Abstract
The application of stem cells for cell therapy has been extensively studied in recent years. Among the various types of stem cells, human adipose tissue-derived stem cells (ASCs) can be obtained in large quantities with relatively few passages, and they possess a stable quality. ASCs can differentiate into a number of cell types, such as adipose cells and ectodermal cells. We therefore focused on the in vitro microenvironment required for such differentiation and attempted to induce the differentiation of human stem cells into microtissues using a microelectromechanical system. We first evaluated the adipogenic differentiation of human ASC spheroids in a three-dimensional (3D) culture. We then created the in vitro microenvironment using a 3D combinatorial TASCL device and attempted to induce the adipogenic differentiation of human ASCs. The differentiation of human ASC spheroids cultured in maintenance medium and those cultured in adipocyte differentiation medium was evaluated via Oil red O staining using lipid droplets based on the quantity of accumulated triglycerides. The differentiation was confirmed in both media, but the human ASCs in the 3D cultures contained higher amounts of triglycerides than those in the 2D cultures. In the short culture period, greater adipogenic differentiation was observed in the 3D cultures than in the 2D cultures. The 3D culture using the TASCL device with adipogenic differentiation medium promoted greater differentiation of human ASCs into adipogenic lineages than either a 2D culture or a culture using a maintenance medium. In summary, the TASCL device created a hospitable in vitro microenvironment and may therefore be a useful tool for the induction of differentiation in 3D culture. The resultant human ASC spheroids were “adipose-like microtissues” that formed spherical aggregation perfectly and are expected to be applicable in regenerative medicine as well as cell transplantation.
Key words: Adipose-derived stem cells (ASCs), Spheroid, Three-dimensional (3D) culture, Tapered stencil for cluster culture (TASCL), Adipogenic differentiation, Microenvironment
INTRODUCTION
A large number of studies in recent years have focused on stem cells and their role in cell therapy. Stem cells are cells with self-renewal and differentiation capabilities, and several different types have been identified, each with the potential to differentiate into specialized cell types, such as embryonic stem cells (ESCs)1 and induced pluripotent stem cells (iPSCs)2,3 with pluripotency, bone marrow-derived mesenchymal stem cells and mesenchymal stem cells (BM-MSCs and MSCs, respectively)4,5, adipose tissue-derived stem cells (ASCs)6,7, and other tissue-derived stem cells with multipotency.
Among these cell types, human ASCs can be obtained from human adipose tissues via relatively low-invasive procedures. Furthermore, human ASCs can be obtained in large quantities over relatively few passages and in high quality with respect to their proliferation and differentiation abilities. Previous reports have cited the high quality of cryopreserved human ASCs8–10, which are an important source of cells for cell transplantation11–13 and regenerative medicine. ASCs can differentiate into a number of cell types, such as endodermal14, mesodermal15, and ectodermal cells16. However, the effective use of human ASCs will require ensuring their function is maintained, including their self-renewal and differentiation abilities.
To maintain stem cells in an undifferentiated state, we need to optimize the culture conditions. The methods for maintaining stem cells in an undifferentiated state in two-dimensional (2D) culture are well established17, but such methods for 3D culture are still being developed, although a few reports18–22 have been published on the matter. Controlling the induction of differentiation into target cells is important, as is the maintenance of stemness. The induction of differentiation is essential in the application of these cells to cell therapy and regenerative medicine. In cells with substantial differentiation capacity, such as human ESCs and iPSCs, the targeted induction of differentiation into specific cells is difficult in both 2D and 3D cultures23,24. In contrast, human tissue-derived stem cells, such as human MSCs4,5 and ASCs7,25, retain their multipotency, and their differentiation is much easier to control in both 2D and 3D cultures. Other factors potentially involved in controlling the differentiation of stem cells include the chemical compounds, low-molecular weight compounds, proteins, nucleic acids, genes, and the culture environment conditions (e.g., levels of O2 and CO2) used for the culture.
Previous reports have underscored the importance of cell–cell interactions in keeping stem cells undifferentiated and in inducing their differentiation into target cells in vitro4,5,24,26–28. The controlled differentiation of stem cells is commonly used in 3D culture, with microenvironments created using a variety of biomaterials and microelectromechanical systems. Creating organs and tissues using stem cells can be difficult in 2D culture. However, 3D culture using stem cells and tissue cells can reliably reproduce organs and tissues in vitro29–36. We therefore focused on the in vitro microenvironment and attempted to differentiate human stem cells into microtissues using a microelectromechanical system.
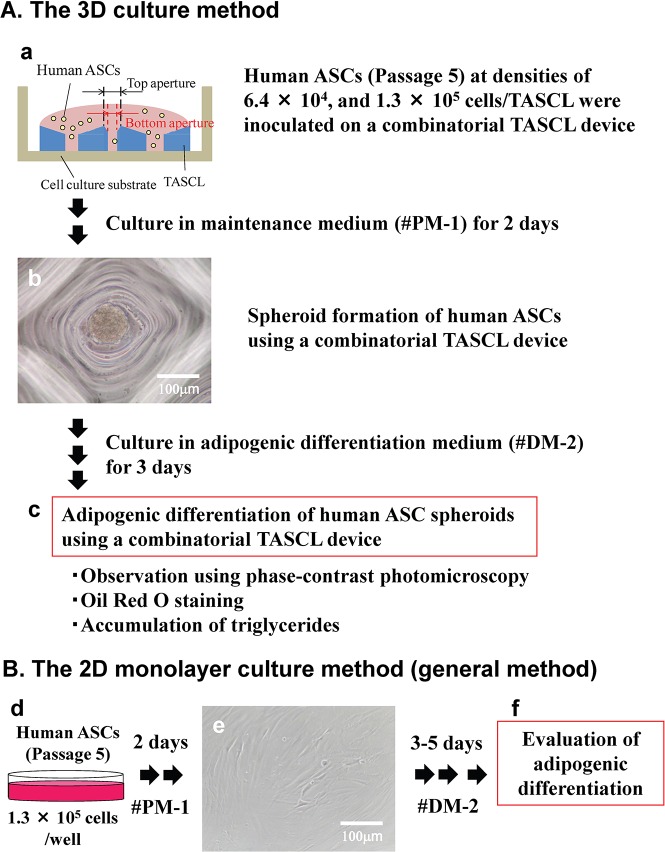
We previously proposed the use of the tapered stencil for cluster culture (TASCL) method (Fig. 1A), an array employing tapered microapertures of varying sizes made of poly(dimethylsiloxane) (PDMS)37,38, compared with 2D culture (Fig. 1B). We were able to use the combinatorial TASCL device to create multiple 3D cell spheroids under several different controlled seeding conditions simultaneously33. The TASCL device can be used quickly and simply and is useful for preparing hepatocyte spheroids33,39. In addition, we prepared uniform embryoid bodies (EBs) from iPSCs on a large scale using the TASCL device and thereby induced the differentiation of EBs into liver cells40.
Figure 1.
The experimental procedure for adipogenic differentiation induction of human adipose tissue-derived stem cells (ASCs) in 3D and 2D cultures. (A) Adipogenic differentiation of human ASCs in the 3D combinatorial tapered stencil for cluster culture (TASCL) device. (a) The TASCL device consisted of microwells measuring 10 mm × 10 mm with a thickness of 0.55 mm. Each microwell in the TASCL device had a top aperture (400 × 400, 600 × 600, and 800 × 800 μm) and bottom aperture (140, 180, 240, and 280 μm in diameter). Human ASCs at a density of 6.4 × 105 or 1.3 × 105 cells/device were seeded onto the TASCL device in a maintenance medium and then were cultured for 2 days. (b) Spheroid formation of human ASCs in a microwell of the TASCL device. Human ASCs at a density of 1.3 × 105 cells/device were inoculated in maintenance medium for 2 days. Scale bar: 100 μm. (c) Evaluation of adipogenic differentiation of human ASC spheroids after 3 days of culture in adipogenic differentiation medium. Adipogenic differentiation of the human ASCs in the TASCL device was determined via Oil red O staining using lipid droplets based on the quantity of accumulated triglycerides. (B) Adipogenic differentiation of human ASCs in the 2D monolayer culture. (d) Human ASCs at a density of 1.3 × 105 cells/well were seeded onto a 12-well plate in maintenance medium and cultured for 2 days. (e) Phase-contrast image of the human ASCs after 2 days of culture. Scale bar: 100 μm. (f) Adipogenic differentiation of the human ASCs in the 2D monolayer culture was evaluated.
We herein report the application of the TASCL device to prepare human ASCs in vitro. The TASCL device will be used to prepare 3D microtissues for cell and tissue transplantation. A 256-microwell 10-mm × 10-mm TASCL device was created, with a top aperture of 400 × 400, 600 × 600, and 800 × 800 μm and bottom diameter of 140, 180, 240, and 280 μm per microwell, respectively. A 60-mm culture dish with an ultralow cell attachment surface under the TASCL device was used in the 3D microtissue preparation. We evaluated the rates of spheroid formation and adipogenic differentiation of the human ASCs in the TASCL device via Oil red O staining using lipid droplets based on the quantity of accumulated triglycerides.
MATERIALS AND METHODS
Materials
Maintenance medium (#PM-1; preadipocyte medium) and adipocyte differentiation medium (#DM-2) were purchased from Zen-Bio, Inc. (Research Triangle Park, NC, USA). Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+ supplementation (D-PBS-free) was purchased from Life Technologies Inc. (Carlsbad, CA, USA). Oil red O reagent and formaldehyde were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other materials and chemicals not specified above were of the highest grade available.
Cells
Cryopreserved human ASCs were obtained from Zen-Bio, Inc. The cryopreserved cells [#ASC-F, Lot ASC062801; sex/age/body mass index (average)/number of patients: female/37/23.29/1] at passage 2 were purchased from Dainippon Sumitomo Pharma (Osaka, Japan).
Preparation of a Combinatorial TASCL Device
These procedures were performed in accordance with a previously reported protocol33,37,38. Briefly, a combinatorial TASCL device was created using PDMS (SYLGARD® 184; Dow Corning, Midland, MI, USA) and coated with an aqueous solution of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer [1% (w/w) Anti-Link; Allvivo Vascular, Inc., Lake Forest, CA, USA] to prevent cell adhesion. After the unconjugated copolymers were washed with D-PBS-free, a TASCL device with a hydrophilic layer was obtained.
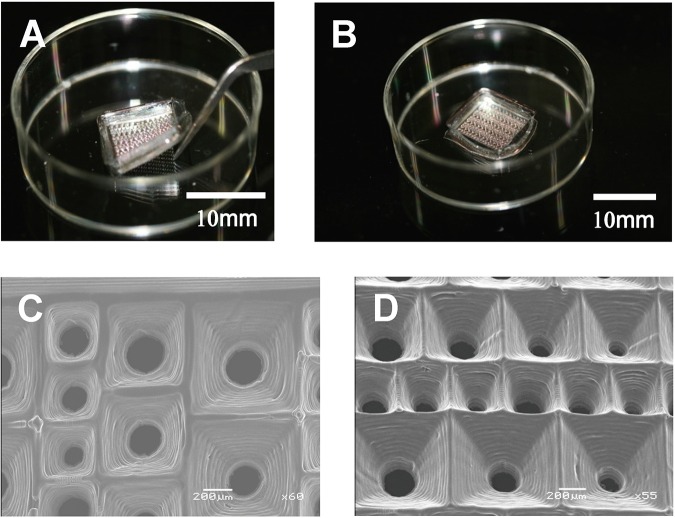
The TASCL device can be easily installed on a 60-mm culture dish with an ultralow cell attachment surface (Corning Inc., Corning, NY, USA) using tweezers (Fig. 2A). The TASCL device has an overall size of 10 mm × 10 mm, with a thickness of 0.55 mm, including microwells with a top aperture of 400 × 400, 600 × 600, and 800 × 800 μm and bottom diameter of 140, 180, 240, and 280 μm per microwell (Fig. 2B). A scanning electron microscope (SEM) was used to visualize the TASCL device. Carbon tape (Nissin Enamel Manufacturing Co. Ltd., Nagoya, Japan) was placed on the SEM stage (JEOL Ltd., Tokyo, Japan), and the TASCL device was attached to the tape. The shape of the TASCL device was confirmed using a field emission SEM (FE-SEM; JEOL JSM-7500F; JEOL Ltd.) at an acceleration voltage of 15 kV (Fig. 2C and D).
Figure 2.
Scanning electron microscope (SEM) images of the combinatorial TASCL device. (A) The TASCL device can be easily installed on a 60-mm culture dish with an ultralow cell attachment surface with tweezers. (B) The gross appearance of the TASCL device consisted of microwells measuring 10 mm × 10 mm with a thickness of 0.55 mm. Each microwell in the TASCL device had a top aperture (400 × 400, 600 × 600, and 800 × 800 μm) and bottom aperture (140, 180, 240, and 280 μm in diameter). Scale bars: 10 mm. (C, D) SEM image of a microwell in the TASCL device. Scale bars: 200 μm.
Thawing and Subculture of Human ASCs
For thawing, 2 × 105 human ASCs were seeded into a T-25 culture flask (NUNC, Roskilde, Denmark) in maintenance medium. The seeded cells attached and spread on the flask and were cultured at 37°C under a humidified 5% CO2 atmosphere to approximately 80%–90% confluence. The cells were then detached from the flask by trypsin (Gibco®; Life Technologies Inc.) treatment and seeded and cultured in a new flask. The cell viability was more than 95%, as determined using the trypan blue dye exclusion test. The final concentration of trypan blue (Gibco®; Life Technologies Inc.) was 0.2%. The cells were passaged two times prior to use in the culture experiments with the TASCL device.
Adipogenic Differentiation of Human ASCs in 3D Culture
Figure 1A shows the experimental procedure of the spheroid formation and adipogenic differentiation induction of human ASCs using the combinatorial TASCL device (3D culture). Human ASCs at passage 5 were inoculated onto the TASCL device at densities of 6.4 × 104 and 1.3 × 105 cells/device to form 3D spheroids. The cells were then cultured in 0.35 ml of maintenance medium for 2 days at 37°C under a humidified 5% CO2 atmosphere. The maintenance medium was partially changed every day. The morphology of human ASC spheroids was observed using a phase-contrast microscope (Olympus, Tokyo, Japan).
Adipogenic differentiation was induced by culturing human ASCs in adipocyte differentiation medium. In the control experiments, human ASC spheroids were cultured in maintenance medium, which was partially changed every day. After the cells had been cultured for 3 days using the TASCL device, they were stained to determine the accumulation of triglycerides by Oil red O staining, as described below. The morphology of the adipogenic differentiated human ASCs spheroids was observed under a phase-contrast microscope.
Adipogenic Differentiation of Human ASCs in 2D Culture
Figure 1B shows the experimental procedure of the monolayer culture and adipogenic differentiation induction of human ASCs in 2D culture. Human ASCs at passage 5 were inoculated onto 12-well plates (BD Falcon, Franklin Lakes, NJ, USA) at densities of 1.3 × 105 cells in 1 ml of maintenance medium. The cells were incubated at 37°C under a humidified 5% CO2 atmosphere, and the medium was changed every day. The morphology of the human ASC monolayer was observed using a phase-contrast microscope. The adipogenic differentiation was performed as described above.
Evaluation of Adipogenic-Differentiated Human ASCs
The adipogenic differentiation of human ASCs was confirmed by microscopic observation of intracellular lipid droplets and Oil red O staining as an indicator of the intracellular lipid accumulation. Briefly, differentiated human ASCs were fixed with 10% formaldehyde in D-PBS-free for 10 min at room temperature and washed with 60% isopropanol (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The cells were then stained with 2% (w/v) Oil red O reagent for 10 min at room temperature followed by repeated washing with distilled water and then destained in isopropanol for 1 min. The Triglyceride E-test™ assay (Wako Pure Chemical Industries Ltd.) was used to determine the accumulated triglycerides in the cell samples, as described previously41,42.
Statistical Analysis
The data are presented as the mean ± standard deviation (SD). Each experiment was repeated three times (n = 3). The statistical significance was determined using the unpaired Student’s t-test for comparisons between the adipogenic induction groups and the control groups. A value of p < 0.05 was considered to be significant.
RESULTS
Spheroid Formation of Human ASCs in the TASCL Device
Human ASCs at two different densities (6.4 × 104 and 1.3 × 105 cells/device) were inoculated into the TASCL device and cultured for 2 days. Cell spheroids approximately 70 μm in diameter were observed in the microwells using a phase-contrast microscope (Fig. 1A, b). Human ASCs in each microwell formed a single spheroid aggregation about 50–200 μm in diameter. In the 2D monolayer culture, human ASCs at densities of 1.3 × 105 cells inoculated onto the 12-well plates and cultured for 2 days formed human fibroblast-like cells (Fig. 1B, e).
Induction of Adipogenic Differentiation of Human ASC Spheroids in the TASCL Device
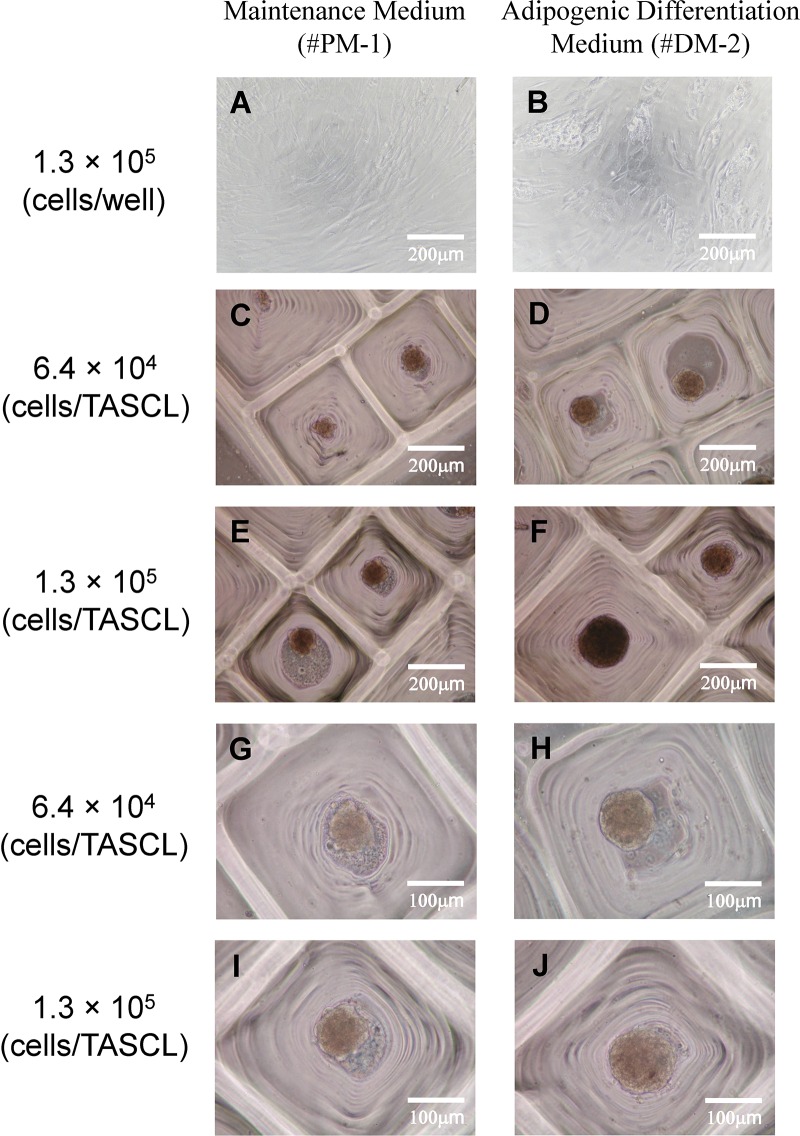
After 2 days of culture in maintenance medium, human ASC spheroids were cultured on the TASCL device for an additional 3 days in adipogenic differentiation medium (Fig. 1A). We observed human ASC spheroids in both the maintenance medium and the adipogenic differentiation medium after 5 days of culture. The morphology of the 3D spheroids was unchanged before and after culture in the adipogenic differentiation medium, continuing to exhibit spherical aggregates (Figs. 1A, b, and 3). Furthermore, no marked differences in the morphology of the human ASC spheroids were noted between cells cultured in the maintenance medium and in the adipocyte differentiation medium under a phase-contrast microscope (Fig. 3C, E, G, and I vs. Fig. 3D, F, H, and J). The size of the 3D spheroids increased both with increasing initial cell seeding density (Fig. 3G and H vs. Fig. 3I and J).
Figure 3.
The adipogenic differentiation of human ASCs in 2D and 3D cultures. The morphology of adipogenic-differentiated human ASCs was observed using a phase-contrast microscope. In 2D culture, human ASCs at a density of 1.3 × 105 cells/well (A, B) were seeded onto a 12-well plate in maintenance medium and cultured for 2 days, and then further cultured in either maintenance medium (A) or adipogenic differentiation induction medium (B) for 3 days. In 3D culture, human ASCs were seeded onto the TASCL device at a density of 6.4 × 104 and 1.3 × 105 cells/device and cultured in maintenance medium for 2 days, and then further cultured in either maintenance medium (C, E, G, I) or adipogenic differentiation induction medium (D, F, H, J) for 3 days. Scale bars: 200 μm (A–F), 100 μm (G–J).
In the 2D monolayer culture, we observed the morphology of human ASCs cultured in maintenance medium and in adipogenic differentiation medium using an optical microscope. After 5 days of culture in adipogenic differentiation medium, the cells showed round morphology with the accumulation of lipid droplets (Fig. 3B). In contrast, the morphology of the human ASCs in maintenance medium did not change after 5 days of culture (Fig. 3A).
Evaluation of the Adipogenic-Differentiated Human ASC Spheroids in the TASCL Device
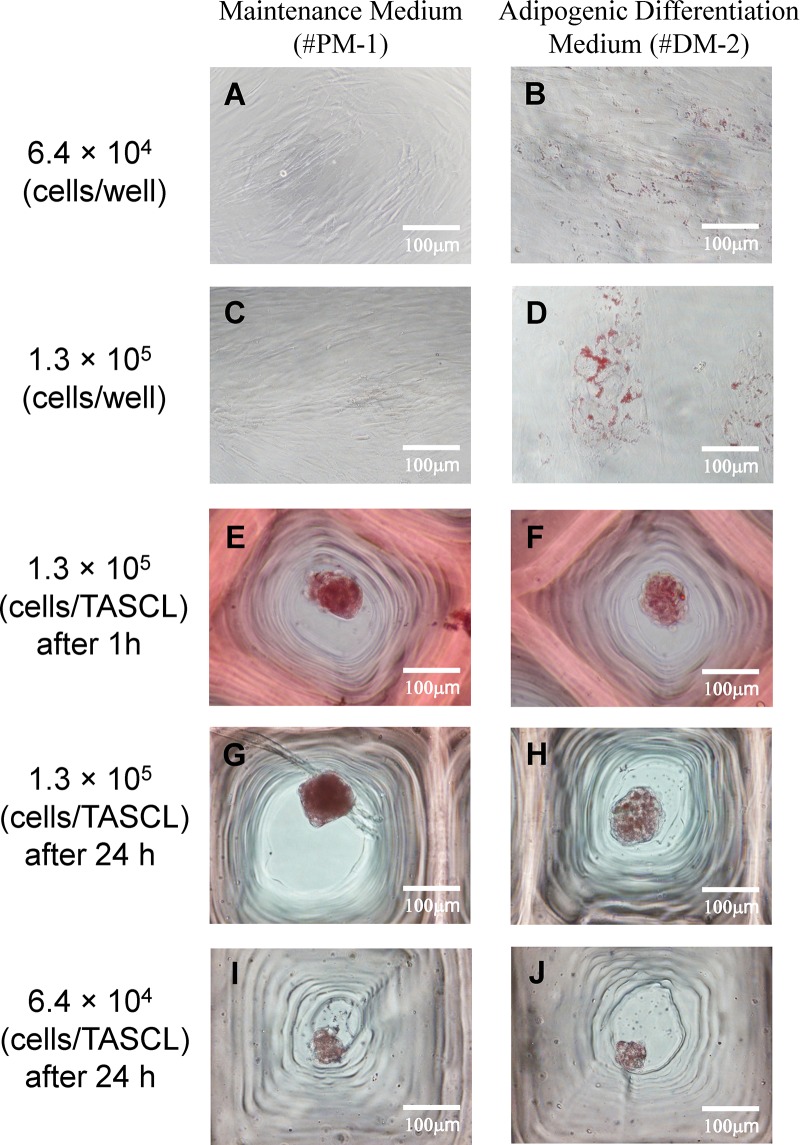
The degree of adipogenic differentiation of human ASC spheroids was evaluated by Oil red O staining of lipid droplets (Fig. 4) and by analyzing the accumulation of triglycerides (Fig. 5). After 1 h of Oil red O staining, positively stained cells were observed in both the maintenance medium and adipogenic differentiation medium (Fig. 4E and F). In particular, intracellular lipid droplets were positively stained with Oil red O for human ASC spheroids cultured in adipogenic differentiation medium for an hour and stained more intensely after 24 h of staining (Fig. 4G and H). In addition, the intracellular lipid droplets were positively stained with Oil red O for human ASC spheroids cultured in maintenance medium for 24 h and stained more intensely as the initial cell seeding density decreased (Fig. 4G and I).
Figure 4.
Phase-contrast images of Oil red O staining after adipogenic differentiation induction of human ASCs in 2D and 3D cultures. In 2D culture, human ASCs at a density of 6.4 × 104 and 1.3 × 105 cells/well were seeded onto a 12-well plate and cultured in maintenance medium for 2 days and then further cultured in either maintenance medium (A, C) or adipogenic differentiation induction medium (B, D) for 3 days. In 3D culture, human ASCs were seeded onto the TASCL device at a density of 6.4 × 104 (I, J) and 1.3 × 105 cells/device (E–H) and then cultured in either or both maintenance medium or adipogenic induction medium. The intracellular lipid droplets were stained with Oil red O after 1 h (A–F) and 24 h (G–J). Scale bars: 100 μm.
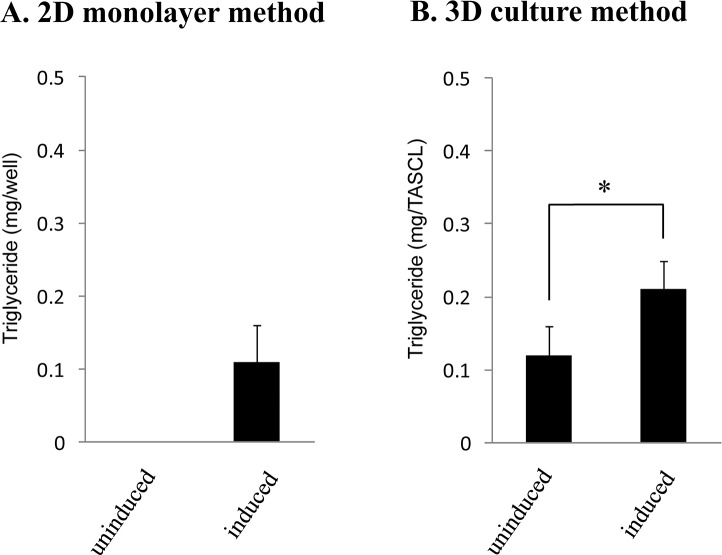
Figure 5.
A quantitative analysis of the accumulated triglycerides after adipogenic differentiation of human ASCs in 2D and 3D cultures. Human ASCs seeded at a density of 1.3 × 105 cells/well (or device) were incubated in maintenance medium for 2 days and then further cultured in maintenance medium (uninduced) or adipogenic induction medium (induced) for 3 days in the 12-well plate (2D culture) (A) and in the combinatorial TASCL device (3D culture) (B). The amount of triglycerides accumulated in the cells was assayed using the Triglyceride E-test™ assay as described previously.
In the 2D monolayer culture, the adipogenic differentiation of the human ASCs in the adipogenic differentiation medium was confirmed by positive Oil red O staining (Fig. 4B and D). The differentiated cells had a round morphology with lipid droplets (Fig. 4D). In contrast, the human ASCs cultured in the maintenance medium were negatively stained and formed human fibroblast-like cells (Fig. 4A and C).
We quantitatively assayed the accumulated triglycerides in the differentiated cells as described previously41,42. Briefly, human ASCs seeded at a density of 1.3 × 105 cells were incubated in maintenance medium for 2 days and then further cultured in either maintenance medium (uninduced) or adipogenic induction medium (induced) for 3 days in a 12-well plate for 2D culture (Fig. 5A) or in the TASCL device for 3D culture (Fig. 5B). In the 2D culture, triglyceride accumulation was observed only for human ASCs cultured in adipogenic induction medium (Fig. 5A). In the 3D culture, triglyceride accumulation in human ASCs cultured in adipogenic induction medium was approximately twofold higher than that in cells cultured in maintenance medium (Fig. 5B).
DISCUSSION
In this study, we evaluated the adipogenic differentiation of human ASC spheroids in 3D culture. We created an in vitro microenvironment using the combinatorial TASCL device and attempted to differentiate the human ASCs. We observed greater adipogenic differentiation in the 3D cultures than in the 2D cultures. Culturing in adipocyte differentiation medium using the TASCL device promoted greater differentiation of human ASCs into adipogenic lineages than culturing in maintenance medium. These results indicated that the TASCL device is effective for creating in vitro microenvironments.
The 3D spheroids of human ASCs perfectly formed spherical aggregations in the TASCL device after 2 days of culture. Given that the size of each microwell in the TASCL device is different, the single cluster sizes of human ASCs varied (about 50–200 μm in diameter) (Figs. 3C–J and 4E–J). The TASCL device was coated with a hydrophilic layer, and an ultralow cell attachment surface on the bottom of each microwell in the TASCL device prevented cell adhesion. By creating a microenvironment in which the cells had difficulty adhering to the TASCL device itself, the cell–cell interactions of the human ASCs favored spherical aggregation. The cell scaffold (extracellular matrix as collagen, synthetic polymers as polystyrene, etc.) of a fabricated device for creating a microenvironment is extremely important4,5,24–30,33,37–39. We previously reported that type I collagen-coated plates and polystyrene plates used the same style of well bottom as the TASCL device33. However, the TASCL device is additionally treated with a hydrophilic layer. The cell attachment to cell scaffolds differs by cell type and culture conditions, and the resultant cell constructs can form spheroids, monolayers, and mixtures of both33.
Human ASCs were cultured in the TASCL device for 5 days (Fig. 3) and then evaluated by Oil red O staining of lipid droplets (Fig. 4). The human ASC spheroids formed spherical aggregations, with no marked differences in shape noted between the maintenance medium and adipocyte differentiation medium. However, the intracellular lipid droplets were more strongly stained for human ASC spheroids (at 1.3 × 105 cells/TASCL) cultured in adipocyte differentiation medium (Fig. 4F and H) than for those cultured in maintenance medium (Fig. 4E and G).
The intracellular lipid droplets were positively stained with Oil red O for human ASC spheroids cultured in maintenance medium for 1 h (Fig. 4E) and 24 h (Fig. 4G). While the peripheral portion of the clusters promotes differentiation, the central portion of the clusters might not (Fig. 4G). We therefore performed the same staining experiment as above but changed the size of the human ASC spheroids (Fig. 4I and J). After 24 h of Oil red O staining, adipogenic differentiation of the human ASC spheroids was demonstrated by positive staining with both the maintenance medium and the adipocyte differentiation medium (Fig. 4I and J). In the 2D monolayer culture, the adipogenic differentiation of human ASCs cultured for 5 days was demonstrated by positive Oil red O staining with adipocyte differentiation medium (Fig. 4B and D). As the culture progressed (7–10 days), the intracellular lipid droplets were positively stained with Oil red O more intensely.
We also confirmed the adipogenic differentiation of human ASCs by measuring the levels of triglycerides. The human ASCs in the 3D culture had greater triglyceride accumulation than those in the 2D culture. Furthermore, in the short culture period, the adipogenic differentiation proceeded more efficiently in the 3D culture than in the 2D culture. Although the culture medium in 3D culture is important for the efficient induction of differentiation, we also successfully induced adipogenic differentiation using only the TASCL device. These results indicate that the TASCL device is effective in creating an in vitro microenvironment.
In summary, a TASCL device effectively created an in vitro microenvironment and may be a useful tool for the induction of differentiation in 3D culture. The TASCL device functioned in the same way as other cell scaffolds for the adipogenic differentiation of human ASCs. The resultant human ASC spheroids were “adipose-like microtissues” that perfectly formed spherical aggregations and are expected to prove useful in regenerative medicine and cell transplantation.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Rina Yokota and Ms. Yumie Koshidaka at Nagoya University for their technical assistance. This work was supported in part by JSPS KAKENHI Grant Nos. 25560248 and 25600054 (Grant-in-Aid for Challenging Exploratory Research), 15H03039 (Scientific Research [B] [General]), and the JST PRESTO program. The authors declare no conflicts of interest.
REFERENCES
- 1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 1998;282(5391):1145–7. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131(5):861–72. [DOI] [PubMed] [Google Scholar]
- 3. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126(4):663–76. [DOI] [PubMed] [Google Scholar]
- 4. Djouad F, Delorme B, Maurice M, Bony C, Apparailly F, Louis-Plence P, Canovas F, Charbord P, Noël D, Jorgensen C. Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007;9(2):R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyagawa Y, Okita H, Hiroyama M, Sakamoto R, Kobayashi M, Nakajima H, Katagiri YU, Fujimoto J, Hata J, Umezawa A, Kiyokawa N. A microfabricated scaffold induces the spheroid formation of human bone marrow-derived mesenchymal progenitor cells and promotes efficient adipogenic differentiation. Tissue Eng Part A 2011;17(3–4):513–21. [DOI] [PubMed] [Google Scholar]
- 6. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T. Stem cells for hepatic regeneration: The role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther. 2010;5(2):182–9. [DOI] [PubMed] [Google Scholar]
- 8. Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: Maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1(4):322–4. [DOI] [PubMed] [Google Scholar]
- 9. Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, Koshima I, Yoshimura K. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121:401–10. [DOI] [PubMed] [Google Scholar]
- 10. Miyamoto Y, Oishi K, Yukawa H, Noguchi H, Sasaki M, Iwata H, Hayashi S. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 2012;21(2–3):617–22. [DOI] [PubMed] [Google Scholar]
- 11. Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: Isolation, characterization and applications in surgery. ANZ J Surg. 2009;79(4):235–44. [DOI] [PubMed] [Google Scholar]
- 12. Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34(9):1178–85. [DOI] [PubMed] [Google Scholar]
- 14. Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24(1):70–7. [DOI] [PubMed] [Google Scholar]
- 15. Baer PC, Brzoska M, Geiger H. Epithelial differentiation of human adipose-derived stem cells. Methods Mol Biol. 2011;702:289–98. [DOI] [PubMed] [Google Scholar]
- 16. Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galié M, Sbarbati A, Krampera M, Belluzzi O, Bonetti B. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(5):909–16. [DOI] [PubMed] [Google Scholar]
- 17. Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olmer R, Haase A, Merkert S, Cui W, Palecek J, Ran C, Kirschning A, Scheper T, Glage S, Miller K, Curnow EC, Hayes ES, Martin U. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010;5(1):51–64. [DOI] [PubMed] [Google Scholar]
- 19. Singh H, Mok P, Balakrishnan T, Rahmat SN, Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4(3):165–79. [DOI] [PubMed] [Google Scholar]
- 20. Amit M, Laevsky I, Miropolsky Y, Shariki K, Peri M, Itskovitz-Eldor J. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat Protoc. 2011;6(5):572–9. [DOI] [PubMed] [Google Scholar]
- 21. Chen VC, Couture SM, Ye J, Lin Z, Hua G, Huang HI, Wu J, Hsu D, Carpenter MK, Couture LA. Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res. 2012;8(3):388–402. [DOI] [PubMed] [Google Scholar]
- 22. Fluri DA, Tonge PD, Song H, Baptista RP, Shakiba N, Shukla S, Clarke G, Nagy A, Zandstra PW. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods 2012;9(5):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Alvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 2009;136:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Soto-Gutiérrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Yamatsuji T, Shirakawa Y, Tanaka N, Basma H, Fox IJ, Kobayashi N. Instant hepatic differentiation of human embryonic stem cells using activin A and a deleted variant of HGF. Cell Transplant. 2006;15:865–71. [DOI] [PubMed] [Google Scholar]
- 25. Naderi N, Wilde C, Haque T, Francis W, Seifalian AM, Thornton CA, Xia Z, Whitaker IS. Adipogenic differentiation of adipose-derived stem cells in 3-dimensional spheroid cultures (microtissue): Implications for the reconstructive surgeon. J Plast Reconstr Aesthet Surg. 2014;67(12):1726–34. [DOI] [PubMed] [Google Scholar]
- 26. Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods 2006;3(5):369–75. [DOI] [PubMed] [Google Scholar]
- 27. Bhatia SN, Balis UJ, Yarmush ML, Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: Role of homotypic cell interactions. Biotechnol Prog. 1998;14(3):378–87. [DOI] [PubMed] [Google Scholar]
- 28. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126(4):677–89. [DOI] [PubMed] [Google Scholar]
- 29. Enosawa S, Miyamoto Y, Kubota H, Jomura T, Ikeya T. Construction of artificial hepatic lobule-like spheroids on a three-dimensional culture. Cell Med. 2012;3(1–3):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng ZQ, Chu X, Huang NP, Wang T, Wang Y, Shi X, Ding Y, Gu ZZ. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009;30(14):2753–63. [DOI] [PubMed] [Google Scholar]
- 31. Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA 2002;99(25):16105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janorkar AV. Polymeric scaffold materials for two-dimensional and three-dimensional in vitro culture of hepatocytes. In: Kulshrestha AS, Mahapatro A, Henderson LA, editors. Biomaterials. Washington (DC): ACS Publications; 2010. p. 1–32. [Google Scholar]
- 33. Miyamoto Y, Ikeuchi M, Noguchi H, Yagi T, Hayashi S. Three-dimensional in vitro hepatic constructs formed using combinatorial tapered stencil for spheroid culture (TASCL) device. Cell Med. 2015;7:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otsuka H, Hirano A, Nagasaki Y, Okano T, Horiike Y, Kataoka K. Two-dimensional multiarray formation of hepatocytes spheroids on a microfabricated PEG-brush surface. Chembiochem 2004;5:850–5. [DOI] [PubMed] [Google Scholar]
- 35. Sakai Y, Nakazawa K. Technique for the control of spheroid diameter using microfabricated chips. Acta Biomater. 2007;3(6):1033–40. [DOI] [PubMed] [Google Scholar]
- 36. Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One 2008;3(2):e1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikeuchi M, Ikuta K. Method for producing different populations of molecules or fine particles with arbitrary distribution forms and distribution densities simultaneously and in quantity, and masking member therefor. United States Patent Application, US 2011/0229953 A1.
- 38. Ikeuchi M, Oishi K, Noguchi H, Hayashi S, Ikuta K. Soft tapered stencil mask for combinatorial 3D spheroid formation of stem cells. Proc μTAS 2010; 641–3. [Google Scholar]
- 39. Miyamoto Y, Ikeuchi M, Noguchi H, Yagi T, Hayashi S. Spheroid formation and evaluation of hepatic cells in a three-dimensional culture device. Cell Med. 2015;8(1–2):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yukawa H, Ikeuchi M, Noguchi H, Miyamoto Y, Ikuta K, Hayashi S. Embryonic body formation using the tapered soft stencil for spheroid culture device. Biomaterials 2011;32(15):3729–38. [DOI] [PubMed] [Google Scholar]
- 41. Oishi K, Noguchi H, Yukawa H, Miyazaki T, Kato R, Kitagawa Y, Ueda M, Hayashi S. Cryopreservation of mouse adipose tissue-derived stem/progenitor cells. Cell Transplant. 2008;17(1–2):35–41. [DOI] [PubMed] [Google Scholar]
- 42. Ono M, Aratani Y, Kitagawa I, Kitagawa Y. Ascorbic acid phosphate stimulates type IV collagen synthesis and accelerates adipose conversion of 3T3-L1 cells. Exp Cell Res. 1990;187(2):309–14. [DOI] [PubMed] [Google Scholar]