Abstract
Adipose-derived mesenchymal stem cells (ASCs) have the potential to differentiate into cells of mesodermal origin, such as osteoblasts, adipocytes, myocytes, and chondrocytes, and cryopreservation is currently performed as a routine method for preserving ASCs to safely acquire large numbers of cells. For clinical application of ASCs, serum-free, xeno-free cryopreservation solutions should be used. This study determined the viability and adipo-osteogenic potential of cryopreserved ASCs using four cryopreservation solutions: 10% DMSO, Cell Banker 2 (serum free), Stem Cell Banker (=Cell Banker 3: serum free, xeno free), and TC protector (serum free, xeno free). The viability of the cryopreserved ASCs was over 80% with all cryopreservation solutions. No difference in the adipo-osteogenic potential was found between the cells that did or did not undergo cryopreservation in these cryopreservation solutions. These data suggest that Cell Banker 3 and TC protector are comparable with 10% DMSO and Cell Banker 2 for ASCs, and cryopreserved as well as noncryopreserved ASCs could be applied for regenerative medicine.
Key words: Adipose-derived mesenchymal stem cells (ASCs), Cryopreservation, Dimethyl sulfoxide (DMSO), Cell Banker 3, TC protector
INTRODUCTION
Mesenchymal stem cells (MSCs) maintain the potential to differentiate into cells of mesodermal origin such as osteoblasts, adipocytes, myocytes, and chondrocytes; moreover, MSCs possess immunosuppressive effects, which implies that they may have possible clinical applications in regenerative medicine and in therapies for treatment-resistant immune disorders1–8. Adipose-derived MSCs (ASCs) are normally isolated from subcutaneous adipose tissue, which allows them to be acquired in large numbers, so ASCs have attracted attention as a cell source for regenerative medicine and cell transplantation. These medical treatments, however, require a high quality and quantity for supply of human ASCs. Cryopreservation is currently performed as a routine method for preserving ASCs to safely acquire large numbers of cells.
However, a number of studies have indicated that the cellular activity of ASCs after freezing and thawing is affected by cryopreservation solutions. For instance, dimethyl sulfoxide (DMSO) and glycerol are commonly used as cryopreservation solutions to protect the cells from damage caused by freezing; however, they can also decrease the survival rate or induce cell differentiation9,10. Our group previously compared seven preservation solutions, including maintenance medium + 10% DMSO, Cell Freezing Medium-DMSO, Cell Freezing Medium-Glycerol, Cell Banker 1, Cell Banker 1+, Cell Banker 2, and CP-1, for mouse ASCs and showed that Cell Banker 2 is the most effective cryopreservation solution7. We also reported that the proliferation rate of human ASCs frozen in Cell Banker 2 and Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 medium (serum free) + 10% DMSO, 0.1 mol/L maltose, and 1% sericin was higher than that of the cells frozen in the maintenance medium + 10% DMSO11. For clinical application of human ASCs, serum-free, xeno-free cryopreservation solutions should be used. In this study, we evaluated serum-free, xeno-free cryopreservation solutions including Cell Banker 3 and TC protector.
MATERIALS AND METHODS
Culturing of Human ASCs
Human ASCs from a 40-year-old female [Lot No. 0000353102; body mass index (BMI): 23; passage 1] were obtained from Lonza Japan, Inc. (Tokyo, Japan). For attached cell culture, 4 × 105 ASCs were seeded into a T-75 flask (Asone, Osaka, Japan) with the Poietics™ ADSC-GM BulletKit™ (PT-4505; Lonza Japan) at 37°C in a humidified atmosphere of 5% CO2. The cells attached and spread onto the flask and were cultured until they were approximately 80%–90% confluent. The cells were detached from the culture flask with trypsin-ethylenediaminetetraacetic acid (EDTA) (0.25% w/v; Wako, Osaka, Japan) and repeatedly seeded and cultured onto new culture flasks. The passaging of the cells was repeated from four to eight times, and the cells obtained after each passage were frozen and stored for 14 days. Proliferation rate and differentiation into adipocytes and osteocytes were not significantly different among the ASCs passaged four to eight times.
Cryopreservation and Cell Viability Assays of Human ASCs
At subconfluency, when the cell density was approximately 2.5–6.5 × 104 cells/cm2, the human ASCs were detached from the flasks with trypsin-EDTA, centrifuged, and suspended at a density of 2 × 105 cells/ml in four types of cryopreservation solution. The cryopreservation solutions were 10% DMSO (Sigma-Aldrich, St. Louis, MO, USA), Cell Banker 2 (Takara Bio Inc., Shiga, Japan), Stem Cell Banker (=Cell Banker 3; Takara Bio Inc.), and TC protector (DS Pharma Biomedical, Osaka, Japan). Cells were either frozen in culture media or not cryopreserved (noncryopreserved) as controls. The cells in cryopreservation solution were rapidly cooled and stored at −80°C for 14 days. Immediately after thawing, the cryopreserved cells were diluted with culture medium. Cell suspensions were centrifuged at 200 × g for 3 min and resuspended in 5 ml of culture medium. Cell viability was determined using the trypan blue (Wako) exclusion procedure.
Measurement of the Proliferation Rate
Noncryopreserved or cryopreserved ASCs were seeded onto 12-well plates (Sigma-Aldrich) at a density of 1.0 × 104 cells/well, and the cells were detached from the plates with trypsin-EDTA. The cell number was counted after 3, 5, and 7 days. Viable cells were then counted using the trypan blue exclusion procedure.
Osteogenic Differentiation Assay
Osteogenic differentiation was induced by culturing the cells for 3 weeks in Osteoblast Differentiation Medium (#OB-1; DS Pharma Biomedical). Differentiation was examined by staining for extracellular matrix calcification by Alizarin red S staining (Calcified Nodule Staining Kit; Cosmo Bio Co., Ltd., Tokyo, Japan).
Adipogenic Differentiation Assay
Adipogenic differentiation was induced by culturing the cells for 7 days in adipocyte differentiation medium (#DM-2; DS Pharma Biomedical). The cells were cultured further in adipocyte maintenance medium (#AM-1; DS Pharma Biomedical) for 7 days. Differentiation was confirmed by Oil red O staining of intracellular lipid droplets. Differentiated ASCs were fixed in a 10% solution of formaldehyde (Wako) in phosphate-buffered saline (PBS) (Wako) for at least 10 min, washed with 60% isopropanol (Wako), and stained with Oil red O solution (Wako) for 10 min followed by repeated washing with water and destaining in 100% isopropanol for 1 min. The activity of glycerol-3-phosphate dehydrogenase (GPDH) was assayed using a GPDH assay kit (Takara Bio Inc.).
Statistical Analyses
The values for the data are presented as the means ± SE. To compare the data among the groups, a repeated-measures ANOVA was used. The differences between the groups were considered to be significant for values of p < 0.05.
RESULTS
Cell Viability of Cryopreserved ASCs
Human ASCs at passages 4–8 were used in this study, and the passage was the same for each comparison. ASCs were cryopreserved for 14 days in 10% DMSO, Cell Banker 2, Cell Banker 3, and TC protector. Immediately after thawing of the cells, the cell viability was evaluated using the trypan blue exclusion procedure. Figure 1 demonstrates that the viability of cryopreserved ASCs was over 80% in all four cryopreservation solutions. On the other hand, the viability of ASCs that were cryopreserved in culture medium was less than 20%.
Figure 1.
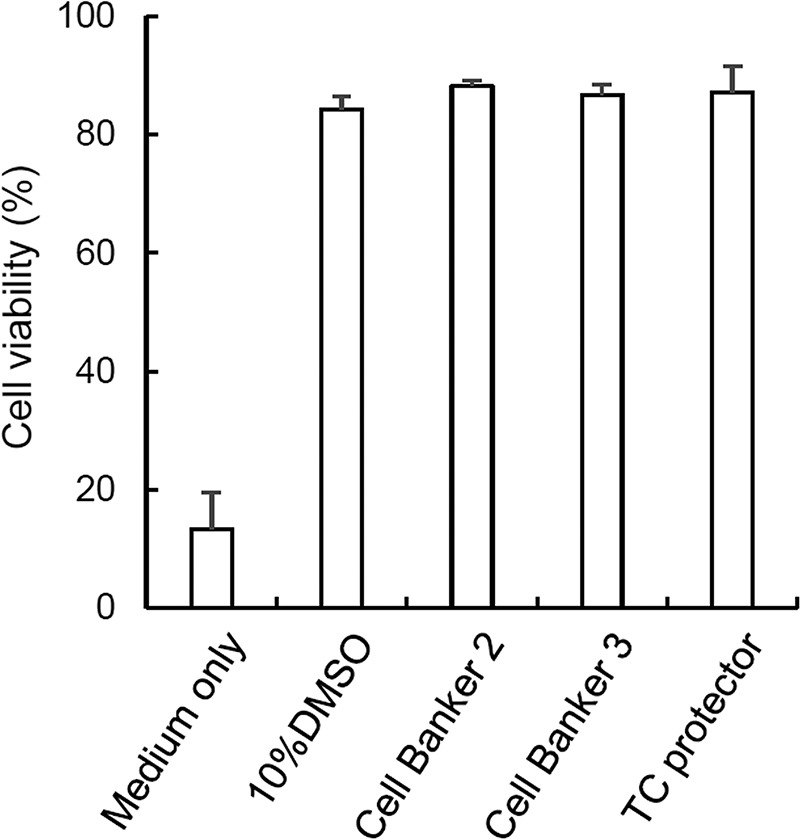
Viability of cryopreserved human adipose-derived mesenchymal stem cells (ASCs). Human ASCs (passages 4–8) cryopreserved in four different solutions, that is, 10% dimethyl sulfoxide (DMSO), Cell Banker 2, Cell Banker 3, and TC protector, were assayed for cell viability. The cell populations were cryopreserved for 7 days. Immediately after thawing of the cells, cell viability was evaluated using the trypan blue exclusion procedure. The averages of three independent assays are indicated with SE bars.
Comparison in the Proliferation Rate Between Cryopreserved and Noncryopreserved ASCs
To examine the proliferation rate of cryopreserved or noncryopreserved ASCs, the cells were counted on days 3, 5, and 7 after culture (Fig. 2). The proliferation rate of cryopreserved ASCs was similar to that of noncryopreserved ASCs.
Figure 2.
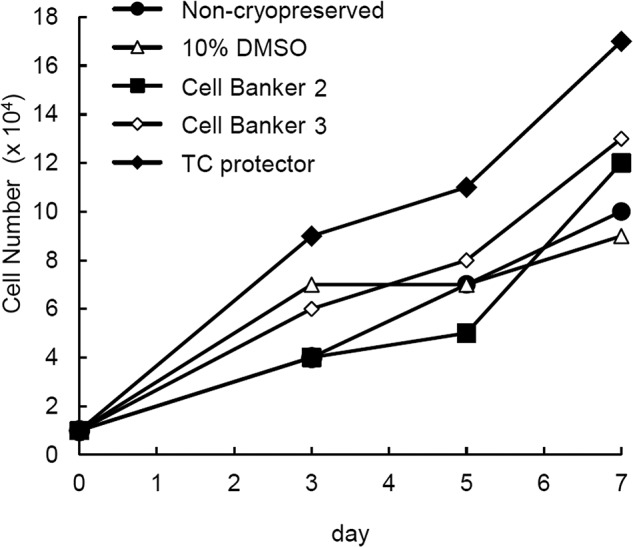
Proliferation of ASCs. Four samples of cryopreserved human ASCs were used for the determination of cell proliferation. The cell populations were cryopreserved for 14 days. The cells were counted on days 3, 5, and 7 after culture. Noncryopreserved cells were used as a control. Since noncryopreserved cells were cultured and passaged for 14 days of the cryopreservation, passage number of noncryopreserved ASCs was two or three times higher than that of cryopreserved ASCs.
Osteogenic Differentiation Capabilities of Cryopreserved ASCs
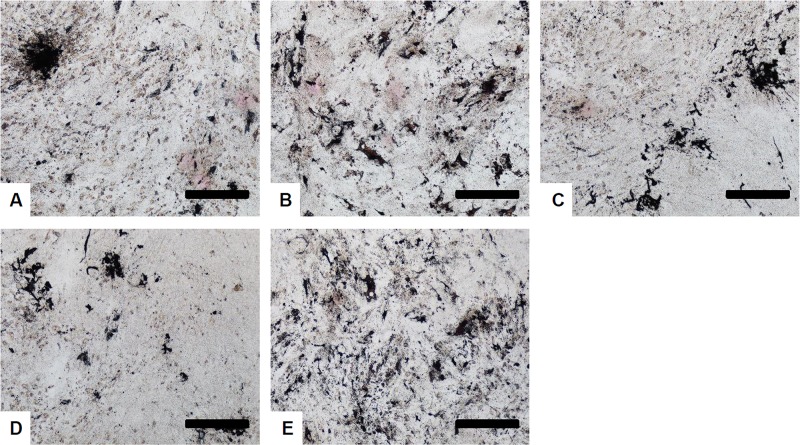
To examine whether the cryopreserved ASCs maintained osteogenic differentiation capabilities, the cells were treated with osteogenic induction medium for 3 weeks, and then Alizarin red S staining was performed. All cryopreserved cells (Fig. 3) were positive for calcified nodule staining, thus suggesting that the cells had osteogenic differentiation capabilities.
Figure 3.
Osteogenic differentiation of cryopreserved human ASCs. Human ASCs were cryopreserved in (A) 10% DMSO, (B) Cell Banker 2, (C) Cell Banker 3, and (D) TC protector for 14 days. The cells were stained with Alizarin red S 3 weeks after osteogenic induction. Noncryopreserved human ASCs (E) were used as a positive control. Scale bars: 500 μm.
Adipogenic Differentiation Capabilities of Cryopreserved ASCs
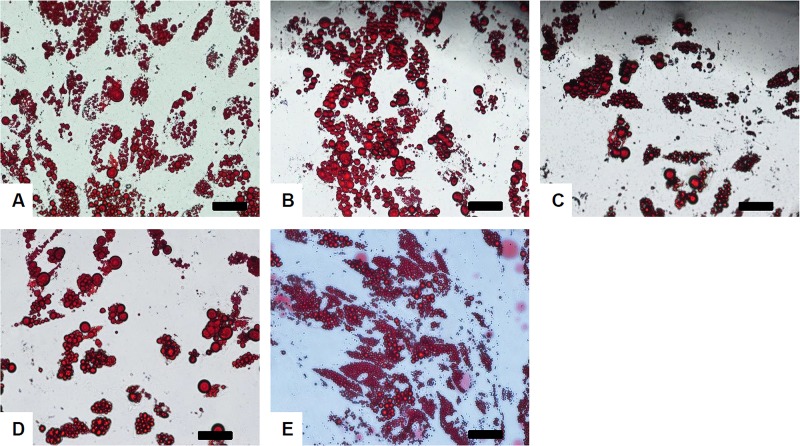
To test whether the cryopreserved ASCs maintained adipogenic differentiation capabilities, the cells were treated with adipogenic induction medium for 7 days and cultured with maintenance medium for an additional 7 days, and then Oil red O staining was performed. All cryopreserved ASCs were positive for Oil red O staining (Fig. 4), suggesting that the cryopreserved cells had adipogenic differentiation capabilities.
Figure 4.
Adipogenic differentiation of cryopreserved ASCs. Human ASCs were cryopreserved in (A) 10% DMSO, (B) Cell Banker 2, (C) Cell Banker 3, and (D) TC protector for 14 days. The cells were stained with Oil red O 7 days after adipogenic induction. Noncryopreserved human ASCs (E) were used as a positive control. Scale bars: 100 μm.
The activity of GPDH in cryopreserved ASCs was assayed using a GPDH assay kit. The GPDH activity in the cells 14 days after adipogenic induction/maintenance showed no apparent difference among the cryopreservation solutions (Fig. 5).
Figure 5.
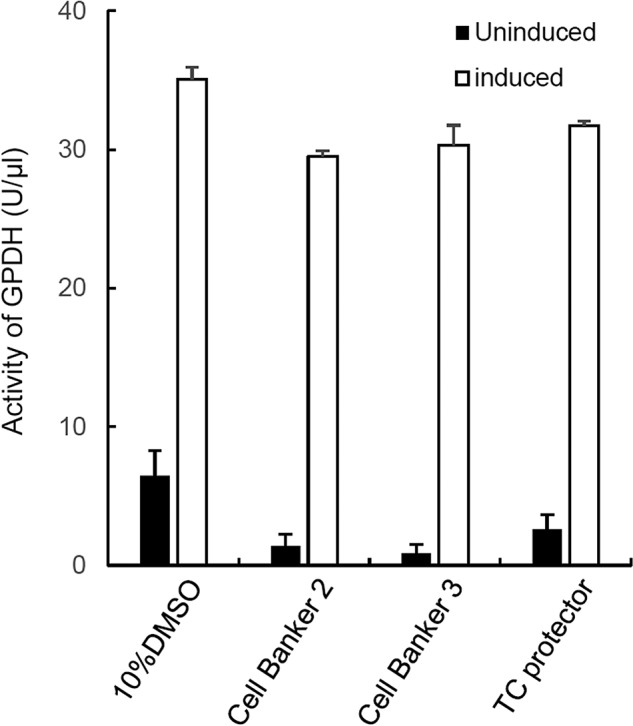
Activity of glycerol-3-phosphate dehydrogenase (GPDH) in cryopreserved ASCs. Human ASCs cryopreserved in four different solutions were assayed for their adipogenic differentiation capabilities. The activity of GPDH in the treated cells was determined using a GPDH assay kit. The averages of three independent assays are indicated with SE bars.
DISCUSSION
DMSO is a solution that is commonly used for the cryopreservation of cultured mammalian cells because it is economical and has a relatively low cell toxicity. However, when the cells will be used for clinical application, negative effects such as a decline in the survival rate following freezing, preserving, and thawing have been reported12–18. Moreover, it has been reported that DMSO induces the differentiation of neuron-like cells4 or cardiac myocytes19 when added to culture medium. Our group previously reported that Cell Banker 2 is an effective cryopreservation solution for mouse and human ASCs7,11. Cell Banker 2 is a serum-free c ryopreservation solution, but is not xeno free. Therefore, we evaluated two serum-free, xeno-free preservation solutions in this study.
The Cell Banker series allows for rapid cell cryopreservation at −80°C and has been shown to achieve a better survival rate following freezing and thawing. Serum-containing Cell Bankers 1 and 1+ can be used for the cryopreservation of nearly all mammalian cells, making them the standard Cell Banker series. Moreover, serum-free Cell Banker 2 is optimal for the cryopreservation of cells in serum-free culture conditions. Stem Cell Banker (Cell Banker 3) is a chemically defined freezing medium optimized for stem cell cryopreservation. It has been successfully used in freezing and thawing a variety of stem cell types, including marrow- or umbilical cord blood-derived stem cells, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs)20. A good manufacturing practice (GMP)-grade version of Cell Banker 3 is currently available. Al-Saqi et al. compared the cell survival rate and differentiation potency of ASCs cryopreserved with 10% DMSO and Cell Banker 3. While there were no differences in the cell morphology before and after freezing and thawing observed between the two types of cryopreservation solutions, the cell survival rate was significantly higher in Cell Banker 3 (90.4 ± 4.5%) than in 10% DMSO (79.9 ± 3.8%). The differentiation potency of ASCs and osteoblasts did not differ significantly between Cell Banker 3 and 10% DMSO21.
TC protector is another cell cryopreservation solution that satisfies the concept of a chemically defined known ingredient that is xeno free and optimized for the preservation performance of valuable cells, such as several human cell lines, somatic stem cells, ESCs, and iPSCs. Miwa et al. reported that there was no apparent difference in the cell growth and morphology between two kinds of human bone marrow MSCs using 10% DMSO or TC protector22.
In conclusion, cryopreserved human ASCs by Cell Banker 3 and TC protector could be applied for regenerative medicine. A number of ASCs can be prepared before the transplantation because the proliferative rate of ASCs is high23,24. Moreover, the antigenicity of ASCs may be low25, suggesting that ASCs could be used in the treatment of several diseases26–29. The serum-free, xeno-free cryopreservation solutions should therefore be widely used in regenerative medicine, cell transplantation, and biological research.
ACKNOWLEDGMENTS
This work was supported in part by the Japan Society for the Promotion of Science, Japan Agency for Medical Research and Development, The Uehara Memorial Foundation, TERUMO Foundation for Life Science and Arts, the Waksman Foundation of Japan Inc., The Ichiro Kanehara Foundation for the Promotion of Medical Science and Medical Care, and University of the Ryukyus Research Project Grant Program. The authors declare no conflicts of interest.
REFERENCES
- 1. Noguchi H. Stem cells for the treatment of diabetes. Endocr J. 2007;54(1):7–16. [DOI] [PubMed] [Google Scholar]
- 2. King SN, Hanson SE, Hematti P, Thibeault SL. Current applications of mesenchymal stem cells for tissue replacement in otolaryngology-head and neck surgery. Am J Stem Cells 2012;1(3):225–38. [PMC free article] [PubMed] [Google Scholar]
- 3. Noguchi H. Recent advances in stem cell research for the treatment of diabetes. World J Stem Cells 2009;1(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu Q, Wang Y, Fu X, Zhang S. Mechanism of in vitro differentiation of bone marrow stromal cells into neuron-like cells. J Huazhong Univ Sci Technolog Med Sci. 2004;24(3):259–61. [DOI] [PubMed] [Google Scholar]
- 5. Noguchi H. Production of pancreatic beta-cells from stem cells. Curr Diabetes Rev. 2010;6(3):184–90. [DOI] [PubMed] [Google Scholar]
- 6. Noguchi H. Stem cell applications in diabetes. J Stem Cells 2012;7(4):229–44. [PubMed] [Google Scholar]
- 7. Oishi K, Noguchi H, Yukawa H, Miyazaki T, Kato R, Kitagawa Y, Ueda M, Hayashi S. Cryopreservation of mouse adipose tissue-derived stem/progenitor cells. Cell Transplant. 2008;17(1–2):35–41. [DOI] [PubMed] [Google Scholar]
- 8. Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M, Noguchi H. Cryopreservation of adipose-derived mesenchymal stem cells. Cell Med. 2015;8(1–2):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuller BJ. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Letters 2004;25(6):375–88. [PubMed] [Google Scholar]
- 10. Meryman HT. Cryopreservation of living cells: Principles and practice. Transfusion 2007;47(5):935–45. [DOI] [PubMed] [Google Scholar]
- 11. Miyamoto Y, Oishi K, Yukawa H, Noguchi H, Sasaki M, Iwata H, Hayashi S. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 2012;21(2–3):617–22. [DOI] [PubMed] [Google Scholar]
- 12. Alessandrino P, Bernasconi P, Caldera D, Colombo A, Bonfichi M, Malcovati L, Klersy C, Martinelli G, Maiocchi M, Pagnucco G, Varettoni M, Perotti C, Bernasconi C. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: Analysis of 126 cases. Bone Marrow Transplant. 1999;23(6):533–7. [DOI] [PubMed] [Google Scholar]
- 13. Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P. Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplant. 2000;25(12):1299–301. [DOI] [PubMed] [Google Scholar]
- 14. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315–7. [DOI] [PubMed] [Google Scholar]
- 15. Higman MA, Port JD, Beauchamp NJ Jr, Chen AR. Reversible leukoencephalopathy associated with re-infusion of DMSO preserved stem cells. Bone Marrow Transplant. 2000;26(7):797–800. [DOI] [PubMed] [Google Scholar]
- 16. Liseth K, Abrahamsen JF, Bjørsvik S, Grøttebø K, Bruserud Ø. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy 2005;7(4):328–33. [DOI] [PubMed] [Google Scholar]
- 17. Windrum P, Morris TC. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31(4):315. [DOI] [PubMed] [Google Scholar]
- 18. Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T; EBMT Chronic Leukaemia Working Party Complications Subcommittee. Variation in dimethyl sulfoxide use in stem cell transplantation: A survey of EBMT centres. Bone Marrow Transplant. 2005;36(7):601–3. [DOI] [PubMed] [Google Scholar]
- 19. Young DA, Gavrilov S, Pennington CJ, Nuttall RK, Edwards DR, Kitsis RN, Clark IM. Expression of metalloproteinases and inhibitors in the differentiation of P19CL6 cells into cardiac myocytes. Biochem Biophys Res Commun. 2004;322(3):759–65. [DOI] [PubMed] [Google Scholar]
- 20. Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Saqi SH, Saliem M, Quezada HC, Ekblad Å, Jonasson AF, Hovatta O, Götherström C. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2015;16(2):181–93. [DOI] [PubMed] [Google Scholar]
- 22. Miwa H, Hashimoto Y, Tensho K, Wakitani S, Takagi M. Xeno-free proliferation of human bone marrow mesenchymal stem cells. Cytotechnology 2012;64(3):301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97(4):744–54. [DOI] [PubMed] [Google Scholar]
- 24. Ohgushi H, Caplan AI. Stem cell technology and bioceramics: From cell to gene engineering. J Biomed Mater Res. 1999;48(6):913–27. [DOI] [PubMed] [Google Scholar]
- 25. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99(10):3838–43. [DOI] [PubMed] [Google Scholar]
- 26. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: Maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1(4):322–4. [DOI] [PubMed] [Google Scholar]
- 28. Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, Koshima I, Yoshimura K. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121(2):401–10. [DOI] [PubMed] [Google Scholar]
- 29. Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: Isolation characterization and applications in surgery. ANZ J Surg. 2009;79(4):235–44. [DOI] [PubMed] [Google Scholar]