Abstract
Introduction:
The optimal ratio of platelets (PLTs) to packed red blood cell (PRBC) in trauma patients requiring massive transfusion protocol (MTP) is still controversial. This report aims to describe the effect of attaining a high PLT:PRBC ratio (≥1:1.5) within 4 h postinjury on the outcomes of trauma patients receiving MTP.
Methods:
Over a 24-month period, records of all adult patients with traumatic injury who received MTP were retrospectively reviewed. Data were analyzed with respect to PLT:PRBC ratio ([high-MTP ≥1:1.5] [HMTP] vs. [low-MTP <1:1.5] [LMTP]) given within the first 4 h postinjury and also between (>4 and 24 h). Baseline demographic, clinical characteristics, complications, and outcomes were compared according to HMTP and LMTP.
Results:
Of the total 3244 trauma patients, PLT:PRBC ratio was attainable in 58 (1.2%) patients who fulfilled the inclusion criteria. The mean age was 32.3 ± 10.7 years; the majority were males (89.6%) with high mean Injury Severity Score (ISS): 31.9 ± 11.5 and Revise Trauma Score (RTS): 5.1 ± 2.2. There was no significant association between age, gender, type of injury, presenting hemoglobin, International Normalized Ratio, ISS, and RTS. The rate of ventilator–associated pneumonia (38.9% vs. 10.8%; P = 0.02) and wound infection (50% vs. 10.8%; P = 0.002) were significantly higher in the HMTP group. However, HMTP was associated with lower rate of multiple organ failure (MOF) (42.1% vs. 87.2%, P = 0.001) and mortality (36.8% vs. 84.6%, P = 0.001) within the first 30 days postinjury.
Conclusions:
Our study revealed that early attainment of high PLT/PRBC ratio within 4 h postinjury is significantly associated with lower MOF and mortality in trauma patients.
Key Words: Complications, mortality, platelet, packed red blood cell, transfusion ratio, trauma
INTRODUCTION
Regardless of gender, race, or economic status, trauma remains a leading cause of death worldwide. Most deaths occur early after hospital admission and are mainly caused by early exsanguinating hemorrhage.[1] This potentially preventable cause of mortality is compounded by a distinct “coagulopathy of trauma” that sets in the early stage of injury.[2,3] Patients with posttraumatic coagulopathy have a 3–4–fold higher mortality in comparison to those who have normal coagulation process and are up to eight times more likely to die on the 1st day posttrauma.[3] This coagulopathy complicates resuscitation and is the target of massive transfusion protocols (MTP) consisting of multiple blood products. Recent military and civilian experience has brought about many shifts in the principles and practice of early trauma resuscitation.[4,5] Prioritizing aggressive blood component therapy has replaced long–institutionalized, crystalloid–dependent fluid resuscitation. There is an increased emphasis on the early and liberal use of balanced blood product ratio, including packed red blood cell (PRBC), fresh–frozen plasma, and platelets (PLT). Despite the widespread implementation of MTPs for trauma, there is still considerable controversy regarding the optimal ratio of PLT:PRBC in trauma patients requiring MTP. This growing debate has evolved to include the time at which the best ratio is achieved. The attainment of high resuscitation ratio as early as 4 h postinjury and their impact on patient outcomes have not been adequately analyzed to date. This report aims to describe the effect of attaining a high PLT:PRBC ratio (≥1:1.5) within 4 h postinjury on the outcomes of trauma patients receiving MTP.
METHODS
All trauma patients who received blood transfusion were retrospectively identified from the blood bank database, and their detailed records were reviewed from the Trauma Registry of the Hamad Trauma Center, Hospital, the National Level I Trauma Referral Center in Qatar between January 2010 and December 2011. All adult (≥18 years) traumatic injury patients who received massive blood transfusion were included in the study. All patients who died on arrival were excluded from the study. Blood bank records were used to identify patients who received >6 units PRBC and particularly screened patients who received >10 units PRBC in the first 24 h of injury. We defined massive transfusion as the infusion of ≥10 units of PRBC over the initial 24 h postinjury.[6] Patient's data regarding demographics, mechanism of injury, initial vitals, laboratory findings, Injury Severity Score (ISS), Revise Trauma Score (RTS), Glasgow Coma Score, hospital length of stay, complications, and outcomes were collected from the trauma registry database. Based on PLT:PRBC ratio, patients who received at least a PLT:PRBC ratio of ≥1:1.5 within 4 h from the time of injury was categorized into the high ratio group (HMTP). Those who received a PLT:PRBC ratio of ≤1:1.5 within 4 h postinjury represented the low ratio group (LMTP). The ratio was then calculated based on the blood products given from 4 to 24 h postinjury. One MTP shipment was defined as the infusion of ≥6 units of PRBC, ≥6 units of plasma, and ≥6 units of PLTs. Patients were followed up to 30-day post injury, hospital discharge, or death; whichever occurred first.
Outcomes measured included overall mortality, postshipment International Normalized Ratio (INR), multiple organ failure (MOF), and infectious complications. The diagnosis of MOF required a maximum Marshall multiple organ dysfunction score >5. The diagnosis of ventilator–associated pneumonia (VAP) was based on quantitative culture threshold of ≥104 CFU/mL for bronchoalveolar lavage specimens. Catheter–related blood stream infections were confirmed by positive peripheral cultures with the identical organism obtained from either a positive semi–quantitative culture (≥15 CFU/segment) or positive quantitative culture (≥103 CFU/segment) from a catheter segment specimen. The diagnosis of urinary tract infection was confirmed if there was ≥105 organisms/ml in the urine specimens.[7] This study was approved by the medical research center at Hamad Medical Corporation, Qatar (IRB #11153/11).
Data were presented as proportions, mean ± standard deviation, or median as appropriate. Baseline demographic, clinical characteristics, laboratory findings, and outcomes were compared according to PLT:PRBC ratio at 4 h (HMTP vs. LMTP) using Chi–square for categorical variables and Student's t–test for continuous variables. The Fisher's exact test was used if the expected cell frequencies were below 5. A two–sided P < 0.05 was considered to be statistically significant. Data analysis was carried out using the Statistical Package for Social Sciences version 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 3244 trauma admissions were admitted during the 2-year study period, of which 100 (2.06%) patients met inclusion criteria. Of these, PLT:PRBC ratio was attainable in 58 patients as the remaining 42 patients did not receive PLTs within the first 4 h postinjury. The mean age of the patients was 32.3 ± 10.7 years; majority were males (89.6%) who sustained severe injuries with a high mean ISS of 31.9 ± 11.5 and RTS of 5.1 ± 2.2. Blunt trauma accounted for most cases (84.5%). The initial vital signs and laboratory findings are shown in Table 1.
Table 1.
Demographic, epidemiologic, and clinical presentation of massive transfusion protocol patients who received platelet transfusion (n=58)
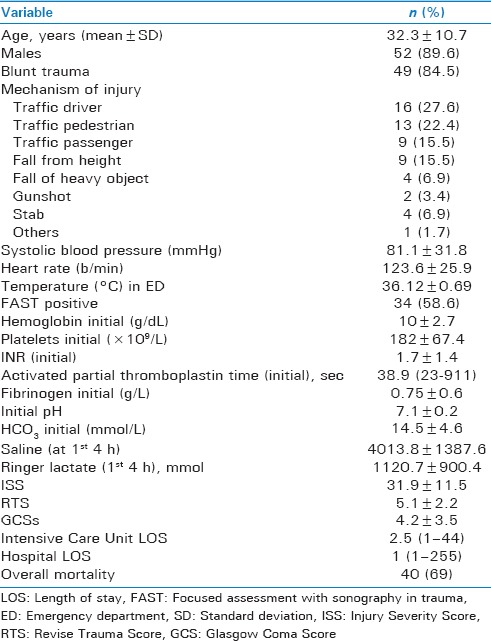
Table 2 demonstrated the baseline comparison of clinical characteristics and injury severity according to PLT:PRBC ratio (HMTP vs. LMTP) within 4 h postinjury. Both the groups were comparable for age, gender, type of injury, presenting hemoglobin level, INR, ISS, and RTS except initial pH. Table 3 shows the comparison of complications and outcomes of patients based on HMTP and LMTP within the first 4 h. No significant differences were noted for the incidence of hyperkalemia, hypomagnesemia, hypocalcemia, abdominal compartment syndrome, blood stream, catheter–related, and urinary tract infections between the two groups. The frequency of MOF was significantly lower in the HMTP group (42.1% vs. 87.2%, P = 0.001). However, the rate of VAP (38.9% vs. 10.8%; P = 0.02) and wound infection (50% vs. 10.8%; P = 0.002) were significantly higher in the HMTP group. The overall mortality was 69%, and the number of deaths was significantly lower in HMTP (36.8%, vs. 84.6% P = 0.001) in comparison to LMTP within the first 30 days.
Table 2.
Baseline comparison of clinical characteristics and injury severity by platelet to packed red blood cell ratio within 4 h postinjury
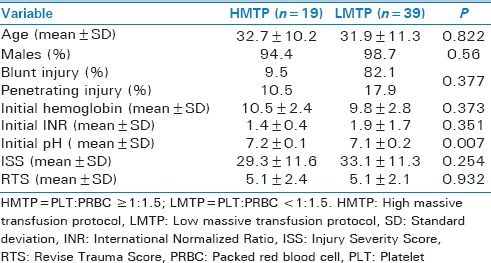
Table 3.
Comparison of complications and clinical outcomes by platelet to packed red blood cell ratio within first 4 h postinjury
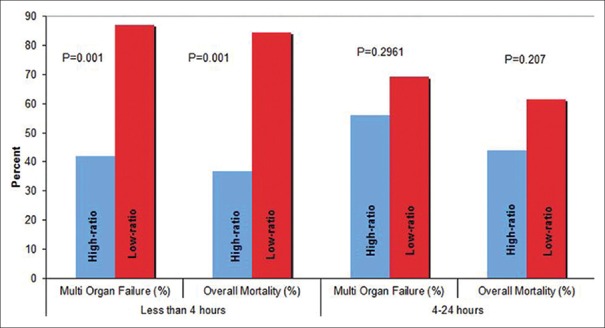
Similarly, in the later period (4–24 h), the frequency of MOF (69.2% vs. 56.0%; P = 0.29) and overall mortality (44% vs. 61.5%, P = 0. 0.207) was nonsignificantly lower in HMTP group as compared to LMTP [Figure 1]. Moreover, no significant difference was observed for the incidence of VAP, wound infection, blood stream infection, catheter–related, or urinary tract infections between the two groups in the later period (4–24 h).
Figure 1.
Mortality and multiple organ failure rates by time and platelet: Packed red blood cell (PBBC) ratio
DISCUSSION
Our study demonstrates the significance of obtaining a high PLT:PRBC ratio early in the resuscitation phase. At the beginning of the 80s, Kashuk et al. had proposed the formation of the lethal triad of coagulopathy, hypothermia, and acidosis secondary to bleeding, cellular shock, and tissue injury.[8] Hypothermia and acidosis are relatively easier to address. Coagulopathy post traumatic injury was once regarded as a secondary outcome, due to dilution and consumption, but now it is recognized as a primary consequence of trauma.[2] About a third of severely injured patients are coagulopathic on arrival.[2] Abnormal coagulation noticed in the exsanguinating trauma victims is often multifactorial in nature with hemodilution, consumption of coagulation factors, activation of inflammatory cascade, hyperfibrinolysis, and PLT dysfunction secondary to acidosis/hypothermia identified as causative or contributory factors.[9,10]
The innate role of PLTs in primary hemostasis is well understood. In addition, activated PLTs promote secondary hemostasis by offering an excellent catalytic surface for the aggregation of enzyme complexes required for optimizing coagulation.[11] The resulting coagulation product is a potent PLT activating agonist. Thus, PLT activation and blood coagulation are mutually dependent processes.
Most guidelines that indicate the use of PLTs in massively bleeding trauma patients are count based. Thrombocytopenia witnessed in massively transfused patients is often misleading as both consumptive factors and dilution can contribute to the same. PLT dysfunction, which is more critical than PLT count, is unaccountable due to lack of a diagnostic test. Despite recent studies that cite the role of point-of-care testing in titrating hemostatic resuscitation efforts,[12] these practical difficulties have led to the continued use of a structured blood product ratio in the form of MTPs. Current literature favors early and aggressive PLT use to attain PLT:PRBC transfusion ratio as high as 1:1. However, early attainment of such high ratio in clinical practice is challenging as evidenced from civilian data. Both military and civilian experience of late have supported the use of higher PLT:PRBC transfusion ratio to improve survival rates in these patients.[4,5] There currently exists no consensus on what constitutes a relevant cutoff between an adequately high and low PLT ratio. Table 4 compares a few recent studies and their outcomes with varied PLT:PRBC cutoff ratio.[13,14,15,16,17,18,19,20]
Table 4.
Comparative studies
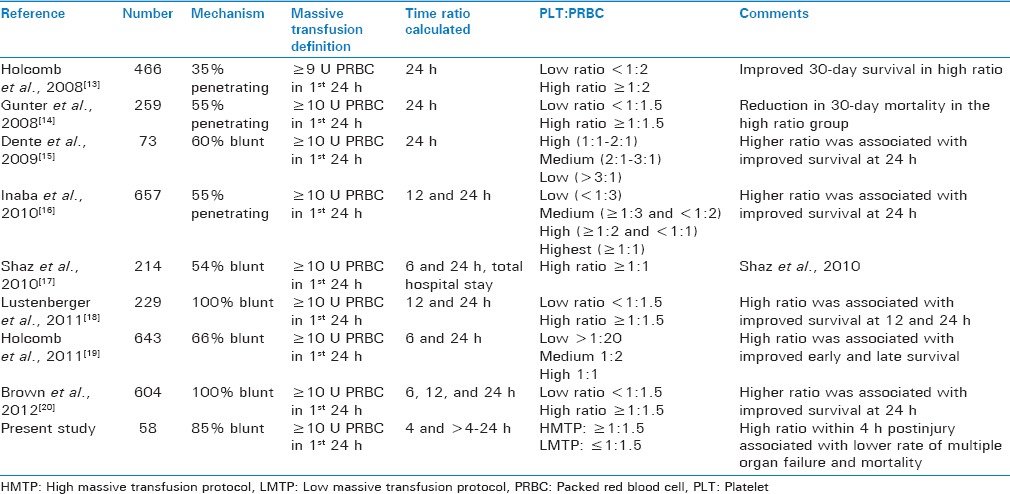
Our study demonstrated a significant improvement in overall mortality and MOF in patients received HMTP within the initial 4 h [Figure 1]. A similar survival benefit has been documented in other studies that have tested high PLT:PRBC ratio.[13,14,15,16,17,18,19,20] However, attainment of this ratio within the first 4 h postinjury and their impact on patient outcome have not been analyzed adequately to date. In our series, the significant improvement in mortality and MOF rates in HMTP group during the early period (<4 h) was not observed during the later period (4–24 h). Hence, we believe the survival benefit of attaining HMTP ratio, documented in other studies, when the ratio was calculated at 24 h is possibly secondary to its “early” rather than “late” attainment. Regardless of how this “early” high PLT:PRBC transfusion ratio is achieved, its survival benefit is noteworthy and a call for a rigidly attained and implemented MTP that can ensure the timely availability and transfusion of these components that are associated with increased survival in trauma patients is in need of MTP.
Usually, earlier studies have categorized patients into high and low PLT:PRBC ratio groups based on the blood products consumed with initial 24 h [Table 4]. The PROMMTT study[21] conceded 60% of hemorrhagic deaths to have occurred within 3 h of admission. Dutton et al.[22] revealed that 80% of those who will bleed to death have done so within 6 h of admission. Thus, 24 h ratio inadequately accounts for and addresses the underlying cause of early deaths. Snyder et al.[23] first raised the question of survival bias in this subgroup of patients as opposed to a survival benefit with a high transfusion ratio. Our meticulous review of records supplemented by blood bank and trauma registry data enabled an hourly blood product transfusion data, describing exactly when PRBCs and PLTs were infused. We were able to account for early deaths by calculating blood product ratio as early as 4 h, whereby reducing possibilities for survival bias.
In the present study, both HMTP and LMTP groups were well matched in terms of initial coagulopathy, injury severity, and demographic characteristics. We also noted progressive improvement in coagulopathy in the HMTP group. These results add to our current understanding of the benefits associated with aggressive PLT use to prevent and treat early onset coagulopathy after injury. The patients in HMTP group had a higher wound infection and pneumonia rates. These could be explained by the potential for a contributory immunodeficiency from this type of resuscitative practice.[24]
Our study is limited to a retrospective analysis of major transfusion practices for an adult and predominantly blunt trauma population from a single center. However, The Hamad Trauma Center is the National Trauma Referral Center in Qatar; as such this is a nationally representative sample of severely injured patients who are the beneficiaries of a single system and consistent standard of trauma care. Small sample size and power are another limitations of our study as it is a single–center study. We also lack information for the management details for coagulopathy. Most studies on massive transfusion ratio have encountered survival bias. We have minimized the confounding effect from this bias by calculating blood product ratio at 4 h, unlike other studies that have calculated ratio at 24 h.
CONCLUSION
The mortality risk associated with LMTP (PLT:PRBC <1:1.5) may occur very early, possibly secondary to ongoing coagulopathy, PLT dysfunction, and hemorrhage. Aggressive attainment of high PLT:PRBC ratio as early as 4 h postinjury can substantially improve coagulopathy and reduce mortality and MOF rates but increases the rate of infectious complications. These results suggest that the survival advantage of HMTP for trauma patients is realized early. We believe that there is a need to conduct a large prospective multi–institutional study to further confirm the significance of attainment of high PLT ratio and improved survival of trauma patients targeted within the first 4 h postinjury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank the entire registry database team in the section of trauma surgery and staff of blood bank. All authors read the manuscript and approved it and had no financial issues to disclose. Medical Research Center (IRB# 11153/11) at Hamad Medical Corporation in Qatar has approved the study.
REFERENCES
- 1.Peng R, Chang C, Gilmore D, Bongard F. Epidemiology of immediate and early trauma deaths at an urban level I trauma center. Am Surg. 1998;64:950–4. [PubMed] [Google Scholar]
- 2.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 3.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–63. doi: 10.1097/TA.0b013e318174e8bc. [DOI] [PubMed] [Google Scholar]
- 4.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 5.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, et al. An FFP: PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 6.Peralta R, Vijay A, El-Menyar A, Consunji R, Abdelrahman H, Parchani A, et al. Trauma resuscitation requiring massive transfusion: A descriptive analysis of the role of ratio and time. World J Emerg Surg. 2015;10:36. doi: 10.1186/s13017-015-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minei JP, Nathens AB, West M, Harbrecht BG, Moore EE, Shapiro MB, et al. Inflammation and the host response to injury, a large–scale collaborative project: Patient–oriented research core – Standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator–associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–13. doi: 10.1097/01.ta.0000220424.34835.f1. [DOI] [PubMed] [Google Scholar]
- 8.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma – A unified approach. J Trauma. 1982;22:672–9. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32:659–65. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: Its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–64. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 11.Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–93. [PubMed] [Google Scholar]
- 12.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed hemostatic resuscitation of trauma–induced coagulopathy: A pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 14.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: Identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–34. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 15.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–24. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 16.Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PG, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010;210:957–65. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50:493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 18.Lustenberger T, Frischknecht A, Brüesch M, Keel MJ. Blood component ratios in massively transfused, blunt trauma patients – A time–dependent covariate analysis. J Trauma. 2011;71:1144–50. doi: 10.1097/TA.0b013e318230e89b. [DOI] [PubMed] [Google Scholar]
- 19.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, et al. Increased platelet: RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–28. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 20.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, et al. Debunking the survival bias myth: Characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73:358–64. doi: 10.1097/TA.0b013e31825889ba. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: Comparative effectiveness of a time–varying treatment with competing risks. JAMA Surg. 2013;148:127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutton RP, Shih D, Edelman BB, Hess J, Scalea TM. Safety of uncrossmatched type–O red cells for resuscitation from hemorrhagic shock. J Trauma. 2005;59:1445–9. doi: 10.1097/01.ta.0000198373.97217.94. [DOI] [PubMed] [Google Scholar]
- 23.Snyder CW, Weinberg JA, McGwin G, Jr, Melton SM, George RL, Reiff DA, et al. The relationship of blood product ratio to mortality: Survival benefit or survival bias? J Trauma. 2009;66:358–62. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 24.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]