Abstract
Cell therapy with adipose tissue-derived stem cells (ASCs) is expected to be a candidate for the treatment of fulminant hepatic failure (FHF), which is caused by excessive immune responses. In order to evaluate the therapeutic effects of ASCs on FHF, the in vitro and in vivo immunomodulatory effects of ASCs were examined in detail in the mouse model. The in vitro effects of ASCs were examined by assessing their influence on the proliferation of lymphomononuclear cells (LMCs) stimulated with three kinds of mitogens: phorbol 12-myristate 13-acetate (PMA) plus ionomycin, concanavalin A (ConA), and lipopolysaccharide (LPS). The proliferation of LMCs was efficiently suppressed in a dose-dependent manner by ASCs in the cases of PMA plus ionomycin stimulation and ConA stimulation, but not in the case of LPS stimulation. The in vivo effects of transplanted ASCs were examined in the murine FHF model induced by ConA administration. The ALT levels and histological inflammatory changes in the ConA-administered mice were apparently relieved by the transplantation of ASCs. The analysis of mRNA expression patterns in the livers indicated that the expressions of the cytokines such as Il-6, Il-10, Ifn-γ, and Tnf-α, and the cell surface markers such as Cd3γ, Cd4, Cd8α, Cd11b, and Cd11c were downregulated in the ASC-transplanted mice. The immunomodulatory and therapeutic effects of ASCs were confirmed in the mouse model both in vitro and in vivo. These suggest that the cell therapy with ASCs is beneficial for the treatment of FHF.
Key words: Adipose tissue-derived stem cells (ASCs), Immunomodulatory effect, Fulminant hepatic failure (FHF), Concanavalin A (Con A)
INTRODUCTION
Acute liver failures including fulminant hepatic failure (FHF) are incurable diseases with significant morbidity and mortality. The pathological basis of FHF is massive destruction of hepatocytes due to excessive immune responses provoked by viral infection, autoimmunity, or chemical reactions in the liver. Therefore, the inhibition of the excessive immune responses is extremely important for the treatment of FHF. If patients do not receive prompt and appropriate treatments, FHF progresses rapidly toward death1–3. While there are a number of treatments aiming the alleviation of liver inflammation and the recovery of liver function, orthotopic liver transplantation is the only effective remedy proven to improve the survival rate of patients with FHF. Liver transplantation has many problems upon implementation, such as lack of donors, operative damages, risks of rejection, and side effects of immunosuppressants4,5. Thus, the establishment of alternative therapeutic approaches is necessary to improve this situation.
Recently, cell transplantation therapy using adipose tissue-derived stem cells (ASCs) has received much attention because ASCs possess immunomodulatory properties as well as multilineage differentiation ability6–14. Considering the clinical application of cell therapy, ASCs have several advantages in comparison to mature cells or other stem cells. ASCs could be prepared by minimal invasive procedure: removing and processing subcutaneous fat in which precursors of ASCs are frequently included. They have been proven to grow faster in vitro and to produce various cytokines and growth factors relating to their immunomodulatory effects more abundantly, in comparison to other mesenchymal stem cells (MSCs)15,16. In addition, the regulatory hurdles of clinical applications for ASCs are substantially lower than those for embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). Thus, ASCs may be a good candidate for the source of cell therapies of FHF with excessive immune responses. However, the immunomodulatory effects of ASCs on the excessive immune responses in FHF remain poorly understood.
Three kinds of mitogens are known to influence the proliferation of lymphomononuclear cells (LMCs) in vitro, which are phorbol 12-myristate 13-acetate (PMA) plus ionomycin [for the activation of both T cells and B cells by protein kinase C (PKC) phosphorylation], concanavalin A (ConA) [for the activation of T cells by indirect activation T-cell receptor (TCR) cross-linking], and lipopolysaccharide (LPS) [for the activation of B cells by Toll-like receptor 4 (TLR4)/myeloid differentiation factor 2 (MD2), cluster of differentiation 180 (CD180)/MD1 activation]. In addition, a number of animal models for acute liver failures including FHF have been reported to date, such as ConA-induced, galactosamine/LPS-induced, carbon tetrachloride (CCl4)-induced liver injury, and so on17–21. ConA-induced liver injury has been used as a representative model for acute liver failure including FHF because the pathogenic mechanism of liver injury is principally immune dependent and well characterized. In ConA-induced liver injury, T cells and natural killer T (NKT) cells are thought to be primarily activated by ConA, and cytokines produced by these activated cells, such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), are considered to act as exacerbating factors22–24. Moreover, the damaged hepatocytes generate free radicals, thereby activating Kupffer cells (liver macrophages) to produce various cytokines that accelerate progression of liver inflammation25,26.
In this study, we assessed the effect of ASCs on the inhibition of the proliferation of LMCs in vitro using three kinds of mitogens, such as PMA plus ionomycin, LPS, and ConA. Moreover, we examined the therapeutic effects of transplanted ASCs on FHF with excessive immune responses using ConA-induced acute liver failure mice. Through these analyses, we evaluated the therapeutic efficacy of ASCs on acute liver failures including FHF.
MATERIALS AND METHODS
Animals
C57BL/6 mice (6- to 10-week-old males and females; Chubu Kagaku Shizai Co. Ltd., Nagoya, Japan) were housed in a controlled environment and fed a standard diet with water ad libitum. All conditions and handing of animals in this study were conducted under protocols approved by the Nagoya University Committee on Animal Use and Care (#025-018).
Isolation and Culture of ASCs
Subcutaneous adipose tissues were taken from two 6- to 8-week-old female C57BL/6 mice, and then the tissues were collected and placed in Dulbecco’s modified Eagle’s medium (DMEM; Wako Pure Chemical Industries Ltd., Osaka, Japan) containing 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT, USA), minced finely with adding 1 ml of Hank’s balanced salt solution (HBSS; Life Technologies, Grand Island, NY, USA). The pooled minced tissues were incubated in 10 ml of HBSS containing 0.1% collagenase (type I; Worthington Biochemical Co., Lakewood, NJ, USA) at 37°C for 60 min. Cells were collected from the digested tissue through a 100-μm mesh (Thermo Fisher Scientific Inc., Waltham, MA, USA) after being suspended in DMEM. After centrifugation at 300 × g for 10 min, the precipitated cells were washed twice with DMEM and resuspended in a culture medium containing 20% FBS as described below. Then the cells were seeded in fibronectin (Sigma-Aldrich, St. Louis, MO, USA)-coated 75-cm2 flasks (Thermo Fisher Scientific Inc.), and the attached fibroblast-like cells were cultured as the progenitors of ASCs. The basal medium was a 3:2 mixture of DMEM and MCDB 201 medium (Sigma-Aldrich), supplemented with 1 ng/ml linoleic acid–albumin (Sigma-Aldrich), 1× insulin, transferrin, selenium (ITS) supplement (Sigma-Aldrich), 0.1 mM ascorbic acid phosphate ester magnesium salt (Wako Pure Chemical Industries), 50 U/ml penicillin and 50 mg/ml streptomycin (Wako Pure Chemical Industries), and 10 ng/ml human fibroblast growth factor-2 (FGF-2) (PeproTech Inc., Rocky Hill, NJ, USA).
Flow Cytometric Analysis of ASCs
ASCs were incubated with antibodies for 30 min on ice. The antibodies used for detecting cell surface markers were as follows: phycoerythrin (PE)-conjugated anti-mouse CD29 (0.2 mg/ml, #562801), CD105 (0.2 mg/ml, #562759), lymphocyte antigen 6A/E (Ly-6A/E; also known as stem cell antigen-1 or Sca-1; 0.2 mg/ml, #553108) antibodies (BD Biosciences, Tokyo, Japan), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD45 (0.5 mg/ml, #553080), CD90 (0.5 mg/ml, #554897), CD117 antibodies (0.5 mg/ml, #553354; BD Biosciences), and allophycocyanin (APC)-conjugated anti-mouse CD44 antibodies (0.2 mg/ml, #559250; BD Biosciences). CD45 is a marker for pan-hematopoietic cells. CD29 (β1 integrin) and CD44 (phagocytic glycoprotein-1) are adhesion molecules and used as markers for MSCs. CD90 (Thy-1) and CD105 (Endoglin) are markers for a variety of stem cells including MSCs. PE-, FITC-, and APC-conjugated rat IgG (0.2 mg/ml, #553930; 0.5 mg/ml, #553988; 0.2 mg/ml, #553991, respectively; BD Biosciences) were used as isotype controls. Flow cytometry was performed using the fluorescence-activated cell sorter (FACSCaliber; BD Biosciences).
Isolation of Murine Lymphomononuclear Cells (LMCs)
Spleens collected from three female C57BL/6 mice (6 to 8 weeks old) were minced and washed with Roswell Park Memorial Institute (RPMI)-1640 (Sigma-Aldrich). After lysing erythrocytes with Tris NH4Cl, the isolated splenocytes served as LMCs. The LMCs were counted and then resuspended at a concentration of 2–4 × 106 viable cells/ml in a complete medium.
Effect of ASCs on LMC Proliferation
To analyze the influence of ASCs on LMC proliferation, murine LMCs (2 × 105 cells) were seeded in a 96-well plate containing RPMI-1640 (Thermo Fisher Scientific Inc.) with 10% FBS, 50 U/ml penicillin, and 50 mg/ml streptomycin. They were stimulated with 50 ng/ml PMA (Sigma-Aldrich) plus 1 μg/ml ionomycin (Sigma-Aldrich), 5 μg/ml ConA, or 10 μg/ml LPS (Sigma-Aldrich). The ratios of ASCs to LMCs were 1:10, 1:20, 1:40, and 1:80 in the coculture condition. After 48 h of incubation, the floating cells, almost all of which were LMCs, were collected by gentle pipetting, seeded in a 96-well plate, and subjected to the bromodeoxyuridine (BrdU) incorporation assay (Roche Diagnostics Japan, Tokyo, Japan) according to the manufacturer’s instructions. In the BrdU incorporation assay, the cells were incubated with BrdU for 2 or 18 h, depending on the experiments. The chemiluminescence of the individual wells was measured using the Wallac 1420 multilabel counter (PerkinElmer Japan Co. Ltd., Yokohama, Japan).
To examine the influence of soluble factors produced by ASCs, murine LMCs (2 × 105 cells) stimulated with PMA plus ionomycin, ConA, or LPS were cultured in the culture supernatants of ASCs (1 × 105 cells). After 48 h of incubation, the cells were subjected to BrdU incorporation assay.
Analysis of Population Rate of LMCs Cocultured With ASCs
To examine the cell population of LMCs cocultured with ASCs, flow cytometric analysis was performed. Culture and stimulation conditions were the same as the BrdU incorporation assay. After 72 h of incubation, LMCs were labeled with FITC-conjugated anti-mouse CD3, CD19 (0.5 mg/ml, #11-0032-82; 0.5 mg/ml, #11-0193-82, respectively; eBioscience Inc., San Diego, CA, USA), APC-conjugated anti-mouse CD4, natural killer 1.1. (NK1.1) antibodies (0.2 mg/ml, #17-0041-82; 0.2 mg/ml, #17-5941-82, respectively; eBioscience), or PE-conjugated anti-mouse CD8a antibody (0.2 mg/ml, #553032; BD Biosciences). FITC-, APC-, PE-conjugated rat and mouse IgG (BD Biosciences) were used as isotype controls, respectively. Flow cytometry was performed using the FACSCaliber. Simultaneously, viable and dead cells were counted by visualizing dead cells with trypan blue staining (Sigma-Aldrich).
Quantitative Production Analysis of Hormone/Growth Factor
The media of passage 3 ASCs at about 90% confluence were replaced with fresh DMEM containing 10% FBS. After 24 h of incubation, the culture media were collected and used for the analysis of hormone/growth factor levels using commercially available enzyme-linked immunosorbent assay (ELISA) kits: Quantikine mouse hepatocyte growth factor (HGF) or vascular endothelial growth factor (VEGF) immunoassay (R&D Systems, Minneapolis, MN, USA) for HGF or VEGF detection, and mouse prostaglandin E2 (PGE2) ELISA kit (Cusabio Biotech Co. Ltd., Wuhan, P.R. China) for PGE2 detection. The assays were conducted according to the manufacturer’s instructions.
Experimental Models of Acute Liver Injury and Treatment With ASCs
Twenty-four 8- to 10-week-old male C57BL/6 mice were treated with overnight food/water deprivation and were injected intravenously with 15 mg/kg body weight of ConA diluted in phosphate-buffered saline (PBS; Wako Pure Chemical Industries). The prepared ASCs (1.0 × 106 cells/195 μl of PBS) were mixed with 5 μl of Novo-Heparin (10,000 U/10 ml; Mochida Pharmaceutical, Tokyo, Japan) and then transplanted into mice via the tail vein at 30 min after ConA administration. As the control group (without ASC transfer), 200 μl of PBS was injected into ConA-administered mice at the same time point. Eight mice were used for each treatment group.
Measurement of Liver Enzymes
Mice were anesthetized by diethyl ether (Wako Pure Chemical Industries), and the whole blood was collected at 8 and 24 h after ConA administration. Blood samples were allowed to clot for 30 min at room temperature and centrifuged at 1,500 × g for 10 min, and then the sera were collected. Alanine aminotransferase (ALT) in the serum was measured by the BBx system (Nittobo Medical Co. Ltd., Tokyo, Japan).
Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Analysis for Cytokines, Chemokines, and Cell Surface Markers
All the mice were sacrificed at 24 h after ConA or PBS administration, and liver tissues were resected. The six mice with medial ALT elevation at 24 h within the groups were selected, and the left lobe of their liver tissue was individually served for qRT-PCR analysis. The qRT-PCR was performed using total RNA prepared from ASCs (the same batch as those injected into the mice) and liver tissues with an RNeasy minikit (Qiagen, Tokyo, Japan). The RNA was subjected for first-strand cDNA synthesis using PrimeScript RT Master Mix (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s instructions. Then qRT-PCR was performed with the ABI PRISM 7000 sequence detection system (Applied Biosystems Japan Ltd., Tokyo, Japan) using SYBR Premix DimerEraser (Takara Bio Inc.) according to the manufacturer’s instructions. The PCR amplification was performed as follows: an initial denaturing step, 95°C for 30 s; followed by 45 cycles of 95°C for 5 s, 55°C for 30 s, and 72°C for 31 s. Cytokines and chemokine analyzed were as follows: interleukin-1α (Il-1α), Il-4, Il-6, Il-10, Ifn-γ, Tnf-α, transforming growth factor-β (Tgf-β ), C–X–C motif chemokine 12 (Cxcl12)/stromal cell-derived factor-1 (Sdf-1), inducible nitric oxide synthase (iNos), indoleamine 2, 3-dioxygenase 1 (Ido1), Cd3γ, Cd4, Cd8, Cd11b, and Cd11c. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as an internal control. All the primers were synthesized by Takara Bio Inc. (Table 1).
Table 1.
Primer Sequences Used for Real-Time RT-PCR
| Gene | Sense Primer | Antisense Primer |
|---|---|---|
| Gapdh | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| Il-1a | TGGTTAAATGACCTGCAACAGGAA | AGGTCGGTCTCACTACCTGTGATG |
| Il-4 | ACGGAGATGGATGTGCCAAAC | AGCACCTTGGAAGCCCTACAGA |
| Il-6 | CCACTTCACAAGTCGGAGGCTTA | CCAGTTTGGTAGCATCCATCATTTC |
| Il-10 | GCCAGAGCCACATGCTCCTA | GATAAGGCTTGGCAACCCAAGTAA |
| Ifn-γ | CGGCACAGTCATTGAAAGCCTA | GTTGCTGATGGCCTGATTGTC |
| Tnf | TATGGCCCAGACCCTCACA | GGAGTAGACAAGGTACAACCCATC |
| Tgf-β | GTGTGGAGCAACATGTGGAACTCTA | CGCTGAATCGAAAGCCCTGTA |
| Cxcl12 | TGACGGACCAATGCTGCAA | CAGGATGGTCTGGCTCCATTCTA |
| Nos2 | CAAGCTGAACTTGAGCGAGGA | TTTACTCAGTGCCAGAAGCTGGA |
| Ido1 | CACCATGGCGTATGTGTGGAA | TGCCAGGACACAGTCTGCATAA |
| Cd3γ | CTGGGCAACAATGCCAAAGA | AGCCGGATATGGTGCCTATGTTTA |
| Cd4 | CAACCTGACTCTGACTCTGGACAA | AGGTAGGTCCCATCACCTCACA |
| Cd8a | GTACTTCAGTTCTGTCGTGCCAGTC | TCGCAGCACTGGCTTGGTA |
| Cd11b | CCACTCATTGTGGGCAGCTC | GGGCAGCTTCATTCATCATGTC |
| Cd11c | AGGTCTGCTGCTGCTGGCTA | GGTCCCGTCTGAGACAAACTG |
Histological Analysis
Liver tissues were resected from all the mice (sacrificed at 24 h after ConA administration) and divided as described (left lobe for qRT-PCR and the remaining part for histological analysis). The tissues for histological analysis from all the mice were fixed with 10% formalin (Wako Pure Chemical Industries) and embedded in paraffin. Then the specimens were sectioned at 3-μm thickness and stained with hematoxylin (Wako Pure Chemical Industries) and eosin (Muto Pure Chemicals Co. Ltd., Tokyo, Japan). The stained liver specimens were observed with All-in-One Fluorescence Microscope (BZ-9000 model; Keyence, Osaka, Japan).
Statistical Analysis
Data are expressed as the mean ± SD. Statistical significance was determined by Dunnett’s test and a one-way analysis of variance (ANOVA) test followed by Bonferroni multiple comparison test using SPSS for Windows v.14.0 (IBM, Armonk, NY, USA). Significant difference between the experimental and control groups was considered by a value of p < 0.05.
RESULTS
Characteristics of ASCs
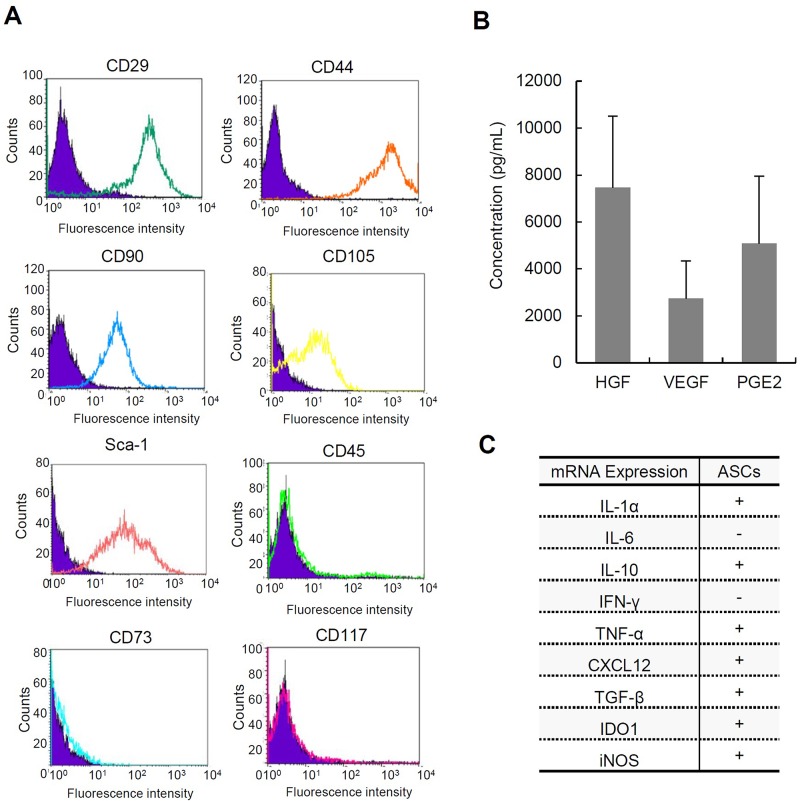
To confirm the characterization of ASCs, we analyzed cell surface markers, cytokine/chemokine expression patterns, and hormone/growth factor production of ASCs using flow cytometry, qRT-PCR, and ELISA, respectively. As shown in Figure 1A, the expressions of CD29, CD44, CD90, CD105, and Sca-1 on ASCs were observed, while the expressions of CD45, CD73, and CD117 were not observed in ASCs; these results are mostly consistent with those of the previous reports27,28. The production of HGF, VEGF, and PGE2 was examined by ELISA using supernatants of ASC cultures, and all of these were confirmed to be produced by ASCs29 (Fig. 1B). Moreover, mRNA expressions of Il-1α, Il-10, Tnf-α, Cxcl12/Sdf-1, Tgf-β, Ido1, and iNos were examined by qRT-PCR using cellular RNA extracts of ASCs. It is of note that the expressions of cytokines with immunomodulatory functions, such as Il-10 and Tgf-β, were detected in the examined ASCs, consistent with the previous reports27,30 (Fig. 1C). These data suggested that the cells established from C57BL/6 mice have characteristics of ASCs with immunomodulatory functions.
Figure 1.
Characteristics of adipose tissue-derived stem cells (ASCs). (A) Cell surface markers of ASCs analyzed by flow cytometry. The assays were performed in triplicate. CD29, cluster of differentiation 29; Sca-1, stem cell antigen-1. (B) The concentrations of hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and prostaglandin synthase E2 (PGE2) produced by ASCs incubated for 24 h in the culture medium (n = 5). The data are shown as mean ± SD values. (C) The expression of cytokines and chemokines in ASCs analyzed by qPCR. IL-1, interleukin-1; IFN, interferon; TNF, tumor necrosis factor; CXCL12, chemokine C–X–C motif ligand 12; TGF, transforming growth factor; IDO1, indoleamine 2, 3-dioxygenase; iNOS, inducible nitric oxide synthase.
Suppressive Effects of ASCs on LMCs Proliferation
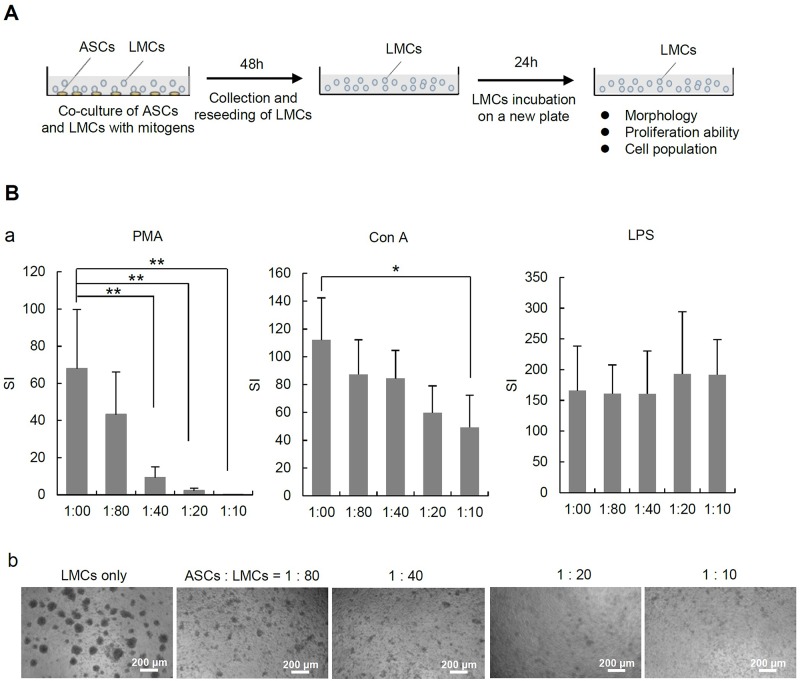
The suppressive effects of ASCs on the proliferation of LMCs stimulated by PMA plus ionomycin, ConA, or LPS were assessed by BrdU incorporation assay (Fig. 2A). Although the number of ASCs cocultured with LMCs was relatively small (ASCs/LMCs = 1:80, 1:40, 1:20, and 1:10), the proliferation of LMCs was efficiently suppressed in a dose-dependent manner by ASCs in the cases of PMA plus ionomycin stimulation and ConA stimulation. On the other hand, this suppressive effect of ASCs on the proliferation of LMCs was not observed in the LPS-stimulated case (Fig. 2B, a and b).
Figure 2.
Effect of ASCs and the culture supernatant on LMC proliferation. (A) The scheme of in vitro experiment. Murine lymphomononuclear cells (LMCs) of 2 × 105/well were placed in a 96-well plate. They were stimulated with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml) plus ionomycin (1 μg/ml), concanavalin A (ConA; 5 μg/ml), or lipopolysaccharide (LPS; 10 μg/ml) with or without ASCs. In the coculture case, the ratios of ASCs to LMCs were 1:10, 1:20, 1:40, and 1:80. After 48 h of incubation, the LMCs were collected and replated in a 96-well plate and subjected to bromodeoxyuridine (BrdU) incorporation assay according to the manufacturer’s instructions (incubation time with BrdU was 2 h). The chemiluminescence of the individual wells was measured using the Wallac 1420 multilabel counter. (B) The proliferation ability (a) and the morphologies (b) of LMCs stimulated with ConA, PMA plus ionomycin, and LPS under coculture with ASCs. Immunosuppressive effects of murine ASCs on stimulated LMCs were evaluated by BrdU incorporation assay. Stimulation index (SI) values were determined by calculating the ratio between stimulated chemiluminescence and basal chemiluminescence activity (wells without mitogen stimulation). The data are shown as the means ± SD values (*p < 0.05 and **p < 0.001).
Survival Rates of LMCs Cocultured With ASCs
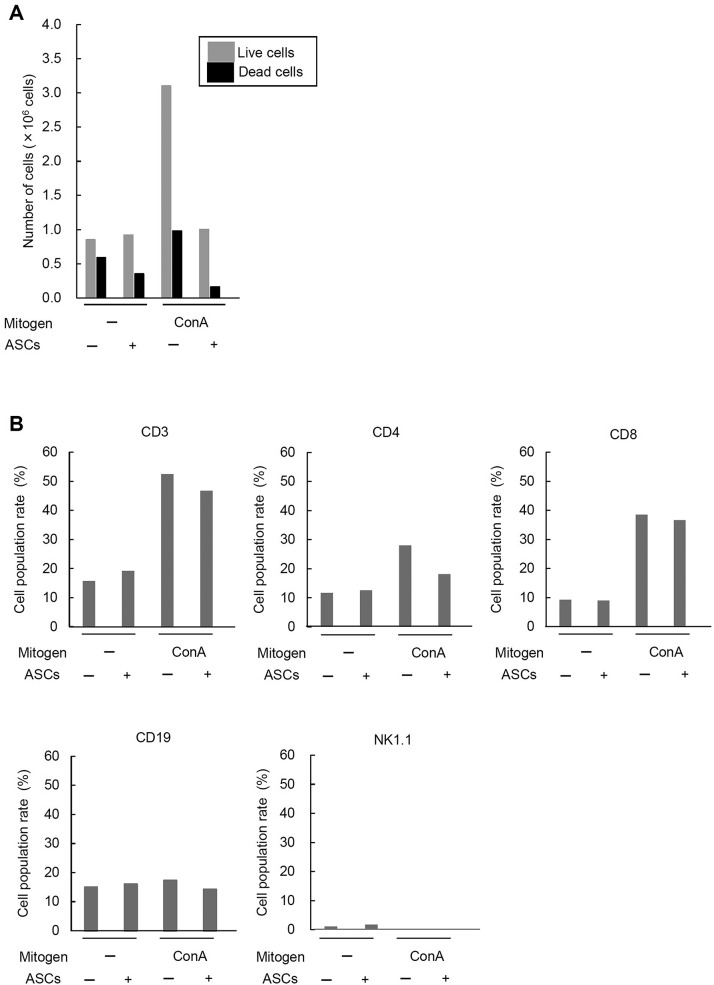
To rule out the possibility that the suppression of LMC proliferation was related to the death of LMCs cocultured with ASCs, the number of viable and dead LMCs was counted in the coculture with ASCs (ASC/LMCs = 1:20). In the culture without stimulation, the number of live LMCs was mostly the same regardless of the presence of ASCs, while the number of dead LMCs was decreased in the presence of ASCs. In the culture with ConA stimulation, the number of live and dead LMCs was dramatically increased, while the number of live and dead LMCs was decreased in the presence of ASCs. The results indicate that the inhibitory effect of ASCs on LMC proliferation is not due to the induction of cell death of LMCs (Fig. 3A).
Figure 3.
Viability and cell population changes of LMCs cocultured with ASCs. (A) The number of viable and dead LMCs cocultured with ASCs. The LMCs were cultured with ASCs in the same condition as the case of the proliferation assay (ASC-to-splenocyte ratio, 1:20) and counted after 48 h of coculture. (B) The cell population changes of mitogen-stimulated LMCs cocultured with ASCs by flow cytometric analysis. Culture and stimulation conditions were the same as the BrdU incorporation assay. NK1.1, natural killer 1.1.
Change of Cell Population in LMCs Cocultured With ASCs
To examine the changes of cell population of LMCs with or without coculture with ASCs and ConA stimulation, the cultured LMCs were subjected to flow cytometric analysis. The levels of the CD3+ population (T-cell marker) in the LMCs without ConA stimulation were about 15%–20% regardless of the coculture with ASCs, whereas those with ConA stimulation were more than 40%, showing a dramatic increase compared to those without stimulation. In the LMCs stimulated with ConA, the level of the CD3+ population was decreased by the coculture with ASCs.
On the other hand, the CD19+ population (B-cell marker) in LMCs remained at the same level in all the culture conditions. The levels of the NK1.1+ population (NK-cell marker) in LMCs without ConA stimulation was lower than 5% and was further decreased with ConA stimulation. The levels of the NK1.1+ population were unchanged regardless of the coculture with ASCs. These data suggest that ConA stimulation contributes to the increase in the number of T cells in LMCs, and ASCs may have the ability to inhibit the proliferation of T cells or the stimulated cells in LMCs (Fig. 3B).
Effects of Transplanted ASCs on ConA-Induced Liver Injury
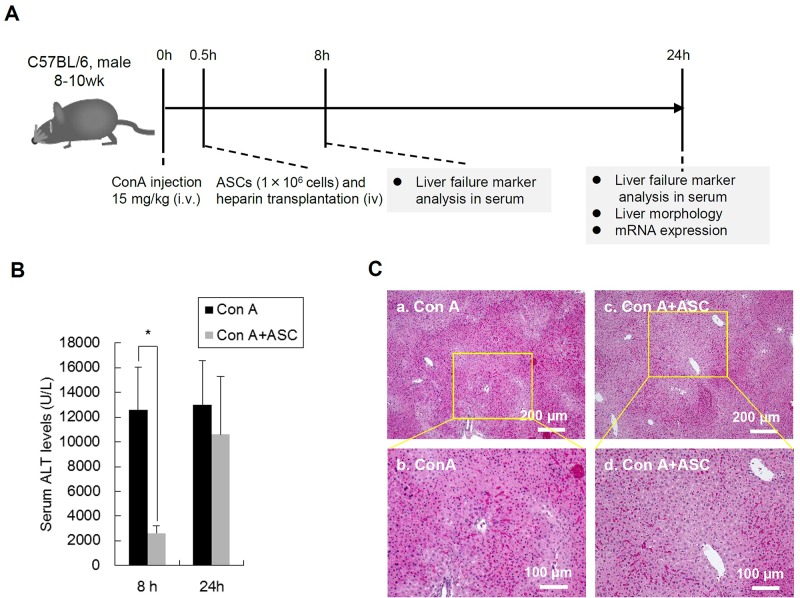
In order to evaluate the effects of transplanted ASCs on liver injury induced by ConA, the levels of serum ALT in mice were evaluated at 8 and 24 h after ConA administration (Fig. 4A). The ALT levels at 8 h after ConA administration (ConA group) were markedly elevated (12,617 ± 3,431 U/L); however, those in the ASC-transplanted group (ConA + ASCs group) at the same time point were significantly decreased (2,633 ± 578 U/L). The ALT levels in the ConA + ASCs group at 24 h after ConA administration were elevated (10,617 ± 4,684 U/L) compared to those at 8 h; however, they were still lower than those of the ConA group at the same time point (13,017 ± 3,563 U/L) (Fig. 4B).
Figure 4.
Effect of ASC transplantation on ConA-induced liver injury in mice. (A) The scheme of the in vivo experiment of murine liver injury model. Mice were injected with ConA to induce acute liver failure. After 0.5 h, the transplantation of ASCs was performed. Sera were collected at 8 and 24 h after ConA administration. At 24 h after ConA administration, mice were sacrificed and liver tissue specimens were resected for histological and mRNA expression analysis. (B) The level of serum alanine aminotransferase (ALT) in ConA-induced mice with or without ASC transplantation. Sera were harvested at 8 and 24 h after ConA injection (n = 6). The data are shown as the means ± SD values (*p < 0.05). (C) Hematoxylin and eosin (H&E) staining of histological sections of the livers in mice treater with ConA (a, b) and ConA + ASCs (c, d). Scale bars: 200 μm (a, c), 100 μm (b, d). Representative images are presented (n = 3).
Moreover, histological changes of the liver at 24 h after ConA administration were observed microscopically, and the degree of liver damage was compared between the ConA group and the ConA + ASCs group. The necrotic areas with hepatocytes showing acidophilic cytoplasm and condensed nuclei were widely observed in the ConA group, while those areas were relatively small in the ConA + ASCs group (Fig. 4C). These changes in ALT levels and liver histology by ASC transplantation suggest that ASC transplantation is useful for the treatment of FHF initiated by excessive immune responses.
Cytokines and Cell Surface Lineage Markers Expressed in the Injured Liver
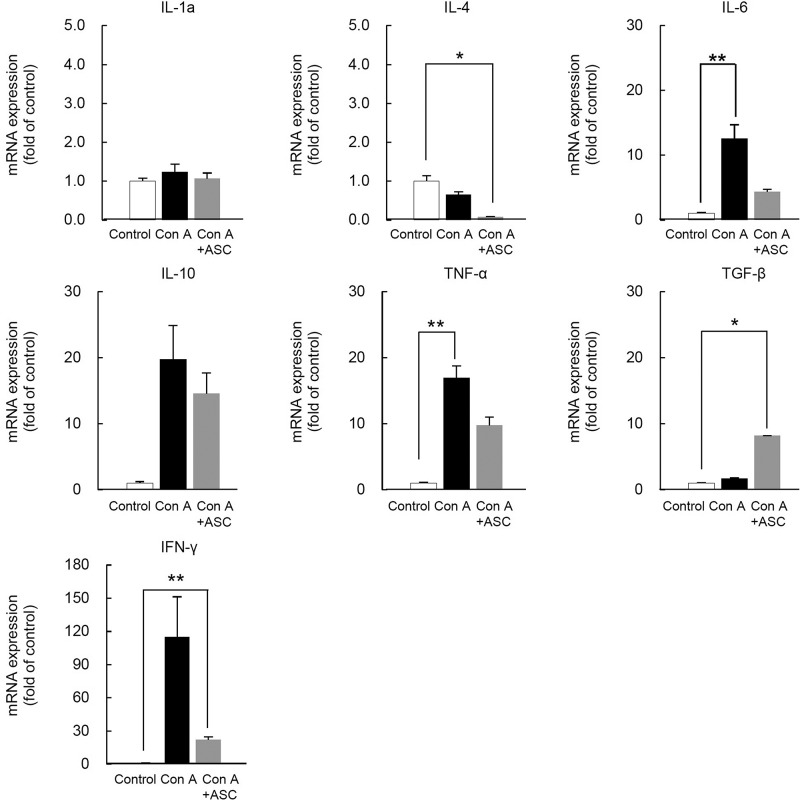
To elucidate the mechanism of action of ASCs for ameliorating liver injury, hepatic mRNA expression of various cytokines and cell lineage markers were evaluated with qRT-PCR using liver samples obtained at 24 h after ConA administration. The mRNA expressions such as Il-6, Il-10, Ifn-γ, and Tnf-α related to inflammation tended to be downregulated in the ConA + ASCs group compared with the ConA group. In addition, the expression of Tgf-β, which may act on suppressing the inflammatory reaction and cell proliferation, tended to be upregulated in the ConA + ASCs group compared with the ConA group, though none of the above genes were significantly altered (Fig. 5).
Figure 5.
mRNA expression of cytokines in ConA-induced mice liver after ASC transplantation. mRNA levels of cytokines of liver in ConA-induced mice after ASC transplantation. The liver samples were harvested at 24 h after ConA administration. Three samples of each group were analyzed and compared with the control (n = 3). The data are shown as the means ± SE values (*p < 0.05, **p < 0.001).
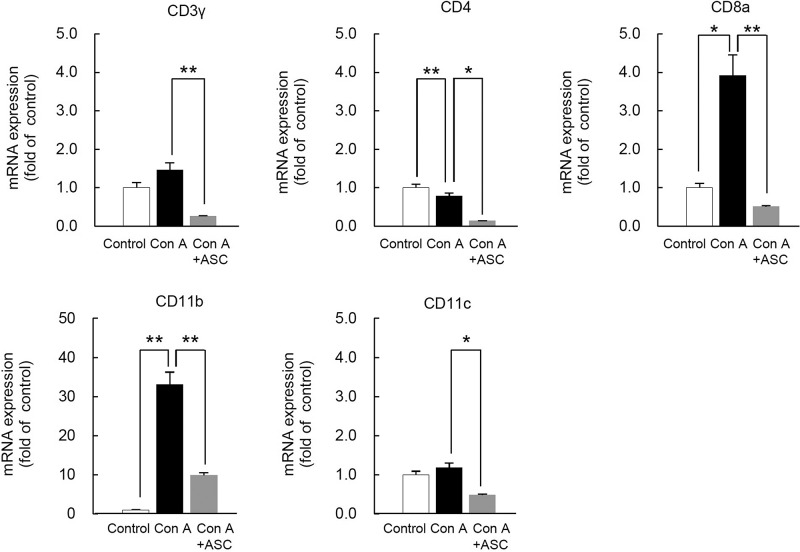
In addition, cell lineage markers such as CD3γ, CD4, CD8α, CD11b, and CD11c were significantly downregulated in the ConA + ASCs group compared with the ConA group. These data suggest that the actions of ASCs for ameliorating FHF induced by excessive immune responses are mediated by suppressing inflammatory reactions and by suppressing invasion or proliferation of inflammatory cells including T cells in the liver (Fig. 6).
Figure 6.
mRNA expression of cell lineage markers in ConA-induced mice liver after ASC transplantation. mRNA levels of cell lineage markers of liver in ConA-induced mice after ASC transplantation. The liver samples were harvested at 24 h after ConA administration. Three samples of each groups were analyzed and compared with the control (n = 3). The data are shown at the means ± SE values (*p < 0.05, **p < 0.001).
DISCUSSION
The expression of surface CD markers of the isolated ASCs was analyzed using flow cytometry, and they were confirmed to be mostly consistent with those of previous reports27,28. The patterns of cytokine expression and growth factor/hormone production of ASCs were also consistent with those of previous reports29. In addition, the differentiation efficiency of ASCs such as for adipocytes and osteocytes could be ascertained comparable to that of previous studies (data not shown). Thus, these data suggest that the established cells in the current study possess most of the feature of ASCs as previously reported.
In the current study, ASCs were confirmed to suppress the proliferation of LMCs stimulated with PMA plus ionomycin (T- and B-cell stimulus) and ConA (T-cell stimulus). However, this suppressive effect of ASCs on LMC proliferation was not observed in the case of LPS stimulation (B-cell stimulus). In addition, through the experiment adding culture supernatant of ASCs to LMC culture instead of coculture with ASCs, it was suggested that the soluble substances such as cytokines and hormones were not responsible for this effect and that direct interaction between ASCs and LMCs was required for exertion of this suppressive function. Recently, Saka et al. reported the suppressive effects of ASCs on mitogen-stimulated proliferation of lymphocytes in vitro. They used human ASCs grown in low-serum culture (hLASCs) and found out that hLASCs suppressed phytohemagglutinin (PHA)-induced proliferation of T cells and B cells. They also reported that this suppressive effect was partly observed even when hLASCs and lymphocytes were placed in a Transwell chamber. However, they conclude that intercellular communication was necessary for hLASCs to exert maximal suppressive effect30. Our results are mostly consistent with their findings, and the differences between the two studies may be caused by the differences in the origins and culture method of the cells and the mitogens used.
In the current study, it was confirmed that the suppression of LMC proliferation was not due to the death of LMCs. Furthermore, in the case of ConA stimulation, which leads to T-cell proliferation, the rate of T cells in LMCs tended to be decreased by the coculture with ASCs. These data suggested that ASCs may mainly affect T cells or the stimulated cells without inducing death to these cells. Since the mechanisms of these effects of ASCs have not yet been elucidated to date, additional analyses for the immunosuppressive function of ASCs, including the necessity of direct interaction to the target cells and specificity to the target cells, are necessary.
The ConA-induced liver injury model in mice is a well-characterized, representative model of acute liver injury; it is caused by excess immune responses and recognized as a model for autoimmune hepatitis in humans20. Therefore, we investigated the anti-inflammatory effects of transplanted ASCs using the ConA-induced liver injury model. Previous reports have shown that ConA-induced liver injury was mainly caused by the activation of NKT cells and T cells that produce TNF-α and IFN-γ31. In this study, the transplantation of ASCs induced the reduction of ALT levels at 8 and 24 h after ConA injection and narrowed the area of massive necrosis at 24 h after ConA injection. However, there were no significant differences in the serum ALT levels at 24 h after ConA injection between with and without ASC transplantation. In the early stage of ConA-induced hepatitis mouse model, the liver injury is triggered by activated T cells and NKT cells that produced TNF-α and IFN-γ31. In a later stage, thrombotic microangiopathy caused by the tissue factor (TF)-activated coagulation system is the main mechanism of liver damage26. Thus, we suppose that ASCs exerted the immunosuppressive effects by inhibiting the proliferation of immune cells as indicated in the in vitro study and by reducing the production of inflammatory cytokines in the early stage, whereas ASCs could not contribute to the improvement of the liver injury in the latter stage. The downregulation of cell surface markers expressed in the live MSCs induced by ConA may support this hypothesis. There is another report by Kubo et al. that describes the effect of ASCs on ConA-induced liver injury model23. The effect of ASCs to ameliorate liver injury is more profound in their report than in our current study; however, the results are mostly consistent with each other. We think that our study confirmed their results and successfully appended theoretical bases of ASC therapy for intractable inflammatory diseases.
In our previous studies, we reported the in vivo fluorescence imaging of transplanted ASCs labeled with quantum dots (effective fluorescence agents) in the mice with acute liver failure16. With regard to the behavior of the transplanted ASCs in mice, we confirmed that the transplanted ASCs accumulated in the injured liver. In addition, there are some reports that described the importance of CXCL12/SDF-1 and C-X-C chemokine receptor 4 (CXCR4: CD184) for the homing of stem cells into the inflamed tissues32,33. Thus, we concluded that the transplanted ASCs could efficiently contact with inflammatory cells, such as T cells, and produce immunosuppressive effects in the liver. However, little is known about the molecular mechanism of stem cell homing; therefore, we should examine this in a further study using in vivo imaging technologies.
In conclusion, we herein investigated the immunomodulatory effects of ASCs on FHF with excessive immune responses. The proliferation of LMCs was found to be efficiently inhibited in a dose-dependent manner by ASCs with direct interaction in the cases of PMA plus ionomycin stimulation (T- and B-cell activation) and ConA stimulation (T-cell activation). Moreover, the therapeutic effects of transplanted ASCs on ConA-induced liver injury were confirmed by the reduction of ALT levels, the improvement of liver histology, and the reduction of mRNA expression of cytokines (Il-6, Il-10, Ifn-γ, and Tnf-α) and cell surface lineage markers (Cd3γ, Cd4, Cd8α, Cd11b, and Cd11c). Our findings suggest that ASCs exert strong immunomodulatory abilities both in vitro and in vivo and can be a good candidate for the source of cell therapies of FHF.
ACKNOWLEDGMENTS
The authors thank Dr. Kazuhiro Furuhashi and Dr. Shoichi Maruyama (Division of Nephrology, Nagoya University Graduate School of Medicine) for technical advice and helpful discussions. This work is partly supported by a grant-in-aid for scientific research (C) from the Ministry of Education, Culture, Sports, Science, and Technology. The authors declare no conflicts of interest.
REFERENCES
- 1. Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010;376:190–201. [DOI] [PubMed] [Google Scholar]
- 2. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: Current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–98. [DOI] [PubMed] [Google Scholar]
- 3. Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl. 2008;14(Suppl. 2):67–79. [DOI] [PubMed] [Google Scholar]
- 4. Lopez MM, Valenzuela JE, Alvarez FC, Lopez-Alvarez MR, Cecilia GS, Paricio PP. Long-term problems related to immunosuppression. Transpl Immunol. 2006;17:31–5. [DOI] [PubMed] [Google Scholar]
- 5. Navarro-Alvarez N, Soto-Gutierrez A, Kobayashi N. Hepatocyte transplantation: A step forward. Curr Opin Organ Transplant. 2007;12:652–8. [Google Scholar]
- 6. Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–7. [DOI] [PubMed] [Google Scholar]
- 7. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001;98:2396–402. [DOI] [PubMed] [Google Scholar]
- 8. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Physiol. 2006;98:1076–84. [DOI] [PubMed] [Google Scholar]
- 9. Huss R. Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells 2000;18:1–9. [DOI] [PubMed] [Google Scholar]
- 10. Jackson L, Jones DR, Scotting P, Sottile V. Adult mesenchymal stem cells: Differentiation potential and therapeutic applications. J Postgrad Med. 2007;53(2):121–7. [DOI] [PubMed] [Google Scholar]
- 11. Meirelles LD, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–98. [DOI] [PubMed] [Google Scholar]
- 12. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 13. Puissant N, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–29. [DOI] [PubMed] [Google Scholar]
- 14. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008;111:1327–33. [DOI] [PubMed] [Google Scholar]
- 15. Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102(1):77–85. [DOI] [PubMed] [Google Scholar]
- 16. Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials 2012;33:2177–86. [DOI] [PubMed] [Google Scholar]
- 17. Liu GT, Li Y, Wei HL, Zhang H, Xu JY, Yu LH. Mechanism of protective action of bicyclol against CCl4-induced liver injury in mice. Liver Int. 2005;25:872–9. [DOI] [PubMed] [Google Scholar]
- 18. Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. IL-18 accounts for both TNF-α and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961–7. [PubMed] [Google Scholar]
- 19. Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–18. [DOI] [PubMed] [Google Scholar]
- 20. Yamanaka A, Hamano S, Miyazaki Y, Ishii K, Takeda A, Mak TW, Himeno K, Yoshimura A, Yoshida H. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172:3590–6. [DOI] [PubMed] [Google Scholar]
- 21. Yokoyama M, Yokoyama A, Mori S, Takahashi HK, Yoshino T, Watanabe T, Watanabe T, Ohtsu H, Nishibori M. Inducible histamine protects mice from P. acnes-primed and LPS-induced hepatitis through H2-receptor stimulation. Gastroenterology 2004;127:892–902. [DOI] [PubMed] [Google Scholar]
- 22. Iwashima S, Ozaki T, Maruyama S, Saka Y, Kobori M, Omae K, Yamaguchi H, Niimi T, Toriyama K, Kamei Y, Torii S, Murohara T, Yuzawa Y, Kitagawa Y, Matsuo S. Novel culture system of mesenchymal stromal cells from human sub-cutaneous adipose tissue. Stem Cells Dev. 2009;18(4):533–43. [DOI] [PubMed] [Google Scholar]
- 23. Kubo N, Narumi S, Kijima H, Mizukami H, Yagihashi S, Hakamada K, Nakane A. Efficacy of adipose tissue-derived mesenchymal stem cells for fulminant hepatitis in mice induced by concanavalin A. J Gastroenterol Hepatol. 2012;27:165–72. [DOI] [PubMed] [Google Scholar]
- 24. Mizuhara H, Kuno M, Seki N, Yu WG, Yamaoka M, Yamashita M, Ogawa T, Kaneda K, Fujii T, Senoh H, Fujiwara H. Strain difference in the induction of T-cell activation-associated, interferon gamma-dependent hepatic injury in mice. Hepatology 1998;27:513–9. [DOI] [PubMed] [Google Scholar]
- 25. Comporti M. Three models of free radical-induced cell injury. Chem Biol Interact. 1989;72:1–56. [DOI] [PubMed] [Google Scholar]
- 26. Kato J, Okamoto T, Motoyama H, Uchiyama R, Kirchhofer D, Van Rooijen N, Enomoto H, Nishiguchi S, Kawada N, Fujimoto J, Tsutsui H. Interferon gamma-mediated tissue factor expression contributes to T cell-mediated hepatitis via induction of hypercoagulation in mice. Hepatology 2013;57:362–72. [DOI] [PubMed] [Google Scholar]
- 27. Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang X, Semon JA, Zhang S, Strong AL, Scruggs BA, Gimble JM, Bunnell BA. Characterization of adipose-derived stromal/stem cells from the Twitcher mouse model of Krabbe disease. BMC Cell Biol. 2013;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang S, Danchuk SD, Imhof KM, Semon JA, Scruggs BA, Bonvillain RW, Strong AL, Gimble JM, Betancourt AM, Sullivan DE, Bunnell BA. Comparison of the therapeutic effects of human and mouse adipose-derived stem cells in a murine model of lipopolysaccharide-induced acute lung injury. Stem Cell Res Ther. 2013;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saka Y, Furuhashi K, Katsuno T, Kim H, Ozaki T, Iwasaki K, Haneda M, Sato W, Tsuboi N, Ito Y, Matsuo S, Kobayashi T, Maruyama S. Adipose-derived stromal cells cultured in a low-serum medium, but not bone marrow-derived stromal cells, impede xenoantibody production. Xenotransplantation 2011;18:196–208. [DOI] [PubMed] [Google Scholar]
- 31. Fang X, Wang R, Ma J, Ding Y, Shang W, Sun Z. Ameliorated ConA-induced hepatitis in the absence of PKC-theta. PLoS One 2012;7:2: e31174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sengenès C, Miranville A, Maumus M, de Barros S, Busse R, Bouloumié A. Chemotaxis and differentiation of human adipose tissue CD34+/CD31− progenitor cells: Role of stromal derived factor-1 released by adipose tissue capillary endothelial cells. Stem Cells 2007;25:2269–76. [DOI] [PubMed] [Google Scholar]
- 33. So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24(3):339–43. [DOI] [PubMed] [Google Scholar]