Abstract
Epidemiological studies indicate that the increased consumption of sugars including sucrose and fructose in beverages correlate with the prevalence of obesity, type-2 diabetes, insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension in humans. A few reports suggest that fructose extends lifespan in Saccharomyces cerevisiae. In Anopheles gambiae, fructose, glucose, or glucose plus fructose also extended lifespan. New results presented here suggest that fructose extends lifespan in Caenorhabditis elegans (C. elegans) wild type (N2). C. elegans were fed standard laboratory food source (E. coli OP50), maintained in liquid culture. Experimental groups received additional glucose (111 mM), fructose (55 mM, 111 mM, or 555 mM), sucrose (55 mM, 111 mM, or 555 mM), glucose (167 mM) plus fructose (167 mM) (G&F), or high fructose corn syrup (HFCS, 333 mM). In four replicate experiments, fructose dose-dependently increased mean lifespan at 55 mM or 111 m Min N2, but decreased lifespan at 555 mM (P < 0.001). Sucrose did not affect the lifespan. Glucose reduced lifespan (P < 0.001). Equal amount of G&F or HFCS reduced lifespan (P < 0.0001). Intestinal fat deposition (IFD) was increased at a higher dose of fructose (555 mM), glucose (111 mM), and sucrose (55 mM, 111 mM, and 555 mM). Here we report a biphasic effect of fructose increasing lifespan at lower doses and shortening lifespan at higher doses with an inverse effect on IFD. In view of reports that fructose increases lifespan in yeast, mosquitoes and now nematodes, while decreasing fat deposition (in nematodes) at lower concentrations, further research into the relationship of fructose to lifespan and fat accumulation in vertebrates and mammals is indicated.
Keywords: biphasic effect, Caenorhabditis elegans, fructose, high fructose corn syrup (HFCS), lifespan, obesity
INTRODUCTION
In 2012, more than one third of children and adults were estimated to be overweight or obese in the United States (US) (Ogden et al., 2014). Obesity associated health issues are increasing globally, including cardiovascular disease, hypertension, stroke, type 2 diabetes, insulin resistance, impaired glucose tolerance, hyperinsulinemia, hypertriglyceridemia, nonalcoholic fatty liver disease, and ovarian or colorectal cancer (Gerstein, 1997, DiMeglio and Mattes, 2000, Ludwig, Peterson, & Gortmaker, 2001, Elliott et al., 2002, Raben et al., 2002, Bray, Nielsen, & Popkin, 2004, Cordain et al., 2005, Johnson et al., 2007, Schenewerk et al., 2014, Zhang et al., 2014).
Epidemiological studies correlate these obesity-related diseases with sedentary behavior and the consumption of excessive energy-dense diets containing saturated fat, sucrose and high-fructose corn syrup (HFCS) (Burt and Pai, 2001, Ashrafi et al., 2003, Heber, 2010, Mathias, Slining, & Popkin, 2013, Ogden et al., 2014, Zheng et al., 2014, Koutoukidis, Knobf, & Lanceley, 2015, Sample, Martin et al., 2015). Since HFCS intake increased 10-fold in the United States from 1970 to 1990 and accounts for 40% of the consumption of caloric sweeteners, the correlation between increased HFCS consumption and increased obesity has been speculated to have a cause and effect relationship (Bray et al., 2004). Consumption of sucrose decreased 50% as HFCS consumption increased during this time period (DiMeglio and Mattes, 2000, Ludwig et al., 2001, Elliott et al., 2002, Raben et al., 2002, Bray et al., 2004, Johnson et al., 2007, Schenewerk et al., 2014).
The insulin/IGF-1-signaling pathway regulates the lifespan of many organisms including yeast, Caenorhabditis elegans (C. elegans), mice, and humans (Barbieri et al., 2003, Lee, Murphy, & Kenyon, 2009, Kenyon, 2010, Zheng and Greenway, 2012). In yeast studies, either glucose or fructose at a concentration of 0.5% extends the lifespan of Saccharomyces cerevisiae mutants compared with a concentration of 2% due to the effect of caloric restriction (Smith et al., 2007). Interestingly, Anopheles gambiae consuming a diet supplemented with fructose (584 mM) or an equal amount of fructose plus glucose (292 mM) supported life compared with the water only control (Kessler, Vlimant, & Guerin, 2015). Feeding fructose to mice did not change body weight compared to a sugar-free, low-fat dietary control over a 3-month study period, and there was no change in triglycerides, free fatty acids, glucose sensitivity or leptin (Tillman et al., 2014).
C. elegans stores energy along the intestinal tract via intestinal fat deposition (IFD), which can be directly quantified photometrically by lipid staining dyes in the intact animal. In the C. elegans model, glucose as low as 0.1% (5.5 mM) is sufficient to decrease lifespan by decreasing expression of the DAF-16/FOXO gene, a homologue of the human gene FOXO, which regulates lifespan, mediates lipid metabolism, regulates insulin/IGF-1 signaling pathways, and controls heat shock transcription factor (HSF-1) activity (Ogg et al., 1997, Lin et al., 2001, Lee et al., 2009, Solis and Petrascheck, 2011, Zheng et al., 2014, Gao et al., 2015a, Gao et al., 2015b, Koutoukidis et al., 2015). C. elegans is a small, multicellular, transparent, free-living, soil nematode that has a completely sequenced genome and conserves 65% of the genes associated with human disease (Fei et al., 2004, Hostetler et al., 2008, Koutoukidis et al., 2015).
Although many studies have addressed the effect of sugars on obesity and diabetes, little is known about the effect of sugars on lifespan. This study evaluated the effects of fructose, glucose, and sucrose sugars on C. elegans lifespan and quantified intestinal fat deposition (IFD) by fluorescence intensity of lipophilic dye Nile red staining in liquid culture (Solis and Petrascheck, 2011).
MATERIALS AND METHODS
C. elegans
Wild type C. elegans (N2) and the standard lab food source Escherichia coli (E. coli, OP50, Uracilauxotroph) were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN). The C. elegans model does not require Institutional Animal Care and Use Committee (IACUC) approval.
Culture of Escherichia coli (E. coli, OP50)
OP50 were cultured by the standard method described elsewhere (Zheng et al., 2010). Briefly, an E. coli pellet was inoculated into a Luria-Bertani (LB) broth solution containing 0.2% streptomycin, incubated at 37°C overnight, and stored at 4°C, before being plated on petrifilm (3M™ Petrifilm™ E. coli/Coliform Count Plates 6404, 3M Corp., Minneapolis, MN) at 37°C for 24 h. Densities of 5 × 108 to 5 × 1011 colony forming units (cfu/mL) were selected and fed to C. elegans ad libitum (Gruber, Ng et al., 2009, Zheng et al., 2010). The OP50 stock feeding solution was enriched to 2 × 109 cfu/mL by centrifuging at 2,200 g for 10 minutes and washing twice with S-complete buffer (Solis and Petrascheck, 2011).
Culture of C. Elegans
Mature gravid wild type C. elegans (N2, Bristol) were treated with NaOH (1M) and sodium hypochlorite solution (5.25%) at 5:2 ratio to dissolve the body and release viable eggs. Eggs were washed with S-complete solution 3 times and hatched overnight at room temperature. The age-synchronized C. elegans were diluted to 100 animals/mL, plated in liquid culture in 96-well plate (120 µL/well, 10–15 animals) (Solis and Petrascheck, 2011) with OP50 (109 cfu/mL), and incubated in 20°C (N2) low temperature incubators (Revco Tech., Nashville, NC, USA). Thirty microliters of 5-Fluoro-2′-deoxyuridine (FUDR, 0.6 mM) stock solution were added to each well at larvae 4 stage.
Lifespan Assay
Glucose, fructose, and sucrose were purchased from Sigma-Aldrich (St. Louis, MO, USA) for use in investigating their effect in C. elegans (Solis and Petrascheck, 2011). Fifty microliters of the treatments were prepared and fed to the C. elegans on day 3. Each group had 60–90 animals (n = 10–15/well × 6 well). The control animals were fed with E. coli OP50 only. Experimental groups were fed with additional glucose (111 mM), fructose (55 mM, 111 mM, or 555 mM), sucrose (55 mM, 111 mM, or 555 mM), glucose (167 mM) plus fructose (167 mM), or high fructose corn syrup with 60% fructose (HFCS, 333 mM). The 96-well plate was placed on a microtiter plate shaker for 2 min before counting. The numbers of live animals were manually recorded every other day under an inverted microscope with a 4× objective (Nikon Eclipse Ti, Melville, NY, USA) (Lee et al., 2009, Zheng et al., 2014). Animals that did not move were excluded from the survival assay. The animals were returned to the 20°C incubators thereafter.
Fluorescence Microscopy
Lipophilic dye, Nile red, was used to stain for intestinal fat deposition (IFD) and fluorescent intensity was evaluated (Zheng et al., 2010). C. elegans in each group were collected after 3 days of treatments, washed with S-Basal solution twice, fixed with paraformaldehyde (4%) over 2 h at 4°C, and washed with PBS for 5 min three times. Nile red (50 µL) was applied to the specimens for 10 min. Ten microliters of Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL) was applied to a glass slide followed by 20 µL of the medium containing Nile red stained C. elegans. A cover glass was mounted on the glass slide and the slides were viewed with an epifluorescence microscope (Nikon Eclipse Ti, Melville, NY) equipped with a Texas Red filter. Fluorescent micrographs (n = 10–15) were taken with a digital camera (Andor, DU-885k) and analyzed using Nikon-Elements (version 3.22.11). Optical densities (arbitrary units, % of control) of Nile red labeled IFD were determined for C. elegans (larvae 4).
Statistical Analysis
Analyses were completed using SAS/STAT® software, Version 9.4 of the SAS System for Windows (Cary, NC, USA). All results were expressed as means ± standard error of the mean (SEM) Survival curves of lifespan were displayed by binomial probabilities as surrogates for survival probabilities and the mean survival time (mean lifespan) was estimated by Kaplan–Meier analysis. The data for fluorescence intensity was analyzed by ANOVA. All P values were calculated based on the comparisons to control group. Statistical significance was set at P ≤ 0.05.
RESULTS
Fructose Increased Lifespan and Increased IFD at Higher Doses
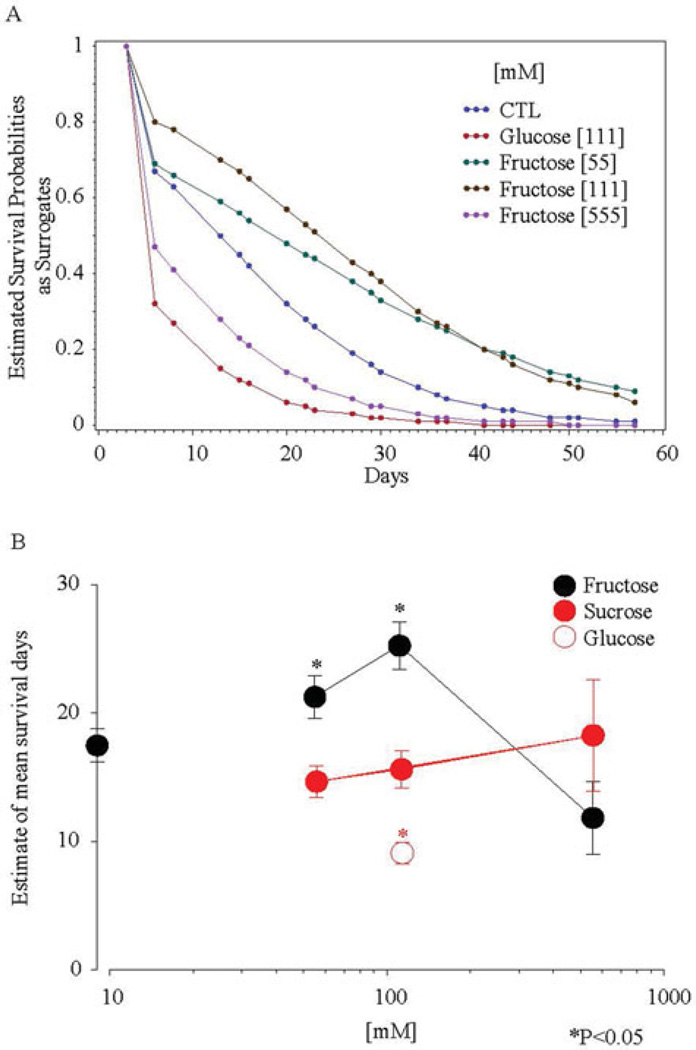
Fructose added to the culture significantly and dose-dependently increased mean lifespan up to the 111 mM dose but reduced lifespan at higher doses in a biphasic dose–response relationship. The average probability of survival across the lifespan was increased at lower doses and decreased at a higher dose (Figure 1a). The mean lifespan was increased at lower doses from 17.3 to 21.1 (55 mM, P < 0.0001) or to 25.2 (100 mM, P = 0.0002) days, and was decreased at a higher dose from 25.2 to 11.8 (555 mM) days (P < 0.00001, Figure 1b).
FIGURE 1.
Glucose, fructose, and sucrose affected lifespan of wild-type C. elegans (N2) and their mean survival days. (A) Survival curves of lifespan were displayed by binomial probabilities as surrogates for survival probabilities. (B) The mean survival time (mean lifespan) was estimated by Kaplan–Meier analysis. Low doses of fructose (55 mM and 111 mM) increased mean lifespan (P < 0.05). The P values of lifespan reflect the Kaplan–Meier probabilities (*P < 0.05).
The intestinal fat deposition (IFD) of C. elegans measured by Nile red staining was not affected by the lower doses of fructose (55 mM or 111 mM, P > 0.05), while it was increased 2-fold at the higher dose (555 mM, P < 0.0001, Figure 2a & b).
FIGURE 2.
Glucose, fructose, and sucrose affected intestinal fat deposition (IFD, *P < 0.05). High dose of fructose (555 mM) increased IFD (P < 0.05). All three doses of sucrose (55 mM, 111 mM & 555 mM) increased IFD, and glucose (111 mM) also increased IFD (P < 0.05).
Sucrose Increased IFD
The average probability of survival across the lifespan was mildly decreased in sucrose treated groups (Figure 3). The mean lifespan was slightly increased from 17.3 to 18.2 (555 mM) days in a dose-dependent trend (P > 0.05, Figure 3 & Figure 1b). Inversely to lifespan extension, the IFD was increased by 120% (55 mM), 180% (111 mM), or 90% (555 mM, P < 0.0001, Figure 2a & b).
FIGURE 3.
Sucrose did not significantly alter the mean survival time (mean lifespan) estimated by Kaplan-Meier analysis. Survival curves of lifespan were displayed by binomial probabilities as surrogates for survival probabilities.
Glucose Reduced Lifespan and Increased IFD
The average probability of survival across the lifespan was decreased in the glucose treated group (Figure 1a). The mean lifespan was reduced from 17.3 to 9.2 days (111 mM, P < 0.05, Figure 1b). The IFD was increased by 20%, P < 0.05, Figure 2a & b).
Equal Amount of Glucose Plus Fructose (G&F) or High Fructose Corn Syrup (HFCS)-Reduced Lifespan
The average probability of survival across the lifespan was decreased in the G&F (167 mM & 167 mM) or HFCS (333 mM) treated group (Figure 4a). The mean lifespan was reduced from 11.8 to 8.2 days for each group (P < 0.05, Figure 4b).
FIGURE 4.
Glucose plus fructose (G&F) or high fructose corn syrup (HFCS) reduced lifespan. (A) Survival curves of lifespan were displayed by binomial probabilities as surrogates for survival probabilities. (B) The mean survival time (mean lifespan) was estimated by Kaplan–Meier analysis. G167 mM & F167 mM and HFCS 333 mM decreased the mean lifespan (P < 0.05). The P values of lifespan reflect the Kaplan–Meier probabilities (*P < 0.05).
DISCUSSION
In this study, the effects of fructose, sucrose, glucose, glucose plus fructose, or HFCS consumption were evaluated in the C. elegans model by measuring alterations in lifespan and changes in IFD determined by the fluorescent intensity of Nile red. Lifespan was reduced in the glucose treated group as previously reported (Lee et al., 2009, Gao et al., 2015a, Gao et al., 2015b). Here we report a biphasic effect of fructose increasing lifespan at lower doses and shortening lifespan at a higher dose. Sucrose had no significant effect on mean lifespan. The mixed glucose & fructose or HFCS treatment reduced lifespan. IFD was increased at the higher dose and by glucose and sucrose in this study.
Although sugars are important energy sources for animals and humans, overconsumption can threaten health. Consumption of sugar-sweetened beverages can increase hyperinsulinemia, heart rate, advanced oxidation of protein products, triacylglycerol in the blood, and oxidative stress markers in rodents (Gurecka et al., 2015). Glucose is transported into cells by GLUT-4, an insulin-dependent transport system, while fructose is transported by GLUT-5 (Basaranoglu, Basaranoglu, & Bugianesi, 2015). A high ratio of fructose to glucose can lead to glucose addictive behavior and metabolic disorders in the rat (Levy, Marshall et al., 2015). An increased fructose-to-glucose ratio in humans increases fasting triglycerides when daily consumption is over 100 g per day, even without a change in body weight (Livesey and Taylor, 2008). Elevation of blood glucose in humans stimulates insulin secretion which can lead to weight gain. Rats fed a 32% sugar supplemented diet gain significantly more retroperitoneal fat compared to a standard diet (Kanarek and Orthen-Gambill, 1982).
Similarly, consumption of fructose is linked tomany adverse events and diseases in humans. Fructose consumption is a risk factor for metabolic syndrome, an accelerated aging process, myocardial infarction, gout, fatty liver disease, impaired adiponectin secretion, cardiovascular disease, hypertension, diabetes, and cancer (Perheentupa and Raivio, 1967, Ames, Cathcart et al., 1981, Hwang, Ho et al., 1987, Sato et al., 1996, Kang et al., 2005, Khosla et al., 2005, Johnson et al., 2007, Sautin, Nakagawa et al., 2007, Gul, Rahman, & Hasnain, 2009, Perez-Pozo, Schold et al., 2010, Van Horn and Dietary Guidelines Advisory, 2010, Suresh and Das, 2012, Kavanagh et al., 2013, Basaranoglu et al., 2015, Levy et al., 2015). Fructose does not stimulate insulin or leptin secretion in vitro and has a limited ability to inhibit food intake, because it is not able to prevent depletion of ATP, which is necessary for a homeostatic response in the brain (Perheentupa and Raivio, 1967, Johnson et al., 2007). Fructose leads to the generation of lactic acid and hyperuricemia which stimulates intestinal epithelial cells and vascular smooth muscle cells to proliferate and release chemotactic and inflammatory substances, induce monocyte chemotaxis, and create oxidative stress in adipocytes (Kang et al., 2005, Khosla et al., 2005, Sautin, Nakagawa et al., 2007).
Citing increased fructose consumption as the cause for the increase in obesity and obesity-associated diseases that are correlated with caloric sweetener use is controversial (Sun and Empie, 2007). Epidemiological studies also find that total energy intake in the U.S. population increased by 515 kcal/day (24%) between 1970 and 2008 (USDA-ERS data). Of the 515 kcal/day increase in caloric intake, only 58 kcal/day were estimated to come from sugar and only half of that, 29 kcal/day, came from fructose, which includes fructose from all sources including raw fruits and vegetables (White, 2011, White, 2012, White, 2013). Moreover, dietary use of fructose peaked in 1999 followed by a 13-year decline of 39 ± 4 g/day today (White, 2011, White, 2012, White, 2013). This decrease in fructose consumption was coincident with an increasing prevalence of obesity, metabolic syndrome, elevated uric acid, and a rising Body Mass Index (BMI) (White, 2011, White, 2012, White, 2013). Some researchers believe that experimental fructose intake representing 24% of total energy is beyond the normal range of human consumption (≤17.9% of energy at 19–22 years of age) which has been claimed to show no detrimental effect on human health and recommended to diabetic individuals (Anderson, Story et al., 1989, Marriott, Cole,&Lee, 2009, Sun, Anderson et al., 2011, Kavanagh et al., 2013, White, 2013). One clinical trial concluded that fructose and other sugars had no effect on fasting plasma glucose, fasting plasma insulin, LDL and total cholesterol, free fatty acids, or leptin (White, 2013, White, 2013). A recent review of 19 articles from 2006 to 2012 concluded that the “six eligible publications” that linked the fructose in HFCS to childhood obesity were based on “inconclusive scientific evidence” (Morgan, 2013). As the sweetest of the nutritive sweeteners, fructose, a simple monosaccharide found in fruits, vegetables, and honey, is bound to glucose forming the disaccharide sucrose. Fructose has a low glycemic index (GI) and is the least cariogenic of the nutritive sugars compared with sucrose and glucose (Cury, Rebelo et al., 2000, Bantle, 2006, Cozma et al., 2012). Interestingly, in human studies, lower amounts of daily fructose intake (50–100 g) were related to reduced dysglycemia and glycated hemoglobin (HbA1c) (Huttunen, 1976, Livesey and Taylor, 2008). Thus, adverse effects of fructose may only occur with preexisting dyslipidemia, as substitution of fructose in isocaloric diets does not cause adverse effects on lipid metabolism (Chiavaroli et al., 2015).
Many studies have addressed the adverse effects of sugars on obesity and diabetes. With this in mind, we tested three common sugars that are used in foods and beverages. To our surprise, provided that we used equal molar amounts of each sugar, we observed heterogeneous effects of the sugars on lifespan and IFD in C. elegans. The biphasic dose-response curve seen with fructose, which increased lifespan at lower doses and reduced at higher doses, supported the results of some other studies using low or high doses (Smith et al., 2007, Kessler et al., 2015). The reduced lifespan by glucose & fructose or HFCS may be related to the dose that could be in the declining lifespan range of fructose treatment alone. Our results with fructose differ from the conclusions of epidemiological studies.
Similarly as in humans reporting, where lower amounts of daily fructose intake (50–100 g) reduced dysglycemia and HbA1c without having adverse effect on lipid metabolism except preexisting dyslipidemia (Livesey and Taylor, 2008), we observed that lower doses of fructose extended lifespan. Higher dose of fructose reduced lifespan and increased IFD, which is in agreement with results from human studies that have demonstrated elevations of triacylglycerol, body weight, and fat mass at higher doses of fructose (Herman, Zakim, & Stifel, 1970, Elliott et al., 2002). In rats, sucrose supplemented diets shorten mean lifespan and elevate blood pressure. In the fruit fly (Drosophila melanogaster) sucrose reduced lifespan and increased levels of body fat (Preuss et al., 1991, Rovenko et al., 2015). Our results showed no change in lifespan with sucrose. Glucose increased intestinal fat deposition and reduced the lifespan in C. elegans which is consistent with previous studies (Zheng et al., 2014, Gao et al., 2015a).
In summary, glucose increased IFD and shortened lifespan, sucrose increased IFD without affecting lifespan, and fructose increased lifespan while increasing IFD at higher doses in C. elegans. These data, along with results from studies showing increased lifespan with fructose in yeast and mosquitoes, suggest the need to assess the dose response effect of fructose on lifespan and IFD in higher animal models to further evaluate if lower doses of fructose may have implications for humans.
Acknowledgments
The authors would like to thank Dr. Donald Ingram for his thorough and insightful review of this manuscript.
FUNDING
The C. elegans strains were from the Caenorhabditis Genetics Center, which is funded by the National Center for Research Resources (NCRR) of the NIH. Drs. Burton and Johnson are supported by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. The C. elegans model does not require regulation by the Institutional Animal Care and Use Committee (IACUC).
Biographies
Jolene Zheng, MD, PhD, Clinical & Pre-Clinical Research Lab Inc. Research interests include: C. elegans models and human clinical studies on nutrition, neurodegenerative diseases, anti-obesity, and insulin-sensitivity.
Chenfei Gao, PhD, is interested in food sciences and produce.
Mingming Wang, School of Nutrition and Food Sciences, Louisiana State University Agriculture Center, Baton Rouge, Louisiana.
Phuongmai Tran, MS, is a medical school student.
Nancy Mai, MS, is a medical school student.
John W. Finley, PhD, is interested in food sciences, natural food products and/or modulations on anti-oxidative stress, and anti-inflammation.
Steven B. Heymsfield, MD, is interested in human body composition and 3D-imaging, nutrition and anti-obesity, muscle physiology, sarcopenia, and body impedance analyses.
Frank L. Greenway, MD, is interested in human clinical studies on nutrition and anti-obesity and natural products of dietary supplement.
Zhaoping Li, MD, PhD, is interested in human clinical studies on nutrition and anti-obesity and natural products of dietary supplement.
David Heber, MD, is interested in human clinical studies on nutrition and anti-obesity and natural products of dietary supplement.
Jeffrey H. Burton, PhD, is interested in biostatistics.
William D. Johnson, PhD, is interested in biostatistics.
Roger A. Laine, PhD, is interested in biochemistry and biology in glycoprotein, carbohydrates, and neurodegenerative diseases.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ijds.
REFERENCES
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JW, Story LJ, Zettwoch NC, Gustafson NJ, Jefferson BS. Metabolic effects of fructose supplementation in diabetic individuals. Diabetes Care. 1989;12(5):337–344. doi: 10.2337/diacare.12.5.337. [DOI] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421(6920):268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Bantle JP. Is fructose the optimal low glycemic index sweetener? Nestle Nutr Workshop Ser Clin Perform Programme. 2006;11:83–91. doi: 10.1159/000094427. discussion 92-85. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285(5):E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Basaranoglu M, Basaranoglu G, Bugianesi E. Carbohydrate intake and nonalcoholic fatty liver disease: fructose as a weapon of mass destruction. Hepatobiliary Surg Nutr. 2015;4(2):109–116. doi: 10.3978/j.issn.2304-3881.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Burt BA, Pai S. Sugar consumption and caries risk: a systematic review. J Dent Educ. 2001;65(10):1017–1023. [PubMed] [Google Scholar]
- Chiavaroli L, de Souza RJ, Ha V, Cozma AI, Mirrahimi A, Wang DD, et al. Effect of fructose on established lipid targets: A systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc. 2015;4(9) doi: 10.1161/JAHA.114.001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clinic Nutr. 2005;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- Cozma AI, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Wang DD, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012;35(7):1611–1620. doi: 10.2337/dc12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury JA, Rebelo MA, Del BelCury AA, Derbyshire MT, Tabchoury CP. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34(6):491–497. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Fei YJ, Liu JC, Inoue K, Zhuang L, Miyake K, Miyauchi S, et al. Relevance of NAC-2, an Na+-coupled citrate transporter, to life span, body size and fat content in Caenorhabditis elegans. Biochem J. 2004;379(Pt 1):191–198. doi: 10.1042/BJ20031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Gao Z, Greenway FL, Johnson WD, Keenan MJ, Enright FM, et al. Oat consumption reduced intestinal fat deposition and improved health span in Caenorhabditis elegans model. Nutr Res. 2015a;35(9):834–843. doi: 10.1016/j.nutres.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, King ML, Fitzpatrick ZL, Wei W, King JF, Wang M, et al. Prowashonupana barley dietary fibre reduces body fat and increases insulin sensitivity in Caenorhabditis elegans model. J Funct Foods. 2015b;18:11. doi: 10.1016/j.jff.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evidence-Based Cardiovasc Med. 1997;1(4):87–88. doi: 10.1016/s1361-2611(97)80002-4. [DOI] [PubMed] [Google Scholar]
- Gruber J, Ng LF, Poovathingal SK, Halliwell B. Deceptively simple but simply deceptive—Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS Lett. 2009;583(21):3377–3387. doi: 10.1016/j.febslet.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Gul A, Rahman MA, Hasnain SN. Influence of fructose concentration on myocardial infarction in senile diabetic and non-diabetic patients. Exp Clin Endocrinol Diabetes. 2009;117(10):605–609. doi: 10.1055/s-0029-1202793. [DOI] [PubMed] [Google Scholar]
- Gurecka R, Koborova I, Jansakova K, Tabi T, Szoko E, Somoza V, et al. Prenatal dietary load of Maillard reaction products combined with postnatal Coca-Cola drinking affects metabolic status of female Wistar rats. Croat Med J. 2015;56(2):94–103. doi: 10.3325/cmj.2015.56.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber D. An integrative view of obesity. Am J Clin Nutr. 2010;91(1):280S–283S. doi: 10.3945/ajcn.2009.28473B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman RH, Zakim D, Stifel FB. Effect of diet on lipid metabolism in experimental animals and man. Fed Proc. 1970;29(3):1302–1307. [PubMed] [Google Scholar]
- Hostetler HA, Huang H, Kier AB, Schroeder F. Glucose directly links to lipid metabolism through high affinity interaction with peroxisome proliferator-activated receptor alpha. J Biol Chem. 2008;283(4):2246–2254. doi: 10.1074/jbc.M705138200. [DOI] [PubMed] [Google Scholar]
- Huttunen JK. Serum lipids, uric acid and glucose during chronic consumption of fructose and xylitol in healthy human subjects. Int Z Vitam Ernahrungsforsch Beih. 1976;15:105–115. [PubMed] [Google Scholar]
- Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10(5):512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86(4):899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr. 1982;112(8):1546–1554. doi: 10.1093/jn/112.8.1546. [DOI] [PubMed] [Google Scholar]
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, et al. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr. 2013;98(2):349–357. doi: 10.3945/ajcn.112.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kessler S, Vlimant M, Guerin PM. Sugar-sensitive neurone responses and sugar feeding preferences influence lifespan and biting behaviours of the Afrotropicalmalaria mosquito, Anopheles gambiae. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015;201(3):317–329. doi: 10.1007/s00359-015-0978-7. [DOI] [PubMed] [Google Scholar]
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Koutoukidis DA, Knobf MT, Lanceley A. Obesity, diet, physical activity, and health-related quality of life in endometrial cancer survivors. Nutr Rev. 2015;73(6):399–408. doi: 10.1093/nutrit/nuu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by down-regulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metabolism. 2009;10(5):379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Marshall P, Zhou Y, Kreek MJ, Kent K, Daniels S, Shore A, et al. Fructose: glucose ratios-a study of sugar self-administration and associated neural and physiological responses in the rat. Nutrients. 2015;7(5):3869–3890. doi: 10.3390/nu7053869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88(5):1419–1437. doi: 10.3945/ajcn.2007.25700. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139(6):1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- Mathias KC, Slining MM, Popkin BM. Foods and beverages associated with higher intake of sugar-sweetened beverages. Am J Prev Med. 2013;44(4):351–357. doi: 10.1016/j.amepre.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RE. Does consumption of high-fructose corn syrup beverages cause obesity in children? Pediatr Obes. 2013;8(4):249–254. doi: 10.1111/j.2047-6310.2013.00173.x. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34(3):454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- Perheentupa J, Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967;2(7515):528–531. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- Preuss H, el Zein M, Areas J, Podlasek S, Knapka J, Antonovych T, Sabnis S, et al. Effects of excess sucrose ingestion on the life span of hypertensive rats (SHR) Geriatr Nephrol Urol. 1991;1(1):13–20. [Google Scholar]
- Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- Rovenko BM, Kubrak OI, Gospodaryov DV, Perkhulyn NV, Yurkevych IS, Sanz A, et al. High sucrose consumption promotes obesity whereas its low consumption induces oxidative stress in Drosophila melanogaster. J Insect Physiol. 2015;79:42–54. doi: 10.1016/j.jinsphys.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Sample CH, Martin AA, Jones S, Hargrave SL, Davidson TL. Western-style diet impairs stimulus control by food deprivation state cues. Implications for obesogenic environments. Appetite. 2015;93:13–23. doi: 10.1016/j.appet.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ito T, Udaka N, Kanisawa M, Noguchi Y, Cushman SW, et al. Immunohistochemical localization of facilitated-diffusion glucose transporters in rat pancreatic islets. Tissue Cell. 1996;28(6):637–643. doi: 10.1016/s0040-8166(96)80067-x. [DOI] [PubMed] [Google Scholar]
- Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- Schenewerk AL, Ramirez FI, Foote C, Ji T, Martinez-Lemus LA, Rivera RM. Effects of the use of assisted reproduction and high-caloric diet consumption on body weight and cardiovascular health of juvenile mouse offspring. Reproduction. 2014;147(1):111–123. doi: 10.1530/REP-13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6(5):649–662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- Solis GM, Petrascheck M. Measuring Caenorhabditis elegans life span in 96 well micro titer plates. J Vis Exp. 2011;(49):2496. doi: 10.3791/2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SZ, Anderson GH, Flickinger BD, Williamson-Hughes PS, Empie MW. Fructose and non-fructose sugar intakes in the US population and their associations with indicators of metabolic syndrome. Food Chem Toxicol. 2011;49(11):2875–2882. doi: 10.1016/j.fct.2011.07.068. [DOI] [PubMed] [Google Scholar]
- Sun SZ, Empie MW. Lack of findings for the association between obesity risk and usual sugar-sweetened beverage consumption in adults—a primary analysis of databases of CSFII-1989–1991, CSFII-1994–1998, NHANES III, and combined NHANES 1999–2002. Food Chem Toxicol. 2007;45(8):1523–1536. doi: 10.1016/j.fct.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Suresh E, Das P. Recent advances in management of gout. QJM. 2012;105(5):407–417. doi: 10.1093/qjmed/hcr242. [DOI] [PubMed] [Google Scholar]
- Tillman EJ, Morgan DA, Rahmouni K, Swoap SJ. Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice. PLoS One. 2014;9(9):e107206. doi: 10.1371/journal.pone.0107206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn L Dietary Guidelines Advisory C. Development of the 2010 US Dietary Guidelines Advisory Committee Report: perspectives from a registered dietitian. J Am Diet Assoc. 2010;110(11):1638–1645. doi: 10.1016/j.jada.2010.08.018. [DOI] [PubMed] [Google Scholar]
- White JS. Postulated fructose influence on myocardial infarction is unconvincing. Exp Clin Endocrinol Diabetes. 2011;119(2):129. doi: 10.1055/s-0030-1255103. [DOI] [PubMed] [Google Scholar]
- White JS. Fructose as a significant cause of gout is unfounded and premature. 2012 Aug;105(8):809–810. doi: 10.1093/qjmed/hcs095. Epub 2012 May 29. [DOI] [PubMed] [Google Scholar]
- White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr. 2013;4(2):246–256. doi: 10.3945/an.112.003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JS. Comment on: Aeberli et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36:150–156. doi: 10.2337/dc12-0540. Diabetes Care 36(7): e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JS. Primate fructose study misses mark due to preventable design flaws. Am J Clin Nutr. 2013;98(5):1369–1370. doi: 10.3945/ajcn.113.072090. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Yao J, Ji G, Qian L, Wang J, et al. Obesity: pathophysiology and intervention. Nutrients. 2014;6(11):5153–5183. doi: 10.3390/nu6115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Enright F, Keenan M, Finley J, Zhou J, Ye J, et al. Resistant starch, fermented resistant starch, and short-chain fatty acids reduce intestinal fat deposition in Caenorhabditis elegans. J Agric Food Chem. 2010;58(8):4744–4748. doi: 10.1021/jf904583b. [DOI] [PubMed] [Google Scholar]
- Zheng J, Greenway FL. Caenorhabditis elegans as a model for obesity research. Int J Obes (Lond) 2012;36(2):186–194. doi: 10.1038/ijo.2011.93. [DOI] [PubMed] [Google Scholar]
- Zheng J, Greenway FL, Heymsfield SB, Johnson WD, King JF, King MJ, et al. Effects of three intense sweeteners on fat storage in the C. elegans model. Chem-Biol Interact. 2014;215C:1–6. doi: 10.1016/j.cbi.2014.02.016. [DOI] [PubMed] [Google Scholar]