148.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rampon C, Prandini MH, Bouillot S, Pointu H, Tillet E, Frank R, Vernet M, Huber P. Protocadherin 12 (ve-cadherin 2) is expressed in endothelial, trophoblast, and mesangial cells. Exp Cell Res. 2005;302:48–60. doi: 10.1016/j.yexcr.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 112.Telo P, Breviario F, Huber P, Panzeri C, Dejana E. Identification of a novel cadherin (vascular endothelial cadherin-2) located at intercellular junctions in endothelial cells. J Biol Chem. 1998;273:17565–17572. doi: 10.1074/jbc.273.28.17565. [DOI] [PubMed] [Google Scholar]
- 113.Philibert C, Bouillot S, Huber P, Faury G. Protocadherin-12 deficiency leads to modifications in the structure and function of arteries in mice. Pathol Biol (Paris) 2012;60:34–40. doi: 10.1016/j.patbio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 115.Brasch J, Harrison OJ, Ahlsen G, Carnally SM, Henderson RM, Honig B, Shapiro L. Structure and binding mechanism of vascular endothelial cadherin: A divergent classical cadherin. J Mol Biol. 2011;408:57–73. doi: 10.1016/j.jmb.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, Troyanovsky RB, Ben-Shaul A, Frank J, Troyanovsky SM, Shapiro L, Honig B. The extracellular architecture of adherens junctions revealed by crystal structures of type i cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu Y, Jin X, Harrison O, Shapiro L, Honig BH, Ben-Shaul A. Cooperativity between trans and cis interactions in cadherin-mediated junction formation. Proc Natl Acad Sci U S A. 2010;107:17592–17597. doi: 10.1073/pnas.1011247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type ii cadherin ectodomain structures: Implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Sivasankar S, Nelson WJ, Chu S. Resolving cadherin interactions and binding cooperativity at the single-molecule level. Proc Natl Acad Sci U S A. 2009;106:109–114. doi: 10.1073/pnas.0811350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang S, Iring A, Strilic B, Albarran Juarez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Muller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2y(2) and gq/g(1)(1) control blood pressure by mediating endothelial mechanotransduction. J Clin Invest. 2015;125:3077–3086. doi: 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nelson CM, Chen CS. Ve-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J Cell Sci. 2003;116:3571–3581. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 122.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating rhoa. Mol Biol Cell. 2004;15:2943–2953. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial ve-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong S, Troyanovsky RB, Troyanovsky SM. Binding to f-actin guides cadherin cluster assembly, stability, and movement. J Cell Biol. 2013;201:131–143. doi: 10.1083/jcb.201211054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barry AK, Wang N, Leckband DE. Local ve-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J Cell Sci. 2015;128:1341–1351. doi: 10.1242/jcs.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(e)-catenin is an actin-binding and -bundling protein mediating the attachment of f-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mege RM, Yan J. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun. 2014;5:4525. doi: 10.1038/ncomms5525. [DOI] [PubMed] [Google Scholar]
- 129.Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds e-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-f-actin interface. J Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 132.Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature. 1983;302:436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- 133.Kolega J. Cytoplasmic dynamics of myosin iia and iib: Spatial ‘sorting’ of isoforms in locomoting cells. J Cell Sci. 1998;111(Pt 15):2085–2095. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- 134.Kim TJ, Zheng S, Sun J, Muhamed I, Wu J, Lei L, Kong X, Leckband DE, Wang Y. Dynamic visualization of alpha-catenin reveals rapid, reversible conformation switching between tension states. Curr Biol. 2015;25:218–224. doi: 10.1016/j.cub.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Niederman R, Pollard TD. Human platelet myosin. Ii. In vitro assembly and structure of myosin filaments. J Cell Biol. 1975;67:72–92. doi: 10.1083/jcb.67.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovascular research. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 137.Wu SK, Gomez GA, Michael M, Verma S, Cox HL, Lefevre JG, Parton RG, Hamilton NA, Neufeld Z, Yap AS. Cortical f-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol. 2014;16:167–178. doi: 10.1038/ncb2900. [DOI] [PubMed] [Google Scholar]
- 138.Rajput C, Kini V, Smith M, Yazbeck P, Chavez A, Schmidt T, Zhang W, Knezevic N, Komarova Y, Mehta D. Neural wiskott-aldrich syndrome protein (n-wasp)-mediated p120-catenin interaction with arp2-actin complex stabilizes endothelial adherens junctions. J Biol Chem. 2013;288:4241–4250. doi: 10.1074/jbc.M112.440396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tang VW, Brieher WM. Fsgs3/cd2ap is a barbed-end capping protein that stabilizes actin and strengthens adherens junctions. J Cell Biol. 2013;203:815–833. doi: 10.1083/jcb.201304143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annual review of cell and developmental biology. 2014;30:291–315. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- 141.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across ve-cadherin and pecam-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. The Journal of physiology. 1968;199:637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simons M, Wang M, McBride OW, Kawamoto S, Yamakawa K, Gdula D, Adelstein RS, Weir L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ Res. 1991;69:530–539. doi: 10.1161/01.res.69.2.530. [DOI] [PubMed] [Google Scholar]
- 145.Higashi-Fujime S. Active movement of bundles of actin and myosin filaments from muscle: A simple model for cell motility. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):69–75. doi: 10.1101/sqb.1982.046.01.010. [DOI] [PubMed] [Google Scholar]
- 146.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: The relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miller JR, Silver PJ, Stull JT. The role of myosin light chain kinase phosphorylation in beta-adrenergic relaxation of tracheal smooth muscle. Mol Pharmacol. 1983;24:235–242. [PubMed] [Google Scholar]
- 148.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(src) J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 149.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 150.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of rhoa by thrombin in endothelial hyperpermeability: Role of rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- 151.van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007;27:2332–2339. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 152.Reinhard NR, van Helden SF, Anthony EC, Yin T, Wu YI, Goedhart J, Gadella TW, Hordijk PL. Spatiotemporal analysis of rhoa/b/c activation in primary human endothelial cells. Scientific reports. 2016;6:25502. doi: 10.1038/srep25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Szulcek R, Beckers CM, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJ, Minshall RD, van Hinsbergh VW, van Nieuw Amerongen GP. Localized rhoa gtpase activity regulates dynamics of endothelial monolayer integrity. Cardiovascular research. 2013;99:471–482. doi: 10.1093/cvr/cvt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith AL, Dohn MR, Brown MV, Reynolds AB. Association of rho-associated protein kinase 1 with e-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012;23:99–110. doi: 10.1091/mbc.E11-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Priya R, Yap AS, Gomez GA. E-cadherin supports steady-state rho signaling at the epithelial zonula adherens. Differentiation. 2013;86:133–140. doi: 10.1016/j.diff.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 156.Priya R, Gomez GA, Budnar S, Verma S, Cox HL, Hamilton NA, Yap AS. Feedback regulation through myosin ii confers robustness on rhoa signalling at e-cadherin junctions. Nat Cell Biol. 2015;17:1282–1293. doi: 10.1038/ncb3239. [DOI] [PubMed] [Google Scholar]
- 157.Siddiqui MR, Komarova YA, Vogel SM, Gao X, Bonini MG, Rajasingh J, Zhao YY, Brovkovych V, Malik AB. Caveolin-1-enos signaling promotes p190rhogap-a nitration and endothelial permeability. J Cell Biol. 2011;193:841–850. doi: 10.1083/jcb.201012129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chava KR, Tauseef M, Sharma T, Mehta D. Cyclic amp response element-binding protein prevents endothelial permeability increase through transcriptional controlling p190rhogap expression. Blood. 2012;119:308–319. doi: 10.1182/blood-2011-02-339473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG. Interaction of p190rhogap with c-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J Biol Chem. 2013;288:18290–18299. doi: 10.1074/jbc.M112.432757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-mrck and rho-rock signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 161.Vogler G, Liu J, Iafe TW, Migh E, Mihaly J, Bodmer R. Cdc42 and formin activity control non-muscle myosin dynamics during drosophila heart morphogenesis. J Cell Biol. 2014;206:909–922. doi: 10.1083/jcb.201405075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koh W, Mahan RD, Davis GE. Cdc42- and rac1-mediated endothelial lumen formation requires pak2, pak4 and par3, and pkc-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 163.Barry DM, Xu K, Meadows SM, Zheng Y, Norden PR, Davis GE, Cleaver O. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development. 2015;142:3058–3070. doi: 10.1242/dev.125260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126:821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 166.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA. Intramembrane binding of ve-cadherin to vegfr2 and vegfr3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 168.Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of ve-cadherin at vascular endothelial cell junctions: In vivo and in vitro investigations. J Vasc Res. 2005;42:77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 169.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation of ve-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu B, Lu S, Hu YL, Liao X, Ouyang M, Wang Y. Rhoa and membrane fluidity mediates the spatially polarized src/fak activation in response to shear stress. Scientific reports. 2014;4:7008. doi: 10.1038/srep07008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited pecam-1 phosphorylation. J Cell Biol. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of pecam-1 in the shear-stress-induced activation of akt and the endothelial nitric oxide synthase (enos) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 173.Cain RJ, Vanhaesebroeck B, Ridley AJ. The pi3k p110alpha isoform regulates endothelial adherens junctions via pyk2 and rac1. J Cell Biol. 2010;188:863–876. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, Tzima E. A novel pathway spatiotemporally activates rac1 and redox signaling in response to fluid shear stress. J Cell Biol. 2013;201:863–873. doi: 10.1083/jcb.201207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Collins C, Osborne LD, Guilluy C, Chen Z, O’Brien ET, 3rd, Reader JS, Burridge K, Superfine R, Tzima E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat Commun. 2014;5:3984. doi: 10.1038/ncomms4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The rho gefs larg and gef-h1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on pecam-1 elicit a global mechanotransduction response via the integrin-rhoa pathway. Curr Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Whittard JD, Akiyama SK. Positive regulation of cell-cell and cell-substrate adhesion by protein kinase a. J Cell Sci. 2001;114:3265–3272. doi: 10.1242/jcs.114.18.3265. [DOI] [PubMed] [Google Scholar]
- 180.Qiao J, Huang F, Lum H. Pka inhibits rhoa activation: A protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 181.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates rhoa in vivo. J Biol Chem. 2003;278:19023–19031. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 182.Daneshjou N, Sieracki N, van Nieuw Amerongen GP, Schwartz MA, Komarova YA, Malik AB, Conway DE. Rac1 functions as a reversible tension modulator to stabilize ve-cadherin trans-interaction. J Cell Biol. 2015;208:23–32. doi: 10.1083/jcb.201409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. The American journal of pathology. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. P120-catenin regulates clathrin-dependent endocytosis of ve-cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. P120-catenin regulates leukocyte transmigration through an effect on ve-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. P120-catenin prevents neutrophil transmigration independently of rhoa inhibition by impairing src dependent ve-cadherin phosphorylation. American journal of physiology. Cell physiology. 2012;303:C385–395. doi: 10.1152/ajpcell.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heupel WM, Efthymiadis A, Schlegel N, Muller T, Baumer Y, Baumgartner W, Drenckhahn D, Waschke J. Endothelial barrier stabilization by a cyclic tandem peptide targeting ve-cadherin transinteraction in vitro and in vivo. J Cell Sci. 2009;122:1616–1625. doi: 10.1242/jcs.040212. [DOI] [PubMed] [Google Scholar]
- 188.Gavard J, Gutkind JS. Vegf controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of ve-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 189.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. Pkcalpha activation of p120-catenin serine 879 phospho-switch disassembles ve-cadherin junctions and disrupts vascular integrity. Circ Res. 2012;111:739–749. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gong H, Gao X, Feng S, Siddiqui MR, Garcia A, Bonini MG, Komarova Y, Vogel SM, Mehta D, Malik AB. Evidence of a common mechanism of disassembly of adherens junctions through galpha13 targeting of ve-cadherin. J Exp Med. 2014;211:579–591. doi: 10.1084/jem.20131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for src kinases during vegf-induced angiogenesis and vascular permeability. Molecular cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 192.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of e-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 193.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. P120 catenin-associated fer and fyn tyrosine kinases regulate beta-catenin tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Han J, Zhang G, Welch EJ, Liang Y, Fu J, Vogel SM, Lowell CA, Du X, Cheresh DA, Malik AB, Li Z. A critical role for lyn kinase in strengthening endothelial integrity and barrier function. Blood. 2013;122:4140–4149. doi: 10.1182/blood-2013-03-491423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Knezevic N, Tauseef M, Thennes T, Mehta D. The g protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med. 2009;206:2761–2777. doi: 10.1084/jem.20090652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of rhoa activity by focal adhesion kinase-induced activation of p190rhogap: Role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 197.Schmidt TT, Tauseef M, Yue L, Bonini MG, Gothert J, Shen TL, Guan JL, Predescu S, Sadikot R, Mehta D. Conditional deletion of fak in mice endothelium disrupts lung vascular barrier function due to destabilization of rhoa and rac1 activities. Am J Physiol Lung Cell Mol Physiol. 2013;305:L291–300. doi: 10.1152/ajplung.00094.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. Vegf-induced vascular permeability is mediated by fak. Dev Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee J, Borboa AK, Chun HB, Baird A, Eliceiri BP. Conditional deletion of the focal adhesion kinase fak alters remodeling of the blood-brain barrier in glioma. Cancer research. 2010;70:10131–10140. doi: 10.1158/0008-5472.CAN-10-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jean C, Chen XL, Nam JO, Tancioni I, Uryu S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, Turowski P, Dejana E, Weis S, Cheresh DA, Schlaepfer DD. Inhibition of endothelial fak activity prevents tumor metastasis by enhancing barrier function. J Cell Biol. 2014;204:247–263. doi: 10.1083/jcb.201307067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of csk to ve-cadherin and inhibition of cell proliferation. EMBO J. 2005;24:1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jin H, Garmy-Susini B, Avraamides CJ, Stoletov K, Klemke RL, Varner JA. A pka-csk-pp60src signaling pathway regulates the switch between endothelial cell invasion and cell-cell adhesion during vascular sprouting. Blood. 2010;116:5773–5783. doi: 10.1182/blood-2010-07-296210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. Ve-ptp and ve-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D. Ve-ptp maintains the endothelial barrier via plakoglobin and becomes dissociated from ve-cadherin by leukocytes and by vegf. J Exp Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the ve-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Balsamo J, Arregui C, Leung T, Lilien J. The nonreceptor protein tyrosine phosphatase ptp1b binds to the cytoplasmic domain of n-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Timmerman I, Hoogenboezem M, Bennett AM, Geerts D, Hordijk PL, van Buul JD. The tyrosine phosphatase shp2 regulates recovery of endothelial adherens junctions through control of beta-catenin phosphorylation. Mol Biol Cell. 2012;23:4212–4225. doi: 10.1091/mbc.E12-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K, Kojima M, Xia H, Seidel C, Lima e Silva R, Dong A, Hackett SF, Wang J, Howard BW, Vestweber D, Kontos CD, Peters KG, Campochiaro PA. Targeting ve-ptp activates tie2 and stabilizes the ocular vasculature. J Clin Invest. 2014;124:4564–4576. doi: 10.1172/JCI74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.van der Heijden M, van Nieuw Amerongen GP, van Bezu J, Paul MA, Groeneveld AB, van Hinsbergh VW. Opposing effects of the angiopoietins on the thrombin-induced permeability of human pulmonary microvascular endothelial cells. PLoS One. 2011;6:e23448. doi: 10.1371/journal.pone.0023448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D’Angelo DD, Lynch KR, Peach MJ. Molecular cloning and expression of a cdna encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992;267:15274–15276. [PubMed] [Google Scholar]
- 211.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: Molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lamas S, Michel T, Collins T, Brenner BM, Marsden PA. Effects of interferon-gamma on nitric oxide synthase activity and endothelin-1 production by vascular endothelial cells. J Clin Invest. 1992;90:879–887. doi: 10.1172/JCI115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Delisle C, Gratton JP. S-nitrosylation of beta-catenin by enos-derived no promotes vegf-induced endothelial cell permeability. Molecular cell. 2010;39:468–476. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 214.Marin N, Zamorano P, Carrasco R, Mujica P, Gonzalez FG, Quezada C, Meininger CJ, Boric MP, Duran WN, Sanchez FA. S-nitrosation of beta-catenin and p120 catenin: A novel regulatory mechanism in endothelial hyperpermeability. Circ Res. 2012;111:553–563. doi: 10.1161/CIRCRESAHA.112.274548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guequen A, Carrasco R, Zamorano P, Rebolledo L, Burboa P, Sarmiento J, Boric MP, Korayem A, Duran WN, Sanchez FA. S-nitrosylation regulates ve-cadherin phosphorylation and internalization in microvascular permeability. Am J Physiol Heart Circ Physiol. 2016;310:H1039–1044. doi: 10.1152/ajpheart.00063.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wormall A. The immunological specificity of chemically altered proteins : Halogenated and nitrated proteins. J Exp Med. 1930;51:295–317. doi: 10.1084/jem.51.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gonzalez D, Rojas A, Herrera MB, Conlan RS. Inos activation regulates beta-catenin association with its partners in endothelial cells. PLoS One. 2012;7:e52964. doi: 10.1371/journal.pone.0052964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003;278:33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 221.Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biological chemistry Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 222.Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free radical biology & medicine. 2011;50:371–380. doi: 10.1016/j.freeradbiomed.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Verma N, Tripathi SK, Chaudhury I, Das HR, Das RH. Inos-targeted 10–23 dnazyme reduces lps-induced systemic inflammation and mortality in mice. Shock. 2010;33:493–499. doi: 10.1097/SHK.0b013e3181c4ecbb. [DOI] [PubMed] [Google Scholar]
- 224.Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of inos is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H2144–2152. doi: 10.1152/ajpheart.00591.2005. [DOI] [PubMed] [Google Scholar]
- 225.Nachtigal P, Kopecky M, Solichova D, Zdansky P, Semecky V. The changes in the endothelial expression of cell adhesion molecules and inos in the vessel wall after the short-term administration of simvastatin in rabbit model of atherosclerosis. The Journal of pharmacy and pharmacology. 2005;57:197–203. doi: 10.1211/0022357055353. [DOI] [PubMed] [Google Scholar]
- 226.Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the beta-catenin/t-cell factor signaling cascade in vascular remodeling. Circ Res. 2002;90:340–347. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- 227.Yu J, Ma Z, Shetty S, Ma M, Fu J. Selective hdac6 inhibition prevents tnf-alpha-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. Am J Physiol Lung Cell Mol Physiol. 2016;311:L39–47. doi: 10.1152/ajplung.00051.2016. [DOI] [PubMed] [Google Scholar]
- 228.Iaconelli J, Huang JH, Berkovitch SS, Chattopadhyay S, Mazitschek R, Schreiber SL, Haggarty SJ, Karmacharya R. Hdac6 inhibitors modulate lys49 acetylation and membrane localization of beta-catenin in human ipsc-derived neuronal cells. ACS chemical biology. 2015;10:883–890. doi: 10.1021/cb500838r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wolf D, Rodova M, Miska EA, Calvet JP, Kouzarides T. Acetylation of beta-catenin by creb-binding protein (cbp) J Biol Chem. 2002;277:25562–25567. doi: 10.1074/jbc.M201196200. [DOI] [PubMed] [Google Scholar]
- 230.Levy L, Wei Y, Labalette C, Wu Y, Renard CA, Buendia MA, Neuveut C. Acetylation of beta-catenin by p300 regulates beta-catenin-tcf4 interaction. Mol Cell Biol. 2004;24:3404–3414. doi: 10.1128/MCB.24.8.3404-3414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Wetzel JL, Sen GC. Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by irf-3 are regulated by the acetylation and phosphorylation of its coactivators. mBio. 2013:4. doi: 10.1128/mBio.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nguyen LT, Lum H, Tiruppathi C, Malik AB. Site-specific thrombin receptor antibodies inhibit ca2+ signaling and increased endothelial permeability. The American journal of physiology. 1997;273:C1756–1763. doi: 10.1152/ajpcell.1997.273.5.C1756. [DOI] [PubMed] [Google Scholar]
- 233.Geyer M, Huang F, Sun Y, Vogel SM, Malik AB, Taylor CW, Komarova YA. Microtubule-associated protein eb3 regulates ip3 receptor clustering and ca(2+) signaling in endothelial cells. Cell reports. 2015;12:79–89. doi: 10.1016/j.celrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. Tlr4 activation of trpc6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med. 2012;209:1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kini V, Chavez A, Mehta D. A new role for pten in regulating transient receptor potential canonical channel 6-mediated ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem. 2010;285:33082–33091. doi: 10.1074/jbc.M110.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-trpc6-mediated ca2+ entry induces rhoa activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- 237.Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, Michel V, Yew DT, Yao X. Expression of trpc homologs in endothelial cells and smooth muscle layers of human arteries. Histochem Cell Biol. 2004;122:553–561. doi: 10.1007/s00418-004-0720-y. [DOI] [PubMed] [Google Scholar]
- 238.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. Rhoa interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 239.Smedlund K, Vazquez G. Involvement of native trpc3 proteins in atp-dependent expression of vcam-1 and monocyte adherence in coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:2049–2055. doi: 10.1161/ATVBAHA.108.175356. [DOI] [PubMed] [Google Scholar]
- 240.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated ca2+ entry in trpc4(-/-) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 241.Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, Malik AB, Tiruppathi C. The ca(2+) sensor stromal interaction molecule 1 (stim1) is necessary and sufficient for the store-operated ca(2+) entry function of transient receptor potential canonical (trpc) 1 and 4 channels in endothelial cells. Mol Pharmacol. 2012;81:510–526. doi: 10.1124/mol.111.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes vegf-induced increase in endothelial permeability by inhibiting trpc1-dependent ca2 influx. Circ Res. 2005;96:1282–1290. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- 243.Pocock TM, Foster RR, Bates DO. Evidence of a role for trpc channels in vegf-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1015–1026. doi: 10.1152/ajpheart.00826.2003. [DOI] [PubMed] [Google Scholar]
- 244.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiological reviews. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 245.Montell C, Birnbaumer L, Flockerzi V. The trp channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 246.Tauseef M, Farazuddin M, Sukriti S, Rajput C, Meyer JO, Ramasamy SK, Mehta D. Transient receptor potential channel 1 maintains adherens junction plasticity by suppressing sphingosine kinase 1 expression to induce endothelial hyperpermeability. FASEB J. 2016;30:102–110. doi: 10.1096/fj.15-275891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human trpc6 and trpc3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 248.Aires V, Hichami A, Boulay G, Khan NA. Activation of trpc6 calcium channels by diacylglycerol (dag)-containing arachidonic acid: A comparative study with dag-containing docosahexaenoic acid. Biochimie. 2007;89:926–937. doi: 10.1016/j.biochi.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 249.Chen W, Oberwinkler H, Werner F, Gassner B, Nakagawa H, Feil R, Hofmann F, Schlossmann J, Dietrich A, Gudermann T, Nishida M, Del Galdo S, Wieland T, Kuhn M. Atrial natriuretic peptide-mediated inhibition of microcirculatory endothelial ca2+ and permeability response to histamine involves cgmp-dependent protein kinase i and trpc6 channels. Arterioscler Thromb Vasc Biol. 2013;33:2121–2129. doi: 10.1161/ATVBAHA.113.001974. [DOI] [PubMed] [Google Scholar]
- 250.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. Trpc6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med. 2015;212:1883–1899. doi: 10.1084/jem.20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Colbert HA, Smith TL, Bargmann CI. Osm-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 253.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of trpv4 channels (hvrl-2/mtrp12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 255.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of trpv4 channels in a hek293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 256.Yin J, Hoffmann J, Kaestle SM, Neye N, Wang L, Baeurle J, Liedtke W, Wu S, Kuppe H, Pries AR, Kuebler WM. Negative-feedback loop attenuates hydrostatic lung edema via a cgmp-dependent regulation of transient receptor potential vanilloid 4. Circ Res. 2008;102:966–974. doi: 10.1161/CIRCRESAHA.107.168724. [DOI] [PubMed] [Google Scholar]
- 257.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. Adp-ribose gating of the calcium-permeable ltrpc2 channel revealed by nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 258.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of trpm2 channel in mediating h2o2-induced ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 259.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. Trpm4 is a ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 260.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases ap-1 binding activity by enhancing capacitative ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–1245. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- 261.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte ca2+ influx system mediated by ltrpc2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 262.Andersen PR, Devare SG, Tronick SR, Ellis RW, Aaronson SA, Scolnick EM. Generation of balb-musv and ha-musc by type c virus transduction of homologous transforming genes from different species. Cell. 1981;26:129–134. doi: 10.1016/0092-8674(81)90041-6. [DOI] [PubMed] [Google Scholar]
- 263.Hillig RC, Hanzal-Bayer M, Linari M, Becker J, Wittinghofer A, Renault L. Structural and biochemical properties show arl3-gdp as a distinct gtp binding protein. Structure. 2000;8:1239–1245. doi: 10.1016/s0969-2126(00)00531-1. [DOI] [PubMed] [Google Scholar]
- 264.Braga VM, Machesky LM, Hall A, Hotchin NA. The small gtpases rho and rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kiosses WB, McKee NH, Kalnins VI. Relationship between the distribution of stress fibers and centrosomes in endothelial cells of the rat aorta. Cell Motil Cytoskeleton. 1997;36:228–235. doi: 10.1002/(SICI)1097-0169(1997)36:3<228::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 266.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from rho to the actin cytoskeleton through protein kinases rock and lim-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 267.Sells MA, Boyd JT, Chernoff J. P21-activated kinase 1 (pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the rho family gtpases requires src tyrosine kinase activity. Curr Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 269.Yamada S, Nelson WJ. Localized zones of rho and rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. Ve-cadherin regulates endothelial actin activating rac and increasing membrane association of tiam. Mol Biol Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Roll-Mecak A, Cao C, Dever TE, Burley SK. X-ray structures of the universal translation initiation factor if2/eif5b: Conformational changes on gdp and gtp binding. Cell. 2000;103:781–792. doi: 10.1016/s0092-8674(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 272.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of gtp-binding proteins. Nature. 2000;403:567–571. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 273.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 274.Colicelli J. Human ras superfamily proteins and related gtpases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ihara K, Muraguchi S, Kato M, Shimizu T, Shirakawa M, Kuroda S, Kaibuchi K, Hakoshima T. Crystal structure of human rhoa in a dominantly active form complexed with a gtp analogue. J Biol Chem. 1998;273:9656–9666. doi: 10.1074/jbc.273.16.9656. [DOI] [PubMed] [Google Scholar]
- 276.Wei Y, Zhang Y, Derewenda U, Liu X, Minor W, Nakamoto RK, Somlyo AV, Somlyo AP, Derewenda ZS. Crystal structure of rhoa-gdp and its functional implications. Nature structural biology. 1997;4:699–703. doi: 10.1038/nsb0997-699. [DOI] [PubMed] [Google Scholar]
- 277.John J, Frech M, Wittinghofer A. Biochemical properties of ha-ras encoded p21 mutants and mechanism of the autophosphorylation reaction. J Biol Chem. 1988;263:11792–11799. [PubMed] [Google Scholar]
- 278.Neal SE, Eccleston JF, Hall A, Webb MR. Kinetic analysis of the hydrolysis of gtp by p21n-ras. The basal gtpase mechanism. J Biol Chem. 1988;263:19718–19722. [PubMed] [Google Scholar]
- 279.Garrett MD, Self AJ, van Oers C, Hall A. Identification of distinct cytoplasmic targets for ras/r-ras and rho regulatory proteins. J Biol Chem. 1989;264:10–13. [PubMed] [Google Scholar]
- 280.Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of gdp from and the subsequent binding of gtp to smg p25a, a ras p21-like gtp-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- 281.Ueda T, Kikuchi A, Ohga N, Yamamoto J, Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of gdp from and the subsequent binding of gtp to rhob p20, a ras p21-like gtp-binding protein. J Biol Chem. 1990;265:9373–9380. [PubMed] [Google Scholar]
- 282.Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA. Catalysis of guanine nucleotide exchange on the cdc42hs protein by the dbl oncogene product. Nature. 1991;354:311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- 283.Hart MJ, Eva A, Zangrilli D, Aaronson SA, Evans T, Cerione RA, Zheng Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J Biol Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- 284.Hoffman GR, Nassar N, Cerione RA. Structure of the rho family gtp-binding protein cdc42 in complex with the multifunctional regulator rhogdi. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 285.Scheffzek K, Stephan I, Jensen ON, Illenberger D, Gierschik P. The rac-rhogdi complex and the structural basis for the regulation of rho proteins by rhogdi. Nature structural biology. 2000;7:122–126. doi: 10.1038/72392. [DOI] [PubMed] [Google Scholar]
- 286.del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 287.Michaely PA, Mineo C, Ying YS, Anderson RG. Polarized distribution of endogenous rac1 and rhoa at the cell surface. J Biol Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- 288.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ. Rho family gtpase modification and dependence on caax motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem. 1992;267:20033–20038. [PubMed] [Google Scholar]
- 290.Michaelson D, Silletti J, Murphy G, D’Eustachio P, Rush M, Philips MR. Differential localization of rho gtpases in live cells: Regulation by hypervariable regions and rhogdi binding. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Moissoglu K, Kiessling V, Wan C, Hoffman BD, Norambuena A, Tamm LK, Schwartz MA. Regulation of rac1 translocation and activation by membrane domains and their boundaries. J Cell Sci. 2014;127:2565–2576. doi: 10.1242/jcs.149088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wirth A, Schroeter M, Kock-Hauser C, Manser E, Chalovich JM, De Lanerolle P, Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. The Journal of physiology. 2003;549:489–500. doi: 10.1113/jphysiol.2002.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 294.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 295.Even-Faitelson L, Rosenberg M, Ravid S. Pak1 regulates myosin ii-b phosphorylation, filament assembly, localization and cell chemotaxis. Cell Signal. 2005;17:1137–1148. doi: 10.1016/j.cellsig.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 296.Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, Ye RD. A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res. 2012;111:1517–1527. doi: 10.1161/CIRCRESAHA.112.273078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of lim-kinase by pak1 couples rac/cdc42 gtpase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 298.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by lim-kinase 1 and its role in rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 299.Goh WI, Lim KB, Sudhaharan T, Sem KP, Bu W, Chou AM, Ahmed S. Mdia1 and wave2 proteins interact directly with irsp53 in filopodia and are involved in filopodium formation. J Biol Chem. 2012;287:4702–4714. doi: 10.1074/jbc.M111.305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mdia1-dependent and rock-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Broman MT, Kouklis P, Gao X, Ramchandran R, Neamu RF, Minshall RD, Malik AB. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res. 2006;98:73–80. doi: 10.1161/01.RES.0000198387.44395.e9. [DOI] [PubMed] [Google Scholar]
- 302.Ramchandran R, Mehta D, Vogel SM, Mirza MK, Kouklis P, Malik AB. Critical role of cdc42 in mediating endothelial barrier protection in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;295:L363–369. doi: 10.1152/ajplung.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Diebold BA, Fowler B, Lu J, Dinauer MC, Bokoch GM. Antagonistic cross-talk between rac and cdc42 gtpases regulates generation of reactive oxygen species. J Biol Chem. 2004;279:28136–28142. doi: 10.1074/jbc.M313891200. [DOI] [PubMed] [Google Scholar]
- 304.Birukova AA, Tian Y, Dubrovskyi O, Zebda N, Sarich N, Tian X, Wang Y, Birukov KG. Ve-cadherin trans-interactions modulate rac activation and enhancement of lung endothelial barrier by iloprost. J Cell Physiol. 2012;227:3405–3416. doi: 10.1002/jcp.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 306.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 307.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 308.Swart-Mataraza JM, Li Z, Sacks DB. Iqgap1 is a component of cdc42 signaling to the cytoskeleton. J Biol Chem. 2002;277:24753–24763. doi: 10.1074/jbc.M111165200. [DOI] [PubMed] [Google Scholar]
- 309.Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, Zhao YY, Malik AB. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated rac1 and cdc42 signaling. Circ Res. 2009;105:696–704. 698–704. doi: 10.1161/CIRCRESAHA.109.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Papaharalambus C, Sajjad W, Syed A, Zhang C, Bergo MO, Alexander RW, Ahmad M. Tumor necrosis factor alpha stimulation of rac1 activity. Role of isoprenylcysteine carboxylmethyltransferase. J Biol Chem. 2005;280:18790–18796. doi: 10.1074/jbc.M410081200. [DOI] [PubMed] [Google Scholar]
- 311.Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. Tiam1 and rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem. 2009;284:5381–5394. doi: 10.1074/jbc.M808958200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Garrett TA, Van Buul JD, Burridge K. Vegf-induced rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor vav2. Exp Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. P120-catenin and p190rhogap regulate cell-cell adhesion by coordinating antagonism between rac and rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 314.Axelrad TW, Deo DD, Ottino P, Van Kirk J, Bazan NG, Bazan HE, Hunt JD. Platelet-activating factor (paf) induces activation of matrix metalloproteinase 2 activity and vascular endothelial cell invasion and migration. FASEB J. 2004;18:568–570. doi: 10.1096/fj.03-0479fje. [DOI] [PubMed] [Google Scholar]
- 315.Bussolino F, Camussi G, Aglietta M, Braquet P, Bosia A, Pescarmona G, Sanavio F, D’Urso N, Marchisio PC. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987;139:2439–2446. [PubMed] [Google Scholar]
- 316.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 317.Andor A, Trulzsch K, Essler M, Roggenkamp A, Wiedemann A, Heesemann J, Aepfelbacher M. Yope of yersinia, a gap for rho gtpases, selectively modulates rac-dependent actin structures in endothelial cells. Cell Microbiol. 2001;3:301–310. doi: 10.1046/j.1462-5822.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 318.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and rac but not cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 319.Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of rho family gtpases in tnf-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244–249. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- 320.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via rho and its target rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 321.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of rho by rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 322.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates rho activity: Reciprocal balance between both gtpases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L749–760. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- 324.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mdia1 and rock in rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 325.Mong PY, Wang Q. Activation of rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182:2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- 326.Beckers CM, Knezevic N, Valent ET, Tauseef M, Krishnan R, Rajendran K, Hardin CC, Aman J, van Bezu J, Sweetnam P, van Hinsbergh VW, Mehta D, van Nieuw Amerongen GP. Rock2 primes the endothelium for vascular hyperpermeability responses by raising baseline junctional tension. Vascul Pharmacol. 2015;70:45–54. doi: 10.1016/j.vph.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Joshi AD, Dimitropoulou C, Thangjam G, Snead C, Feldman S, Barabutis N, Fulton D, Hou Y, Kumar S, Patel V, Gorshkov B, Verin AD, Black SM, Catravas JD. Heat shock protein 90 inhibitors prevent lps-induced endothelial barrier dysfunction by disrupting rhoa signaling. Am J Respir Cell Mol Biol. 2014;50:170–179. doi: 10.1165/rcmb.2012-0496OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Bogatcheva NV, Zemskova MA, Gorshkov BA, Kim KM, Daglis GA, Poirier C, Verin AD. Ezrin, radixin, and moesin are phosphorylated in response to 2-methoxyestradiol and modulate endothelial hyperpermeability. Am J Respir Cell Mol Biol. 2011;45:1185–1194. doi: 10.1165/rcmb.2011-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- 331.Braga V. The crossroads between cell-cell adhesion and motility. Nat Cell Biol. 2000;2:E182–184. doi: 10.1038/35036433. [DOI] [PubMed] [Google Scholar]
- 332.Chen H, Paradies NE, Fedor-Chaiken M, Brackenbury R. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J Cell Sci. 1997;110(Pt 3):345–356. doi: 10.1242/jcs.110.3.345. [DOI] [PubMed] [Google Scholar]
- 333.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates rho family gtpases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 334.Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 335.Gao Y, Xing J, Streuli M, Leto TL, Zheng Y. Trp(56) of rac1 specifies interaction with a subset of guanine nucleotide exchange factors. J Biol Chem. 2001;276:47530–47541. doi: 10.1074/jbc.M108865200. [DOI] [PubMed] [Google Scholar]
- 336.Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of cdc42, rac1, and rhoa. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- 337.Fukuyama T, Ogita H, Kawakatsu T, Inagaki M, Takai Y. Activation of rac by cadherin through the c-src-rap1-phosphatidylinositol 3-kinase-vav2 pathway. Oncogene. 2006;25:8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- 338.Timmerman I, Heemskerk N, Kroon J, Schaefer A, van Rijssel J, Hoogenboezem M, van Unen J, Goedhart J, Gadella TW, Jr, Yin T, Wu Y, Huveneers S, van Buul JD. A local ve-cadherin and trio-based signaling complex stabilizes endothelial junctions through rac1. J Cell Sci. 2015;128:3514. doi: 10.1242/jcs.179424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kurella VB, Richard JM, Parke CL, Lecour LF, Jr, Bellamy HD, Worthylake DK. Crystal structure of the gtpase-activating protein-related domain from iqgap1. J Biol Chem. 2009;284:14857–14865. doi: 10.1074/jbc.M808974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of iqgap1, a target of the small gtpases cdc42 and rac1, in regulation of e-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 341.David S, Ghosh CC, Mukherjee A, Parikh SM. Angiopoietin-1 requires iq domain gtpase-activating protein 1 to activate rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol. 2011;31:2643–2652. doi: 10.1161/ATVBAHA.111.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A, Burlingame AL, Matthay MA, Sheppard D. Iqgap1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol. 2012;303:L12–19. doi: 10.1152/ajplung.00375.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 rhogap to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–23918. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 344.Roof RW, Haskell MD, Dukes BD, Sherman N, Kinter M, Parsons SJ. Phosphotyrosine (p-tyr)-dependent and -independent mechanisms of p190 rhogap-p120 rasgap interaction: Tyr 1105 of p190, a substrate for c-src, is the sole p-tyr mediator of complex formation. Mol Cell Biol. 1998;18:7052–7063. doi: 10.1128/mcb.18.12.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG. Myosin ii directly binds and inhibits dbl family guanine nucleotide exchange factors: A possible link to rho family gtpases. J Cell Biol. 2010;190:663–674. doi: 10.1083/jcb.201003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci U S A. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of rhoa signalling at epithelial junctions by p114rhogef drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Richnau N, Aspenstrom P. Rich, a rho gtpase-activating protein domain-containing protein involved in signaling by cdc42 and rac1. J Biol Chem. 2001;276:35060–35070. doi: 10.1074/jbc.M103540200. [DOI] [PubMed] [Google Scholar]
- 349.van Buul JD, Geerts D, Huveneers S. Rho gaps and gefs: Controling switches in endothelial cell adhesion. Cell Adh Migr. 2014;8:108–124. doi: 10.4161/cam.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A. 2005;102:15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to rhoa-driven microtubule-independent focal-adhesion sliding. J Cell Sci. 2009;122:3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lee SK, Thomas GH. Rac1 modulation of the apical domain is negatively regulated by beta (heavy)-spectrin. Mech Dev. 2011;128:116–128. doi: 10.1016/j.mod.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 354.Liu KD, Datta A, Yu W, Brakeman PR, Jou TS, Matthay MA, Mostov KE. Rac1 is required for reorientation of polarity and lumen formation through a pi 3-kinase-dependent pathway. Am J Physiol Renal Physiol. 2007;293:F1633–1640. doi: 10.1152/ajprenal.00053.2007. [DOI] [PubMed] [Google Scholar]
- 355.Dipaolo BC, Davidovich N, Kazanietz MG, Margulies SS. Rac1 pathway mediates stretch response in pulmonary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;305:L141–153. doi: 10.1152/ajplung.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Abiko H, Fujiwara S, Ohashi K, Hiatari R, Mashiko T, Sakamoto N, Sato M, Mizuno K. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J Cell Sci. 2015;128:1683–1695. doi: 10.1242/jcs.157503. [DOI] [PubMed] [Google Scholar]
- 357.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by rho and rac but not cdc42 or pi 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. Gef-h1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 360.Van Rijssel J, Timmerman I, Van Alphen FP, Hoogenboezem M, Korchynskyi O, Geerts D, Geissler J, Reedquist KA, Niessen HW, Van Buul JD. The rho-gef trio regulates a novel pro-inflammatory pathway through the transcription factor ets2. Biol Open. 2013;2:569–579. doi: 10.1242/bio.20134382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, Offermanns S, Mochizuki N, Zheng Y, Gutkind JS. Rhoa and rock mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun. 2015;6:6725. doi: 10.1038/ncomms7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sawada N, Itoh H, Miyashita K, Tsujimoto H, Sone M, Yamahara K, Arany ZP, Hofmann F, Nakao K. Cyclic gmp kinase and rhoa ser188 phosphorylation integrate pro- and antifibrotic signals in blood vessels. Mol Cell Biol. 2009;29:6018–6032. doi: 10.1128/MCB.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kato M, Blanton R, Wang GR, Judson TJ, Abe Y, Myoishi M, Karas RH, Mendelsohn ME. Direct binding and regulation of rhoa protein by cyclic gmp-dependent protein kinase ialpha. J Biol Chem. 2012;287:41342–41351. doi: 10.1074/jbc.M112.421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small gtp-binding protein rho binds to and activates a 160 kda ser/thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 365.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. Rock-i and rock-ii, two isoforms of rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 366.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. Rock-i regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 369.Takami A, Iwakubo M, Okada Y, Kawata T, Odai H, Takahashi N, Shindo K, Kimura K, Tagami Y, Miyake M, Fukushima K, Inagaki M, Amano M, Kaibuchi K, Iijima H. Design and synthesis of rho kinase inhibitors (i) Bioorg Med Chem. 2004;12:2115–2137. doi: 10.1016/j.bmc.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 370.Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of rho-kinase-induced myristoylated alanine-rich c kinase substrate (marcks) phosphorylation in human neuronal cells by h-1152, a novel and specific rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 371.Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Fasudil, a rho-kinase inhibitor, attenuates bleomycin-induced pulmonary fibrosis in mice. Int J Mol Sci. 2012;13:8293–8307. doi: 10.3390/ijms13078293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Li Y, Wu Y, Wang Z, Zhang XH, Wu WK. Fasudil attenuates lipopolysaccharide-induced acute lung injury in mice through the rho/rho kinase pathway. Med Sci Monit. 2010;16:BR112–118. [PubMed] [Google Scholar]
- 373.Xiao JW, Zhu XY, Wang QG, Zhang DZ, Cui CS, Zhang P, Chen HY, Meng LL. Acute effects of rho-kinase inhibitor fasudil on pulmonary arterial hypertension in patients with congenital heart defects. Circ J. 2015;79:1342–1348. doi: 10.1253/circj.CJ-14-1015. [DOI] [PubMed] [Google Scholar]
- 374.Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H, et al. Effect of at877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–577. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- 375.Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T, Nakashima M, Kato K. Anti-anginal effect of fasudil, a rho-kinase inhibitor, in patients with stable effort angina: A multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 376.Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652–659. doi: 10.1006/bbrc.2000.2343. [DOI] [PubMed] [Google Scholar]
- 377.Casey DB, Badejo AM, Dhaliwal JS, Sikora JL, Fokin A, Golwala NH, Greco AJ, Murthy SN, Nossaman BD, Hyman AL, Kadowitz PJ. Analysis of responses to the rho-kinase inhibitor y-27632 in the pulmonary and systemic vascular bed of the rat. Am J Physiol Heart Circ Physiol. 2010;299:H184–192. doi: 10.1152/ajpheart.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 379.Shyamsundar M, McKeown ST, O’Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS, McAuley DF. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O’Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (the harp study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 381.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, Johnston P, Hopkins PA, Johnston AJ, McDowell C, McNally C Investigators H, Irish Critical Care Trials G. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 382.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: Roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 384.Chavez A, Schmidt TT, Yazbeck P, Rajput C, Desai B, Sukriti S, Giantsos-Adams K, Knezevic N, Malik AB, Mehta D. S1pr1 tyr143 phosphorylation downregulates endothelial cell surface s1pr1 expression and responsiveness. J Cell Sci. 2015;128:878–887. doi: 10.1242/jcs.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Oo ML, Chang SH, Thangada S, Wu MT, Rezaul K, Blaho V, Hwang SI, Han DK, Hla T. Engagement of s1p(1)-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by fty720 does not require the s1p1 receptor. Cell Signal. 2007;19:1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 388.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator fty720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 389.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 390.Camp SM, Chiang ET, Sun C, Usatyuk PV, Bittman R, Natarajan V, Garcia JG, Dudek SM. Pulmonary endothelial cell barrier enhancement by novel fty720 analogs: Methoxy-fty720, fluoro-fty720, and beta-glucuronide-fty720. Chem Phys Lipids. 2015;191:16–24. doi: 10.1016/j.chemphyslip.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sun X, Ma SF, Wade MS, Acosta-Herrera M, Villar J, Pino-Yanes M, Zhou T, Liu B, Belvitch P, Moitra J, Han YJ, Machado R, Noth I, Natarajan V, Dudek SM, Jacobson JR, Flores C, Garcia JG. Functional promoter variants in sphingosine 1-phosphate receptor 3 associate with susceptibility to sepsis-associated acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2013;305:L467–477. doi: 10.1152/ajplung.00010.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sun X, Singleton PA, Letsiou E, Zhao J, Belvitch P, Sammani S, Chiang ET, Moreno-Vinasco L, Wade MS, Zhou T, Liu B, Parastatidis I, Thomson L, Ischiropoulos H, Natarajan V, Jacobson JR, Machado RF, Dudek SM, Garcia JG. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012;47:628–636. doi: 10.1165/rcmb.2012-0048OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 394.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 396.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-tie2 pathway. Microvasc Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 397.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C. Angiopoietin-2 is increased in severe sepsis: Correlation with inflammatory mediators. Crit Care Med. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 399.Wada T, Jesmin S, Gando S, Yanagida Y, Mizugaki A, Sultana SN, Zaedi S, Yokota H. The role of angiogenic factors and their soluble receptors in acute lung injury (ali)/ acute respiratory distress syndrome (ards) associated with critical illness. J Inflamm (Lond) 2013;10:6. doi: 10.1186/1476-9255-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Diamond JM, Porteous MK, Cantu E, Meyer NJ, Shah RJ, Lederer DJ, Kawut SM, Lee J, Bellamy SL, Palmer SM, Lama VN, Bhorade SM, Crespo M, Demissie E, Wille K, Orens J, Shah PD, Weinacker A, Weill D, Arcasoy S, Wilkes DS, Ware LB, Christie JD Lung Transplant Outcomes G. Elevated plasma angiopoietin-2 levels and primary graft dysfunction after lung transplantation. PLoS One. 2012;7:e51932. doi: 10.1371/journal.pone.0051932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M, Aplenc R, Abramova H, Atochina-Vasserman EN, Beers MF, Calfee CS, Cohen MJ, Pittet JF, Christiani DC, O’Keefe GE, Ware LB, May AK, Wurfel MM, Hakonarson H, Christie JD. Angpt2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med. 2011;183:1344–1353. doi: 10.1164/rccm.201005-0701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 403.Chu D, Sullivan CC, Du L, Cho AJ, Kido M, Wolf PL, Weitzman MD, Jamieson SW, Thistlethwaite PA. A new animal model for pulmonary hypertension based on the overexpression of a single gene, angiopoietin-1. Ann Thorac Surg. 2004;77:449–456. doi: 10.1016/S0003-4975(03)01350-X. discussion 456–447. [DOI] [PubMed] [Google Scholar]
- 404.Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/tie2/serotonin pathway. Proc Natl Acad Sci U S A. 2003;100:12331–12336. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Thistlethwaite PA, Lee SH, Du LL, Wolf PL, Sullivan C, Pradhan S, Deutsch R, Jamieson SW. Human angiopoietin gene expression is a marker for severity of pulmonary hypertension in patients undergoing pulmonary thromboendarterectomy. J Thorac Cardiovasc Surg. 2001;122:65–73. doi: 10.1067/mtc.2001.113753. [DOI] [PubMed] [Google Scholar]
- 406.Young JA, Ting KK, Li J, Moller T, Dunn L, Lu Y, Moses J, Prado-Lourenco L, Khachigian LM, Ng M, Gregory PA, Goodall GJ, Tsykin A, Lichtenstein I, Hahn CN, Tran N, Shackel N, Kench JG, McCaughan G, Vadas MA, Gamble JR. Regulation of vascular leak and recovery from ischemic injury by general and ve-cadherin-restricted mirna antagonists of mir-27. Blood. 2013;122:2911–2919. doi: 10.1182/blood-2012-12-473017. [DOI] [PubMed] [Google Scholar]
- 407.Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q. Lncrna-miat regulates microvascular dysfunction by functioning as a competing endogenous rna. Circ Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 408.Roderburg C, Luedde M, Vargas Cardenas D, Vucur M, Scholten D, Frey N, Koch A, Trautwein C, Tacke F, Luedde T. Circulating microrna-150 serum levels predict survival in patients with critical illness and sepsis. PLoS One. 2013;8:e54612. doi: 10.1371/journal.pone.0054612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rajput C, Tauseef M, Farazuddin M, Yazbeck P, Amin MR, Avin Br V, Sharma T, Mehta D. Microrna-150 suppression of angiopoetin-2 generation and signaling is crucial for resolving vascular injury. Arterioscler Thromb Vasc Biol. 2016;36:380–388. doi: 10.1161/ATVBAHA.115.306997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wary KK, Vogel SM, Garrean S, Zhao YD, Malik AB. Requirement of alpha(4)beta(1) and alpha(5)beta(1) integrin expression in bone-marrow-derived progenitor cells in preventing endotoxin-induced lung vascular injury and edema in mice. Stem Cells. 2009;27:3112–3120. doi: 10.1002/stem.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Jones CP, Pitchford SC, Lloyd CM, Rankin SM. Cxcr2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling. Stem Cells. 2009;27:3074–3081. doi: 10.1002/stem.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Li JW, Wu X. Mesenchymal stem cells ameliorate lps-induced acute lung injury through kgf promoting alveolar fluid clearance of alveolar type ii cells. Eur Rev Med Pharmacol Sci. 2015;19:2368–2378. [PubMed] [Google Scholar]
- 413.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: A severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 414.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]