Abstract
Introduction:
As pulmonary artery catheter (PAC) use declines, search continues for reliable and readily accessible minimally invasive hemodynamic monitoring alternatives. Although the correlation between inferior vena cava collapsibility index (IVC-CI) and central venous pressures (CVP) has been described previously, little information exists regarding the relationship between IVC-CI and pulmonary artery pressures (PAPs). The goal of this study is to bridge this important knowledge gap. We hypothesized that there would be an inverse correlation between IVC-CI and PAPs.
Methods:
A post hoc analysis of prospectively collected hemodynamic data was performed, examining correlations between IVC-CI and PAPs in a convenience sample of adult Surgical Intensive Care Unit patients. Concurrent measurements of IVC-CI and pulmonary arterial systolic (PAS), pulmonary arterial diastolic (PAD), and pulmonary arterial mean (PAM) pressures were performed. IVC-CI was calculated as ([IVCmax − IVCmin]/IVCmax) × 100%. Vena cava measurements were obtained by ultrasound–credentialed providers. For the purpose of correlative analysis, PAP measurements (PAS, PAD, and PAM) were grouped by terciles while the IVC-CI spectrum was divided into thirds (<33, 33–65, ≥66).
Results:
Data from 34 patients (12 women, 22 men, with median age of 59.5 years) were analyzed. Median Acute Physiologic Assessment and Chronic Health Evaluation II score was 9. A total of 76 measurement pairs were recorded, with 57% (43/76) obtained in mechanically ventilated patients. Correlations between IVC-CI and PAS (rs = −0.334), PAD (rs = −0.305), and PAM (rs = −0.329) were poor. Correlations were higher between CVP and PAS (R2 = 0.61), PAD (R2 = 0.68), and PAM (R2 = 0.70). High IVC-CI values (≥66%) consistently correlated with measurements in the lowest PAP ranges. Across all PAP groups (PAS, PAD, and PAM), there were no differences between the mean measurement values for the lower and middle IVC-CI ranges (0%-65%). However, all three groups had significantly lower mean measurement values for the ≥66% IVC-CI group.
Conclusions:
Low PAS, PAD, and PAM measurements show a reasonable correlation with high IVC-CI (≥66%). These findings are consistent with previous descriptions of the relationship between IVC-CI and CVP. Additional research in this area is warranted to better describe the hemodynamic relationship between IVC-CI and PAPs, with the goal of further reduction in the reliance on the use of PACs.
Key Words: Comparison study, correlations, inferior vena cava collapsibility index, pulmonary artery catheter, pulmonary artery pressures
INTRODUCTION
Invasive hemodynamic monitoring approaches are associated with significant complications.[1,2] At the same time, the clinical utility of information provided by various invasive methods remains questionable at best.[3] With declining the use of indwelling intravascular monitoring devices, including the pulmonary artery catheter (PAC),[4,5] search continues for reliable, accessible, and easy-to-use minimally invasive hemodynamic monitoring alternatives.[6,7,8] Although the correlation between inferior vena cava collapsibility index (IVC-CI) and central venous pressures (CVP) has been well described,[9,10,11,12] little information exists regarding the relationship between central vein collapsibility (including pertinent subcomponent measurements) and pulmonary artery pressures (PAPs).[13,14] The aim of this study is to answer important questions regarding correlations between information traditionally provided by the PAC and the corresponding IVC-CI findings. We hypothesized that the relationship between PAPs and IVC-CI will approximate previously published results for CVP and IVC-CI.[9,10]
METHODS
We conducted a post hoc analysis of prospectively collected, single–center data. Correlations between IVC-CI and PAPs were examined using a convenience sample of Surgical Intensive Care Unit (SICU) patients at a major academic regional referral center. Included in the current subset analysis were SICU patients between ages 18 and 90 years who, independently of the current study, met criteria for invasive hemodynamic monitoring using the PAC. Excluded populations included prisoners and patients <18 years or >90 years old (standard Institutional Review Board [IRB] exclusions for our institution).
Recorded clinical data included patient demographics, illness acuity/severity (Acute Physiologic Assessment and Chronic Health Evaluation II [APACHE II] scores), clinical diagnosis associated with PAC placement, and mechanical ventilation status.
Intensivist-performed focused sonographic measurements and corresponding invasive hemodynamic parameters were obtained during weekly SICU ultrasound team rounds. Concurrent measurements of IVC-CI and pulmonary arterial systolic (PAS), pulmonary arterial diastolic (PAD), and pulmonary arterial mean (PAM) pressures were recorded within 5 min of each other. Vena cava measurements were carried out using a standardized, previously described technique.[9,10,15] IVC collapsibility was calculated for both mechanically ventilated and spontaneously breathing patients as:
IVC-CI = ([IVCmax − IVCmin]/IVCmax) × 100%.
The PAC setup utilized by the institution consists of continuous cardiac output – PAC, as previously described by our group.[16] The portable ultrasound device used to acquire IVC measurements was Sonosite M-Turbo™ (Sonosite Fuji Film, Bothell, WA, USA).
For the purpose of correlative analyses, PAP measurements (PAS, PAD, and PAM) were grouped by terciles, whereas the IVC-CI spectrum was divided into thirds (<33, 33–65, and ≥66). Categorical data were analyzed using Fisher's exact or Chi–square testing (as appropriate) while continuous data were compared using the Kruskal–Wallis test (continuous data were nonnormally distributed). No formal sample size/power analysis was performed due to the post hoc nature of this study and the limited number of patients undergoing PAC placement in the SICU. Bivariate correlations involving noncontinuous data were determined using Spearman's rho (two-tailed). For continuous data, linear regression was utilized. Statistical significance was set at α = 0.05. This study was approved by the IRB.
RESULTS
Data from 34 patients (12 women, 22 men) from noncardiac SICU were analyzed. Median patient age was 59.5 years (range, 31–89). Median APACHE II score was 9 (range, 5–28) at the time of admission to ICU. Septic shock was the most common diagnosis associated with PAC placement (29/34, 85.3%) followed by cardiogenic shock (5/34, 14.7%). Twenty–six of 34 (76.5%) patients were mechanically ventilated during clinical data acquisition. Prospective data collection took place during weekly interdisciplinary SICU ultrasound rounds between March 26, 2012 and April 15, 2013.
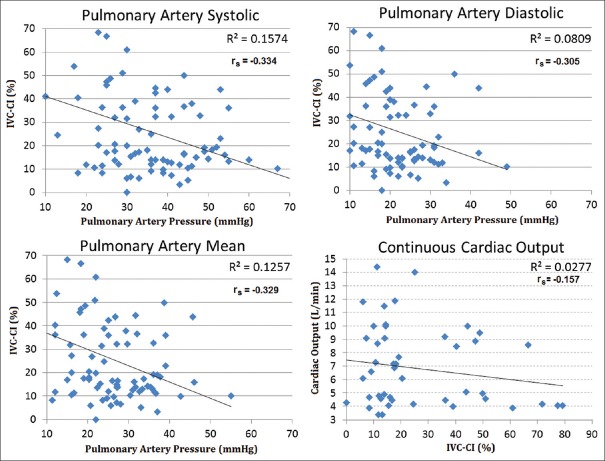
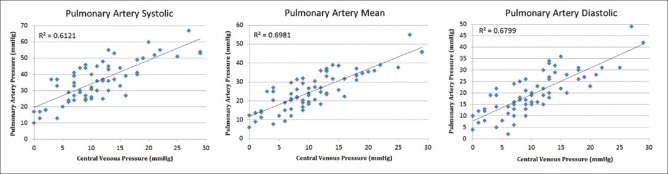
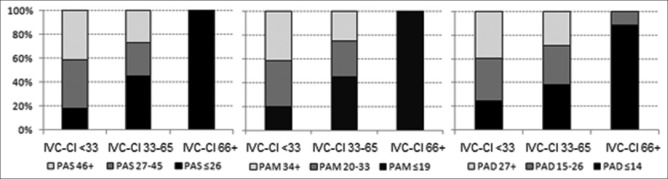
A total of 76 simultaneous “measurement sets” were recorded, with 57% (43/76) obtained in mechanically ventilated patients. Correlations between IVC-CI and PAS (rs = −0.334, P < 0.01), PAD (rs = −0.305, P < 0.01), and PAM (rs = −0.329, P < 0.01) were poor [Figure 1]. Correlations were higher between CVP and PAS (R2 = 0.61, P < 0.01), PAD (R2 = 0.68, P < 0.01), and PAM (R2 = 0.70, P < 0.01) [Figure 2]. High IVC-CI values (>66%) consistently correlated with measurements in the lowest PAP ranges [Figure 3]. In all three PAP groups (PAS, PAD, and PAM), there were no differences between the mean group pressures for the lower and middle IVC-CI ranges (0%-65%). However, all three PAP sub–groups had significantly lower mean composite pressures for the ≥66% IVC-CI segment.
Figure 1.
Correlations between pulmonary artery pressures (mmHg) and IVC-CI (%) (top left and right; bottom left); correlation between continuous cardiac output (L/min) and IVC-CI (%) (bottom right). IVC-CI: Inferior vena cava collapsibility index
Figure 2.
Correlations between pulmonary artery pressures (mmHg) and central venous pressures (mmHg)
Figure 3.
The relationship between IVC-CI and pulmonary artery pressures. Relationship between IVC-CI (<33%, 33%-65%, ≥66%) and PAS pressures (left); relationship between IVC-CI and PAM pressures (middle); relationship between IVC-CI and PAD pressures (right). IVC-CI: Inferior vena cava collapsibility index, PAM: Pulmonary arterial mean, PAS: Pulmonary arterial systolic, PAD: Pulmonary arterial diastolic
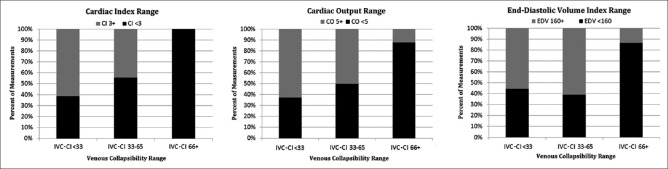
Finally, we compared IVC-CI (grouped by previously outlined ranges) against cardiac output, cardiac index, and end–diastolic volume index (EDVI). Here, “low” intravascular volume (e.g., IVC-CI ≥66%) was associated with a greater proportion of measurements indicating lower cardiac index, cardiac output, and EDVI [Figure 4]. Of note, the overall relationship between IVC-CI and cardiac output was very low (rs = −0.157, P = N/S) [Figure 1].
Figure 4.
Relationships between IVC-CI and CI and CO, respectively (left and middle); IVC-CI and EDV index (right). VCI: Venous collapsibility index, CI: Cardiac index, CO: Cardiac output, EDV: End–diastolic volume index, IVC-CI: Inferior vena cava collapsibility index
DISCUSSION
Accurate hemodynamic and volume status assessments are critical to clinical management optimization in the SICU.[17] One recent trend in critical care medicine is the abandonment of invasive hemodynamic monitoring tools, such as the PAC, in favor of minimally or noninvasive substitutes.[18,19] The rationale behind this trend is two-fold: (a) elimination of complications associated with invasive hemodynamic monitoring tools;[2,20] and (b) lack of clinical evidence supporting the use of invasive modalities outside of narrowly defined, often specialized indications.[3,21]
Despite the fact that few of the existing minimally invasive or noninvasive methods of hemodynamic and volume status assessment have the desired reliability or the ability for continuous, uninterrupted monitoring, the trend away from invasive modalities marches forward.[22] Intensivist–performed focused bedside sonographic assessment of intravascular volume status is increasing in popularity due to its relative simplicity, skill standardization, and mounting evidence of clinical utility.[9,10,12,13,22,23,24,25] Given these parallel developments, as well as the need for more evidence–based comparative research, we set out to examine the relationship between IVC-CI and PAPs in a modest sample of patients.
The primary goal of this study was to provide a frame of reference for critical care practitioners who may be familiar and comfortable with both the traditional and more contemporary methods of hemodynamic and volume status assessment. More specifically, practitioners may find it useful to be able to relate the “new and unknown” to the “old and known.” We believe that this study provides such fundamental information.
Despite poor correlations between IVC-CI and most of the PAC–derived parameters, important generalizations can be made based on our data. First, high IVC-CI (≥66%) appears to correlate well with low cardiac output/index. Same can be said about high IVC-CI as an indicator of “low” EDVI. Finally, all three of the PAP measurement categories (PAS, PAD, and PAD) show inverse stepwise correlation with increasing IVC-CI ranges. This fits well with previous observations that higher degrees of central vein collapsibility tend to be associated with hypovolemic states.[9,10] Moreover, the demonstration of an inverse relationship between IVC-CI and continuous cardiac output measurements may also prove helpful in clinical decision-making.
The sonographic approach utilized in the current investigation constitutes only a small part of the repertoire of tools available to the modern critical care practitioner. Other minimally invasive tools include arterial waveform–based monitors, impedance plethysmography devices, as well as other ultrasound–based methodologies (e.g., transesophageal echocardiography, transthoracic echocardiography, and esophageal Doppler monitors).[26,27,28,29] Thus far, none of the above tools has proven to be the panacea for the problem of accurate hemodynamic and volume status assessment.[1,30] Much like in the decades past, the practitioner is left with his or her clinical “gestalt,” a set of supporting triage mechanisms, and a number of tools that have the potential to influence the ultimate clinical decision.[31,32,33,34] Monitoring dynamic trends and changes within parameters (including appropriate cross–checking of information from various sources and repeated measurements of resuscitation endpoints) may be the only proven method of avoiding costly diagnostic and management mistakes in the ICU.[35]
Limitations of this post hoc analysis include the purely observational nature of the study precluding any standardization beyond measurement acquisition, and the inability to examine dynamic parameter responses to real–time interventions. In addition, the study was a priori defined to examine only those patients with clinical indications for PACs (and, presumably, the abnormal PAPs inherently overrepresented in such a group). The study population is, therefore, heterogeneous, and our findings do not apply to the use of IVC-CI in patients who are not critically ill or who are not suspected of having abnormal PAC measurements. Consequently, the authors stress that it is important for the reader to remember that patient outcomes depend on interventions based on hemodynamic information and not the presence of monitoring devices.[29,36] The gradual abandonment of PACs at this institution made this project especially challenging, severely limiting the sample size. In addition, the increasing emphasis on cardiac output/flow and tissue perfusion characteristics diminishes the significance of central venous and PAP measurements. Strengths of this study include the evaluation of variables rarely used in clinical medicine and their correlation to sonographic measurements of intravascular volume status that are utilized at an increasing rate across both emergency and intensive care settings. Finally, despite the intuitive nature of our findings, descriptive data in this area continue to be very limited.
CONCLUSIONS
This study found that low PAS, PAD, and PAM measurements consistently correlate with high IVC-CI (≥66%) values. In addition, elevated IVC-CI appears to be linked to greater overall proportion of associated low cardiac output measurements. These findings corroborate previous descriptions of the relationship between IVC-CI and intravascular volume status. The association between high IVC-CI and “low cardiac output” provides potentially important hemodynamic information to the clinician who desires a rapid noninvasive test for low cardiac output in the setting of hypovolemia. Further research in this area is needed to better define the relationship between IVC-CI and PAP, with the goal of continued reduction in the use of pulmonary artery catheters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge the generous help of Creagh T. Boulger, Eric J. Adkins, Daniel S. Eiferman, David E. Lindsey, Chinedu Njoku, Charles H. Cook, and Steven M. Steinberg in providing logistical support, collecting clinical measurements, and assistance with data interpretation.
REFERENCES
- 1.Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, et al. Clinical review: Update on hemodynamic monitoring – A consensus of 16. Crit Care. 2011;15:229. doi: 10.1186/cc10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DC, Doraiswamy VA, Prosciak MP, Silviera M, Seamon MJ, Rodriguez Funes V, et al. Complications associated with pulmonary artery catheters: A comprehensive clinical review. Scand J Surg. 2009;98:199–208. doi: 10.1177/145749690909800402. [DOI] [PubMed] [Google Scholar]
- 3.Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomised controlled trial. Lancet. 2005;366:472–7. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 4.Gershengorn HB, Wunsch H. Understanding changes in established practice: Pulmonary artery catheter use in critically ill patients. Crit Care Med. 2013;41:2667–76. doi: 10.1097/CCM.0b013e318298a41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidwani UK, Mohanty B, Chatterjee K. The pulmonary artery catheter: A critical reappraisal. Cardiol Clin. 2013;31:545–65, viii. doi: 10.1016/j.ccl.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Malbrain M, De Potter T, Deeren D. Yearbook of Intensive Care and Emergency Medicine 2005. New York: Springer; 2005. Cost-effectiveness of minimally invasive hemodynamic monitoring; pp. 603–31. [Google Scholar]
- 7.Porhomayon J, Zadeii G, Congello S, Nader ND. Applications of minimally invasive cardiac output monitors. Int J Emerg Med. 2012;5:18. doi: 10.1186/1865-1380-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benes J, Kasal E. Annual Update in Intensive Care and Emergency Medicine 2015. New York: Springer; 2015. New fully non–invasive hemodynamic monitoring technologies: Groovy or paltry tools; pp. 249–58. [Google Scholar]
- 9.Stawicki SP, Adkins EJ, Eiferman DS, Evans DC, Ali NA, Njoku C, et al. Prospective evaluation of intravascular volume status in critically ill patients: Does inferior vena cava collapsibility correlate with central venous pressure? J Trauma Acute Care Surg. 2014;76:956–63. doi: 10.1097/TA.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 10.Stawicki SP, Braslow BM, Panebianco NL, Kirkpatrick JN, Gracias VH, Hayden GE, et al. Intensivist use of hand–carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: Correlations with CVP. J Am Coll Surg. 2009;209:55–61. doi: 10.1016/j.jamcollsurg.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 11.Stawicki S, Bahner D. Evidence tables: Inferior vena cava collapsibility index (IVCCI) OPUS 12 Scientist. 2012;6:3–5. [Google Scholar]
- 12.Stawicki SP, Kent A, Patil P, Jones C, Stoltzfus JC, Vira A, et al. Dynamic behavior of venous collapsibility and central venous pressure during standardized crystalloid bolus: A prospective, observational, pilot study. Int J Crit Illn Inj Sci. 2015;5:80–4. doi: 10.4103/2229-5151.158392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stawicki SP, Panebianco NL, Kirkpatrick J, Gracias VH, Hayden G, Dean AJ. Intensivist use of hand–carried ultrasound to measure E/e’ and IVC collapsibility in estimating volume status: Correlations with pulmonary artery and central venous pressures. South Med J. 2008;101:861. [Google Scholar]
- 14.Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734–9. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 15.Kent A, Patil P, Davila V, Bailey JK, Jones C, Evans DC, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10:44–9. doi: 10.4103/1817-1737.146872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiferman DS, Davido HT, Howard JM, Gerckens J, Evans DC, Cook CH, et al. Two methods of hemodynamic and volume status assessment in critically Ill patients: A study of disagreement. J Intensive Care Med. 2016;31:113–7. doi: 10.1177/0885066614530085. [DOI] [PubMed] [Google Scholar]
- 17.Perel A, Saugel B, Teboul JL, Malbrain ML, Belda FJ, et al. The effects of advanced monitoring on hemodynamic management in critically ill patients: A pre and post questionnaire study. J Clin Monit Comput. 2016;30:511–8. doi: 10.1007/s10877-015-9811-7. [DOI] [PubMed] [Google Scholar]
- 18.Cooper AS. Pulmonary artery catheters for adult patients in intensive care. Crit Care Nurse. 2016;36:80–2. doi: 10.4037/ccn2016883. [DOI] [PubMed] [Google Scholar]
- 19.Isakow W, Schuster DP. Extravascular lung water measurements and hemodynamic monitoring in the critically ill: Bedside alternatives to the pulmonary artery catheter. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1118–31. doi: 10.1152/ajplung.00277.2006. [DOI] [PubMed] [Google Scholar]
- 20.Tilton D. Central venous access device infections in the critical care unit. Crit Care Nurs Q. 2006;29:117–22. doi: 10.1097/00002727-200604000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013:CD003408. doi: 10.1002/14651858.CD003408.pub3. [Doi: 10.1002/14651858.CD003408.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly N, Esteve R, Papadimos TJ, Sharpe RP, Keeney SA, DeQuevedo R, et al. Clinician-performed ultrasound in hemodynamic and cardiac assessment: A synopsis of current indications and limitations. Eur J Trauma Emerg Surg. 2015;41:469–80. doi: 10.1007/s00068-014-0492-6. [DOI] [PubMed] [Google Scholar]
- 23.Stawicki SP, Bahner DP. Modern sonology and the bedside practitioner: Evolution of ultrasound from curious novelty to essential clinical tool. Eur J Trauma Emerg Surg. 2015;41:457–60. doi: 10.1007/s00068-014-0464-x. [DOI] [PubMed] [Google Scholar]
- 24.Stawicki SP, Seamon MJ, Kim PK, Meredith DM, Chovanes J, Schwab CW, et al. Transthoracic echocardiography for pulmonary embolism in the ICU: Finding the “right” findings. J Am Coll Surg. 2008;206:42–7. doi: 10.1016/j.jamcollsurg.2007.06.293. [DOI] [PubMed] [Google Scholar]
- 25.Kent A, Bahner DP, Boulger CT, Eiferman DS, Adkins EJ, Evans DC, et al. Sonographic evaluation of intravascular volume status in the surgical intensive care unit: A prospective comparison of subclavian vein and inferior vena cava collapsibility index. J Surg Res. 2013;184:561–6. doi: 10.1016/j.jss.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 26.DeMaria S, Jr, Mooney T, Kam J. Monitoring in Anesthesia and Perioperative Care. New York: Cambridge University Press; 2011. Appendix: Monitoring recommendations for common types of surgical procedures; p. 397. [Google Scholar]
- 27.Thiele RH, Bartels K, Gan TJ. Monitoring Technologies in Acute Care Environments. New York: Springer; 2014. Noninvasive cardiac output monitoring; pp. 65–72. [Google Scholar]
- 28.Hadian M, Kim HK, Severyn DA, Pinsky MR. Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO, FloTrac and pulmonary artery catheters. Crit Care. 2010;14:R212. doi: 10.1186/cc9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCanny P, Colreavy F, Bakker J, Hofer C, Zimmerman J. Haemodynamic monitoring and management. 2013. [Last accessed on 2016 Nov 27]. Available from: http://pact.esicm.org/media/HaemMon%20and%20Mgt%208%20April%202013%20final.pdf .
- 30.Pinsky MR. Functional hemodynamic monitoring: Current concepts in critical care. Curr Opin Crit Care. 2014;20:288. [Google Scholar]
- 31.Kline JA, Stubblefield WB. Clinician gestalt estimate of pretest probability for acute coronary syndrome and pulmonary embolism in patients with chest pain and dyspnea. Ann Emerg Med. 2014;63:275–80. doi: 10.1016/j.annemergmed.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Goerlich CE, Wade CE, McCarthy JJ, Holcomb JB, Moore LJ. Validation of sepsis screening tool using StO2 in emergency department patients. J Surg Res. 2014;190:270–5. doi: 10.1016/j.jss.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Hams SP. A gut feeling? Intuition and critical care nursing. Intensive Crit Care Nurs. 2000;16:310–8. doi: 10.1054/iccn.2000.1500. [DOI] [PubMed] [Google Scholar]
- 34.Kabrhel C, Camargo CA, Jr, Goldhaber SZ. Clinical gestalt and the diagnosis of pulmonary embolism: Does experience matter? Chest. 2005;127:1627–30. doi: 10.1378/chest.127.5.1627. [DOI] [PubMed] [Google Scholar]
- 35.Stawicki PS, Braslow B, Gracias VH. Exploring measurement biases associated with esophageal Doppler monitoring in critically ill patients in intensive care unit. Ann Thorac Med. 2007;2:148–53. doi: 10.4103/1817-1737.36548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, et al. Cardiac complications associated with goal–directed therapy in high–risk surgical patients: A meta-analysis. Br J Anaesth. 2014;112:648–59. doi: 10.1093/bja/aet466. [DOI] [PubMed] [Google Scholar]