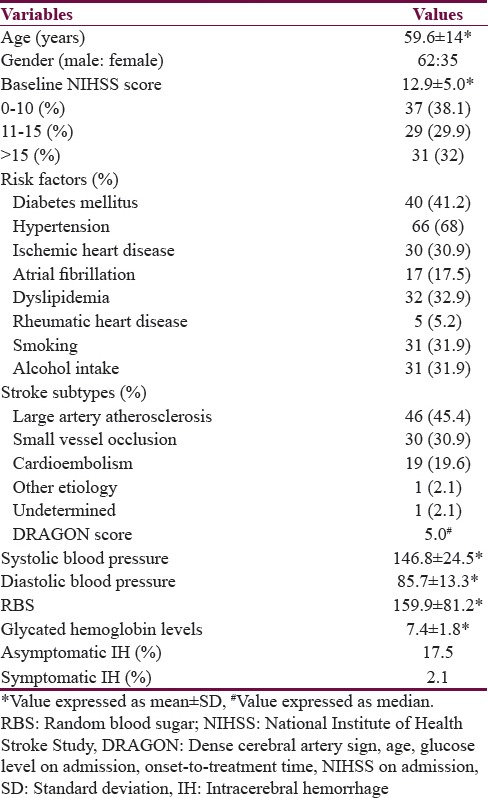
Abstract
Background:
Intravenous thrombolysis (IVT) has now become a standard treatment in eligible patients with acute ischemic stroke (AIS) who present within 4.5 h of symptom onset.
Objective:
To determine the usefulness of IVT and the subset of patients who will benefit from IVT in AIS within 4.5 h.
Materials and Methods:
Patients with AIS within 4.5 h of symptom onset who underwent IVT were studied prospectively. The study period was from October 2011 to October 2015.
Results:
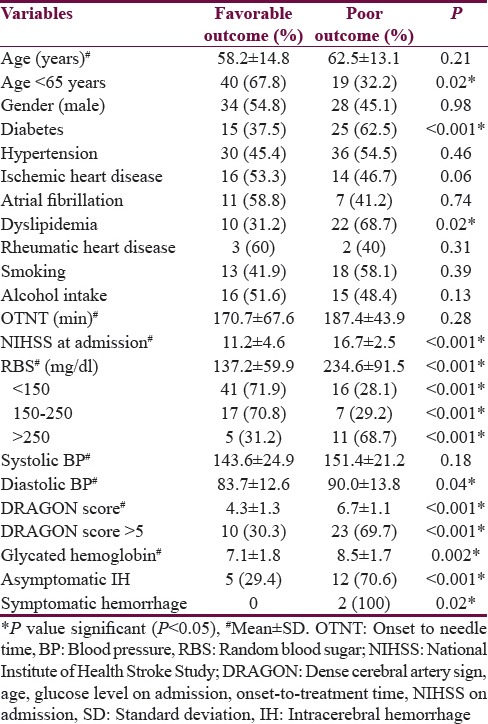
A total of 97 patients were thrombolysed intravenously. The mean onset to needle time in all patients was 177.2 ± 62 min (range: 60–360). At 3 months follow-up, favorable outcome was seen in 65 patients (67.1%) and poor outcome including death in the remaining 32 patients (32.9%). Factors predicting favorable outcome was age <65 years (P = 0.02), the National Institute of Health Stroke Scale (NIHSS) <15 (P < 0.001), small vessel occlusion (P = 0.006), cardioembolism (P = 0.006), and random blood sugar (RBS) <250 mg/dl (P < 0.001). Factors predicting poor outcome was diabetes mellitus (P = 0.01), dyslipidemia (P = 0.01), NIHSS at admission >15 (P = 0.03), RBS >250 mg/dl (P = 0.01), Dense cerebral artery sign, age, glucose level on admission, onset-to-treatment time, NIHSS on admission score >5 (P = 0.03), and occlusion of large artery (P = 0.02).
Conclusion:
Milder baseline stroke severity, blood glucose <250 mg/dL, younger patients (<65 years), cardioembolic stroke, and small vessel occlusion benefit from recombinant tissue plasminogen activator.
KEYWORDS: Acute ischemic stroke, diabetes, intravenous thrombolysis, outcome, recombinant tissue plasminogen activator
INTRODUCTION
The administration of intravenous recombinant tissue plasminogen activator (rt-PA) in acute ischemic stroke (AIS) within 3 h of symptom onset has been known to improve the outcome as per The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study.[1] There was 30% relative risk reduction of death or disability at the 90-day follow-up with rt-PA treatment.[1] The window period was extended to 4.5 h following the results of the European Cooperative Acute Stroke Study III.[2] However, with the advent of intra-arterial thrombolysis/endovascular mechanical thrombectomy and demonstration of their superiority in the recent studies with respect to recanalization of occluded vessels and outcome, intravenous thrombolysis (IVT) has lost its sheen among the interventional neurologists worldwide.[3,4] However, IVT still has a role to play in the management of AIS in the resource-poor settings of the developing countries. The present study was aimed at determining the usefulness of IVT and the subset of patients who will benefit in AIS within 4.5 h.
MATERIALS AND Methods
This was a prospective, single center, and observational study. The study was approved by the Institutional Scientific Committee and ethics review board. Patients with AIS within 4.5 h of symptom onset who underwent IVT were studied prospectively. The study period was from October 2011 to October 2015. In total, 97 patients with AIS presenting within 4.5 h, who had received IVT with rt-PA were analyzed. Patients presenting within 4.5 h of symptoms suggestive of stroke were evaluated by a neurologist. Patients underwent nonenhanced computed tomography (CT) scans of the brain which were evaluated by a neurologist or a radiologist or both. Informed consent was obtained from all eligible patients. Inclusion and exclusion criteria were according to guidelines proposed by the American Heart Association/American Stroke Association.[5] All patients were treated with IV rt-PA (alteplase, Boehringer-Ingelheim, Ingelheim, Germany). A dose of 0.9 mg/kg with 10% as a bolus and the rest as an infusion over 1 h was given to the eligible patients. They were admitted in intensive care unit for monitoring for the first 24 h. Follow-up CT scan of brain was done at 24 h postthrombolysis in all patients.
Data
Demographic data and clinical characteristics
Data on the demographic characteristics, presenting symptoms, risk factors such as hypertension, diabetes mellitus (DM), dyslipidemia, ischemic heart disease, atrial fibrillation, rheumatic heart disease, alcohol, and nicotine abuse, etc., time of symptom onset (defined by the time they were “last seen to be well”), time from symptom onset to rt-PA administration (onset to needle time), door to needle time (time from presentation to the hospital to initiation of thrombolysis), dose of rt-PA, Dense cerebral artery sign, age, glucose level on admission, onset-to-treatment time, NIHSS on admission (DRAGON) score,[6] National Institute of Health Stroke Scale (NIHSS) score at admission, at day 1, day 7, and day 90 were recorded.[7] Various subtypes of ischemic stroke as per the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria were determined.[8] Patients were followed up at day 90 for clinical assessment and to record the outcome. The hospital mortality and its causes were recorded. Symptomatic intracerebral hemorrhage (ICH) was defined as per NINDS rt-PA stroke study criteria, which is hemorrhage associated with worsening of ≥1 point on the NIHSS score.[1]
Investigations
Following laboratory data were collected: Hemoglobin level, total leukocyte count, platelet count, packed cell volume, prothrombin time and international normalized ratio, activated plasma thromboplastin time, blood urea nitrogen, serum creatinine, serum electrolytes, random blood sugar (RBS), glycated hemoglobin, fasting lipid profile, serum homocysteine, D-dimer, serological testing for human immunodeficiency virus, hepatitis-B virus, and syphilis. The following imaging characteristics were noted: The presence of signs of AIS at baseline CT scan, vascular territory involved (anterior cerebral, middle cerebral, vertebrobasilar artery, and watershed areas), angiographic findings (normal, occlusion of internal carotid, middle cerebral (M1 and M2), anterior cerebral, and vertebrobasilar artery) and presence of asymptomatic and symptomatic ICH.
Outcome
The functional outcome of each patient was assessed using modified Rankin scale (mRS) and Barthel Index (BI). The mRS score was recorded at day 7 and day 90 and BI at day 90. BI evaluates 10 domains of activities of daily living which include feeding, grooming, bowel, and bladder, bed-to-chair transfer, dressing, mobility, bathing, toilet, and climbing stairs.[9] Favorable outcome was considered as an mRS score of ≤2 and BI score of ≥95.
Statistical analysis
The frequency with percentage was calculated for categorical variables and mean ± standard deviation with range was calculated for continuous variables. Student's unpaired t-test was used for the comparison of continuous variables and the Chi-square test for the proportions. The predictors of outcome were determined by univariate analysis that included the following variables: Demographic (gender, age), risk factors such as hypertension, DM, dyslipidemia, ischemic heart disease, atrial fibrillation, rheumatic heart disease, alcohol and nicotine use, systolic and diastolic blood pressure, NIHSS score at admission, onset to needle time, dose of rt-PA, RBS, glycated hemoglobin, serum homocysteine level, DRAGON score, abnormalities on angiography, asymptomatic and symptomatic hemorrhage, subtypes according to TOAST classification. Variables with a significant P value (≤0.05) in univariate analysis were considered for multivariate analysis (logistic-regression analysis). Statistical analyses were performed using SPSS 17 software, IBM. inc, Texas, USA.
RESULTS
A total of 97 patients were thrombolysed intravenously with rt-PA during the study period. Baseline characteristics of the patients are shown in Table 1. AIS in the anterior circulation was seen in 85 (87.6%) and in posterior circulation in 12 (12.4%) patients. The mean onset to needle time in all patients was 177.2 ± 62 min (range: 60–360). A total of 57 patients (59%) were thrombolysed within 180 min; 36 (37%) in between 180 and 270 min, and 4 (4%) above 270 min of symptom onset. The mean door to needle time in all patients was 45.8 ± 7.0 min (range: 30–55) with median of 45 min. All the patients were thrombolysed within the recommended 60 min or less of their arrival to hospital. The dose of rt-PA used was 50 mg in 65 of the 97 patients. 20 patients received 60 mg, and 12 patients received 70 mg.
Table 1.
Baseline characteristics of 97 patients’ thrombolysed with recombinant tissue plasminogen activator
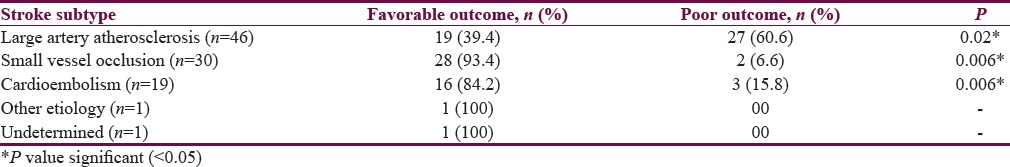
Outcome according to stroke subtype
The outcome of patients following IVT with respect to stroke subtype is shown in Table 2. Cardioembolic stroke and small vessel occlusion subtype had favorable outcome.
Table 2.
Outcome according to the stroke subtype
Outcome at 3 months
At 3 months follow-up, favorable outcome in the form of functional independence (mRS score ≤2) was seen in 65 patients (67.1%) and poor outcome including death (mRS score ≥3) in the remaining 32 patients (32.9%). One patient died within 1 week due to refractory cardiogenic pulmonary edema and another patient within 2 weeks due to sepsis.
Radiological findings
Angiographic abnormalities were noted in 46 (47.4%) patients. Internal carotid artery occlusion was noted in 27 (58.7%) patients, middle cerebral artery occlusion in 13 (28.3%) patients; and vertebrobasilar artery occlusion in 6 (13%) patients. Repeat angiography was done in 4 patients postthrombolysis within 48 h. There was total recanalization in 2 patients [Figures 1 and 2].
Figure 1.
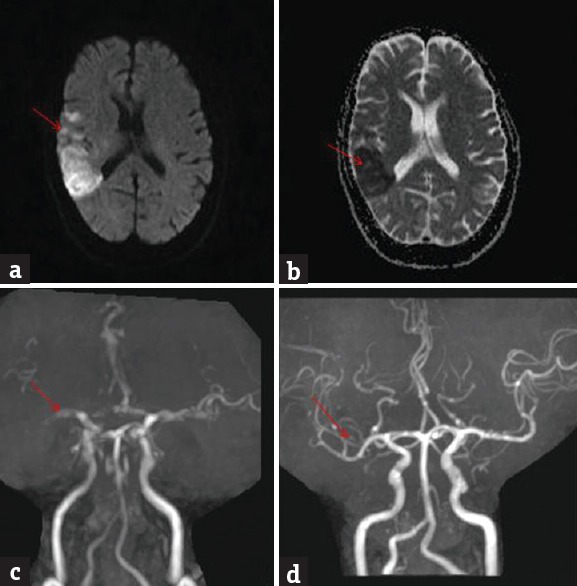
Brain magnetic resonance imaging diffusion-weighted imaging shows bright signal in the right middle cerebral artery territory (a) (red arrow); apparent diffusion coefficient shows corresponding dark signal (b) (red arrow); magnetic resonance angiography at admission shows nonvisualization of M2 (c) (red arrow); magnetic resonance angiography at 48 h postthrombolysis shows recanalization (d) (red arrow)
Figure 2.
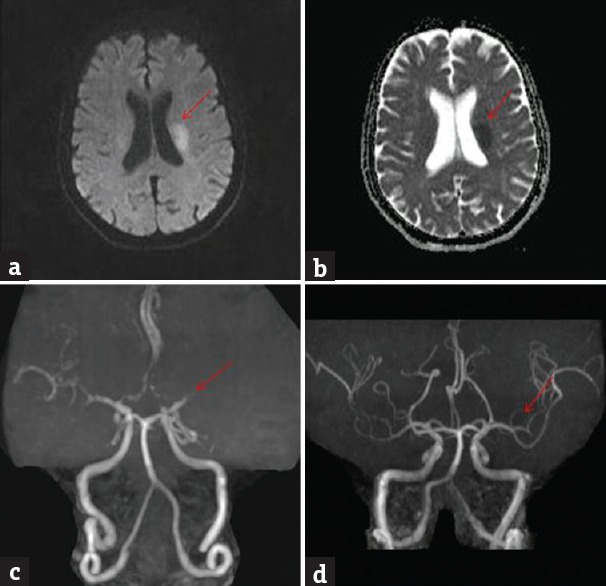
Brain magnetic resonance imaging diffusion-weighted imaging shows bright signal in the left middle cerebral artery territory (a) (red arrow); apparent diffusion coefficient shows corresponding dark signal (b) (red arrow); magnetic resonance angiography at admission shows nonvisualization of M2 (c) (red arrow); magnetic resonance angiography at 48 h postthrombolysis shows recanalization (d) (red arrow)
Predictive factors of outcome
Factors predicting favorable outcome were age <65 years (P = 0.02), NIHSS <15 (P < 0.001), small vessel occlusion (P = 0.006), cardioembolism (P = 0.006), and RBS <250 mg/dl (P < 0.001). Factors predicting poor outcome were DM (P < 0.001), dyslipidemia (P = 0.02), higher NIHSS score at admission (P < 0.001), NIHSS >15 (P < 0.001), higher RBS at admission (P < 0.001), RBS more than 250 mg/dl (P < 0.001), higher diastolic blood pressure (P = 0.04), higher DRAGON score (P < 0.001), DRAGON score >5 (P < 0.001), higher glycated hemoglobin level (P < 0.001), elevated serum homocysteine level (P = 0.004), large artery occlusion (P < 0.001), asymptomatic hemorrhage (P < 0.001), and symptomatic hemorrhage (P = 0.02). The predictive factors of outcome following IVT have been summarized in Table 3. On multivariate analysis, following factors were significant predicting poor outcome: DM (P = 0.01), dyslipidemia (P = 0.01), NIHSS at admission >15 (P = 0.03), RBS >250 mg/dl (P = 0.01), DRAGON score >5 (P = 0.03), and occlusion of large artery (P = 0.02).
Table 3.
Predictive factors of outcome after intravenous thrombolysis
DISCUSSION
Occlusion of cerebral artery cause critical reduction in cerebral perfusion pressure within minutes that results in central infarct core with irreversibly damaged cerebral tissue and surrounding hypoperfused ischemic penumbra with still viable cerebral tissue. The role of thrombolytics is to salvage the ischemic penumbra by restoring the blood supply. The present study was aimed at analyzing the data of 97 patients who underwent IVT which was collected over a 4-year period. We found a favorable outcome at 3-month follow-up in 67% patients. Favorable outcome was seen in 39% patients in the NINDS trial.[1] According to the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST), 54% patients had a good outcome, and study by Litwin et al., 61% patients had favorable outcomes.[10,11] The rate of symptomatic hemorrhage in our study was % as compared to 6.4% in the NINDS study and 3.3% in the Standard Treatment with Alteplase to Reverse Stroke study.[12]
We found that patients with age <65 years had favorable outcome following IVT. However, age more than 65 years did not predict poor outcome either. A meta-analysis of individual patient data from 6756 patients in nine randomized trials comparing alteplase with placebo or open control showed that the proportional benefits were similar for patients aged older than 80 years compared with younger patients.[13] In the study by Caso et al., age was not the independent risk factor for adverse outcome.[14] However, study by Mouradian et al., reported that older patients with more severe baseline stroke had lesser benefit with IVT as compared to younger patients.[15]
The previous reports have shown that women with AIS on conservative treatment have worse functional outcomes as compared with men.[16] A systematic review on the outcome of intravenous rt-PA according to gender by Meseguer et al. showed no evidence of gender differences in outcomes after IV rt-PA therapy.[17] We also found no gender differences in the outcome following IVT.
We found that the patients with cardioembolic stroke and small vessel occlusion had favorable outcome. However, patients with large artery occlusion had poor outcome following IVT. Favorable outcome of cardioembolic stroke post IVT may be related to fresh blood clots that get easily dissolved by thrombolytic agents rather than old blood clots. A study by Molina et al., reported that the rate of complete recanalization at 6 h was higher (P = 0.006) in patients with cardioaortic embolic (CE) stroke compared to those with other stroke subtypes.[18] Another study by Prefasi et al., (2014) reported that CE infarctions might benefit more from IV rt-PA than other etiology.[19] A study by the Helsinki Stroke Thrombolysis Registry Group found that patients with small-vessel disease strokes were likely to have a good outcome.[20] A study by Pan et al. found that patients with small-vessel disease strokes had a good outcome.[21] Favorable outcome in small-vessel disease may be attributed to younger age and milder stroke severity.[22] The occurrence of atheroma and embolism apart from lipohyalinosis alone and perfusion defects on magnetic resonance imaging perfusion scan has been attributed to the beneficiality of thrombolysis in lacunar infarcts.[23,24] There is chance of worsening in patients with lacunar infarcts even though they have milder stroke severity. Early neurological deterioration has been reported to occur in 20–30% patients with lacunar infarcts.[25] A study by Caso et al. reported that patients with carotid artery occlusion had poor outcome.[14]
The onset to treatment time (OTT) is critical to the success of IVT treatment. Earlier the institution of rt-PA better is the outcome as evidenced by several large trials. About two-thirds of patients in our study received rt-PA within 180 min of onset of symptoms. This can explain the higher rate of favorable outcome (67%) in our study. The NIHSS score on admission has been shown to be directly related to patients’ outcomes. We found that patients with NIHSS score <15 had a favorable outcome. A study by Demchuk et al. found that milder baseline stroke severity was an independent predictor of favorable outcome following IVT.[26] A study by Pan et al. found that patients with lower NIHSS score at admission had a good outcome.[21]
Dyslipidemia has also been reported to affect the outcome of patients after receiving IV rt-PA. This is due to the formation of nondissolvable lipid-rich thrombus, which could in turn cause larger infarction and hemorrhagic transformation.[27] We found that patients with dyslipidemia had a poor outcome. A study by Bruno et al. showed that with increasing admission blood glucose and admission mean blood pressure, the odds for favorable outcome progressively decreased.[28] In our study, patients who had poor outcome had significantly higher diastolic blood pressure. We found significantly elevated serum homocysteine level in patients who had poor outcome. A study by Zhong et al. found that high plasma homocysteine level was associated with increased risk of major disability in women but not in men.[29] Hyperglycemia at the onset of acute stroke may adversely affect the outcome. We found that patients who are diabetic and RBS of more than 250 mg/dl had a poor outcome. DM is also associated with reduced fibrinolysis capacity and increased the concentration of serum plasminogen activator inhibitor-1.[30] Patients with DM had less chance of improvement, lower rate of functional independency, and a higher rate of mortality as per SITS-MOST.[10]
The DRAGON score predicts functional outcome in the hyperacute phase of IVT treatment of ischemic stroke patients.[6] The score consists of 6 parameters with a maximum score of 10 that includes: dense cerebral artery sign or early infarct signs on admission CT head scan, age, glucose level on admission, OTT, NIHSS on admission. In our study, patients with DRAGON score of more than 5 had poor outcome.
The limitations of the study are that the sample size was small and was a single-center experience. We did not do repeat angiography in a large number of patients post thrombolysis in whom there were angiographic abnormalities.
CONCLUSION
Intravenous rt-PA has been found to be useful in treatment in AIS within 4.5 h of symptom onset. However, all patients with AIS do not benefit from IV rt-PA. There is need to identify subgroups of patients who will benefit from rt-PA, especially in resource-poor settings. Milder baseline stroke severity, blood glucose <250 mg/dL, younger patients (<65 years), cardioembolic stroke, and small vessel occlusion benefit from rt-PA. The presence of hyperglycemia (RBS >250 mg/dl), dyslipidemia, severe baseline stroke severity, higher DRAGON score, and large artery occlusion have poor outcome with IV rt-PA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira R, et al. SOLITAIRE™ with the intention for thrombectomy (SWIFT) trial: Design of a randomized, controlled, multicenter study comparing the SOLITAIRE™ Flow Restoration device and the MERCI Retriever in acute ischaemic stroke. Int J Stroke. 2014;9:658–68. doi: 10.1111/j.1747-4949.2012.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 5.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–8. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strbian D, Meretoja A, Ahlhelm FJ, Pitkäniemi J, Lyrer P, Kaste M, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: The DRAGON score. Neurology. 2012;78:427–32. doi: 10.1212/WNL.0b013e318245d2a9. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–2. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 10.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): An observational study. Lancet. 2007;369:275–82. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 11.Litwin T, Kobayashi A, Skowronska M, Czlonkowska A. Thrombolysis in acute ischaemic stroke within 3 hours of symptom onset: A report of the first 100 cases. Neurol Neurochir Pol. 2008;42:1–5. [PubMed] [Google Scholar]
- 12.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: The standard treatment with alteplase to reverse stroke (STARS) study. JAMA. 2000;283:1145–50. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 13.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caso V, Paciaroni M, Venti M, Palmerini F, Silvestrelli G, Milia P, et al. Determinants of outcome in patients eligible for thrombolysis for ischemic stroke. Vasc Health Risk Manag. 2007;3:749–54. [PMC free article] [PubMed] [Google Scholar]
- 15.Mouradian MS, Senthilselvan A, Jickling G, McCombe JA, Emery DJ, Dean N, et al. Intravenous rt-PA for acute stroke: Comparing its effectiveness in younger and older patients. J Neurol Neurosurg Psychiatry. 2005;76:1234–7. doi: 10.1136/jnnp.2004.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A International Stroke Trial Collaborative Group. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–8. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 17.Meseguer E, Mazighi M, Labreuche J, Arnaiz C, Cabrejo L, Slaoui T, et al. Outcomes of intravenous recombinant tissue plasminogen activator therapy according to gender: A clinical registry study and systematic review. Stroke. 2009;40:2104–10. doi: 10.1161/STROKEAHA.108.546325. [DOI] [PubMed] [Google Scholar]
- 18.Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke. 2004;35:486–90. doi: 10.1161/01.STR.0000110219.67054.BF. [DOI] [PubMed] [Google Scholar]
- 19.Prefasi D, Fuentes B, Martínez-Sánchez P, Rodríguez-Sanz A, Ruiz-Ares G, Sanz-Cuesta B, et al. Intravenous thrombolysis in stroke patients under 55 years of age: Is there a different effect according to etiology and severity? J Thromb Thrombolysis. 2014;37:557–64. doi: 10.1007/s11239-013-0984-y. [DOI] [PubMed] [Google Scholar]
- 20.Mustanoja S, Meretoja A, Putaala J, Viitanen V, Curtze S, Atula S, et al. Outcome by stroke etiology in patients receiving thrombolytic treatment: Descriptive subtype analysis. Stroke. 2011;42:102–6. doi: 10.1161/STROKEAHA.110.597534. [DOI] [PubMed] [Google Scholar]
- 21.Pan YT, Lee JD, Lin YH, Huang YC, Weng HH, Lee M, et al. Comparisons of outcomes in stroke subtypes after intravenous thrombolysis. Springerplus. 2016;5:47. doi: 10.1186/s40064-016-1666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fluri F, Hatz F, Rutgers MP, Georgiadis D, Sekoranja L, Schwegler G, et al. Intravenous thrombolysis in patients with stroke attributable to small artery occlusion. Eur J Neurol. 2010;17:1054–60. doi: 10.1111/j.1468-1331.2010.02961.x. [DOI] [PubMed] [Google Scholar]
- 23.Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke. 2015;46:687–91. doi: 10.1161/STROKEAHA.114.007466. [DOI] [PubMed] [Google Scholar]
- 24.Poppe AY, Coutts SB, Kosior J, Hill MD, O’Reilly CM, Demchuk AM. Normal magnetic resonance perfusion-weighted imaging in lacunar infarcts predicts a low risk of early deterioration. Cerebrovasc Dis. 2009;28:151–6. doi: 10.1159/000225908. [DOI] [PubMed] [Google Scholar]
- 25.Hwang YH, Seo JG, Lee HW, Park SP, Suh CK. Early neurological deterioration following intravenous recombinant tissue plasminogen activator therapy in patients with acute lacunar stroke. Cerebrovasc Dis. 2008;26:355–9. doi: 10.1159/000151638. [DOI] [PubMed] [Google Scholar]
- 26.Demchuk AM, Tanne D, Hill MD, Kasner SE, Hanson S, Grond M, et al. Predictors of good outcome after intravenous tPA for acute ischemic stroke. Neurology. 2001;57:474–80. doi: 10.1212/wnl.57.3.474. [DOI] [PubMed] [Google Scholar]
- 27.Urbach H, Hartmann A, Pohl C, Omran H, Wilhelm K, Flacke S, et al. Local intra-arterial thrombolysis in the carotid territory: Does recanalization depend on the thromboembolus type? Neuroradiology. 2002;44:695–9. doi: 10.1007/s00234-002-0762-6. [DOI] [PubMed] [Google Scholar]
- 28.Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669–74. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 29.Zhong C, Xu T, Xu T, Peng Y, Wang A, Wang J, et al. Plasma Homocysteine and prognosis of acute ischemic stroke: A gender-specific analysis from CATIS randomized clinical trial. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9799-0. doi:10.1007/s12035-016-9799-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Trost S, Pratley R, Sobel B. Impaired fibrinolysis and risk for cardiovascular disease in the metabolic syndrome and type 2 diabetes. Curr Diab Rep. 2006;6:47–54. doi: 10.1007/s11892-006-0052-5. [DOI] [PubMed] [Google Scholar]


