Abstract
PURPOSE: The incidence of anaplastic lymphoma kinase (ALK) rearrangement in pulmonary sarcomatoid carcinoma (PSC) is controversial. In this study, we aimed to reveal the reliable frequency and the clinical-pathologic characteristics of pulmonary sarcomatoid carcinoma (PSC) with ALK rearrangement in Chinese population, and to provide insight into the translatability of anti-ALK treatment in this treatment-refractory disease. METHODS: Immunohistochemistry (IHC) using a Ventana anti-ALK (D5F3) rabbit monoclonal antibody was performed in 141 PSC specimens collected from multiple medical centers. IHC-positive cases were then confirmed using ALK fluorescent in situ hybridization (FISH). The incidence rates and clinical-pathologic characteristics of ALK-rearranged PSC were then analyzed. Response to ALK inhibitor crizotinib in a patient with ALK-rearranged PSC was evaluated according to the response evaluation criteria for solid tumors (RECIST) version 1.1. RESULTS: Five of 141 (3.5%) of PSCs showed ALK rearrangement-positive by IHC and then were confirmed by FISH. Two were carcinosarcomas and the other three were pulmonary pleomorphic carcinoma (PPC). Strong positive ALK rearrangement was observed in both the epithelioid and sarcomatoid components. The median age of ALK-positive patients was younger than that of ALK-negative patients. PSCs in never-smokers were more likely to harbor ALK rearrangement than those in former or current smokers (P < .05). A 40-year-old woman diagnosed with ALK-rearranged PPC experienced a partial response (−32%) to the ALK inhibitor crizotinib. CONCLUSIONS: The incidence rates of ALK rearrangement in PSC in the Chinese population are similar to those of other subtypes of NSCLC. PSCs in younger never-smokers are more often to harbor ALK rearrangement. ALK inhibitors may serve as an effective treatment for ALK-rearranged PSC.
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare and highly aggressive group of poorly differentiated non-small cell lung cancer (NSCLC) [1], [2]. It approximately accounts for 0.1–0.4% of all lung cancer, and encompasses five different histological subtypes: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma [3], [4], [5]. PSC usually has a higher rate of recurrence and a poorer response to conventional chemotherapy than other NSCLCs [6], [7].
Recent comprehensive genetic studies and clinical observations are starting to uncover the oncogenic underpinnings and potential molecular targets of PSC [8], [9], [10], [11]. Previously, several small sample size studies have reported no anaplastic lymphoma kinase (ALK) rearrangement in PSC, yet recently, some isolated case reports have shown patients of PSC harboring ALK rearrangement [12], [13], [14]. Because the frequency is controversial, the value of routine detection of ALK rearrangement and the clinical benefit from anti-ALK treatment in PSC remain unknown. Larger sample size studies to uncover the accurate incidence rates of ALK rearrangement in PSC are urgent. Therefore, we performed a cohort study in a large series of PSC from multiple centers in China to reveal the frequency and the clinical-pathologic characteristics of PSC with ALK rearrangement.
Materials and Methods
Patients and Specimens
This study was approved by the institutional review board of every participating institution. We collected 167 PSCs from six medical centers in many different areas, including the southern, northern, eastern, and western parts of China, from November 1999 to October 2015. A total of 26 cases were excluded because of lacking enough tumor tissues. Eventually, 141 PSCs were enrolled for ALK rearrangement detection and further analysis. All patients were confirmed through a PSC diagnosis by an experienced pathologist (Y.Z.) according to the 2015 WHO criteria of lung cancer [5]. Clinical parameters and tumor features were tabulated from the medical records of the patients, including EGFR and KRAS mutational status where available. Clinical staging was performed according to the American Joint Committee on Cancer (AJCC, 7th edition).
Immunochemistry staining of ALK rearrangement
Immunohistochemistry (IHC) staining was performed on 4-um-thick slides of FFPE using a Ventana anti-ALK (D5F3) rabbit monoclonal primary antibody combined with the OptiView DAB IHC Detection Kit and the OptiView Amplification Kit (Ventana Medical Systems, Inc. Tucson, AZ). Each patient had a matched negative control slide to assess non-specific background staining and the degree of background staining known to occur as a result of specific tissue elements. The scoring criteria for positive ALK rearrangement was the presence of strong granular cytoplasmic staining in tumor cells (any percentage of positive tumor cells), whereas the absence of strong granular cytoplasmic staining in tumor cells was considered negative ALK rearrangement. All results were confirmed by two experienced pathologists (Y. Z. and J. B. L.).
Fluorescent In Situ Hybridization of ALK Rearrangement
IHC-positive cases were then confirmed using ALK fluorescent in situ hybridization (FISH) analysis. For FISH detection, 4-um-thick slides of FFPE were used with the Vysis ALK Dual Color, Break Apart FISH Probe Kit (Abbott Molecular, Inc. Des Plaines, IL) according to the manufacturer's instructions. All cases were independently scored 100 nuclei on each slide by two different pathologists (F. W. and Y. Z.). The criteria of FISH-positive ALK rearrangement cases were defined as more than 15% tumor cells showing separated green and red signals or a single red signal. The samples showed an isolated green signal, or fused red and green signals, which were considered FISH negative.
Evaluation of the Response to Crizotinib in a Patient With ALK Rearrangement
One patient with ALK-rearranged PSC was treated with crizotinib. Chest and upper abdomen computed tomography (CT) scans were performed at the baseline and every two cycles of crizotinib treatment. Treatment responses were evaluated according to the response evaluation criteria for solid tumors (RECIST) version 1.1.
Statistical Analysis
All data were processed with SPSS version 22.0 (SPSS Inc., Chicago, IL). The Fisher's exact test was used to compare the clinical-pathologic characteristics of ALK arrangement-positive and-negative PSCs. A two-sided P value ≤.05 was considered statistically significant.
Results
Patient Characteristics
A total of 141 histologically confirmed PSCs from six medical centers were enrolled for ALK arrangement analysis. The clinical parameter and tumor features of all patients are shown in Table 1. The median age of patients was 59 (range, 28–84) with 51.1% of patients being older than 60. The majority of patients with PSC were male (87.2%) and had smoking history (66.7%). The most common pathologic subtypes were pleomorphic carcinoma (66.7%), followed by spindle-cell carcinomas (14.6%) and carcinosarcoma (9.7%). EGFR mutations were identified 5 of 32 cases (15.6%), and KRAS mutations were found in 2 of 12 patients (16.7%).
Table 1.
Clinical-pathologic characteristics of all patients (n = 141)
| Characteristic | No. of patients (%) |
|---|---|
| Gender | |
| Male | 123 (87.2) |
| Female | 18 (12.8) |
| Age | |
| ≤60 years | 69 (48.9) |
| >60 years | 72 (51.1) |
| Smoking status | |
| Never-smoker | 48 (34.0) |
| Former/current smoker | 93 (66.0) |
| Stage | |
| I | 39 (22.7) |
| II | 43 (30.5) |
| III | 54 (38.3) |
| IV | 5 (3.5) |
| Histological subtype | |
| Pleomorphic carcinomas | 96 (66.7) |
| Carcinosarcomas | 21 (14.6) |
| Spindle-cell carcinomas | 14 (9.7) |
| Giant-cell carcinomas | 6 (4.2) |
| Pneumoblastoma | 4 (2.8) |
| Mutation status | |
| EGFR (n = 32) | 5 (15.6) |
| KRAS (n = 12) | 2 (16.7) |
EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog.
Frequency and Clinical-Pathologic Features of ALK Rearrangement-Positive PSC
Five of 141 (3.5%) PSCs showed ALK rearrangement positivity by IHC and then were confirmed by FISH. The clinical and pathologic characteristics of all 5 PSCs are shown in Table 2. Two were carcinosarcomas with a stage Ib (pT2bN0M0) tumor and a stage IIIb (pT4N2M0) tumor, respectively, occurring in never-smoked men. The other three were pleomorphic carcinomas, and their clinical stages were stage IV (cT4N1M1a), stage Ia (pT1aN0M0), and stage Ib (pT2N0M0), respectively. Of all five tumors, four of them had adenocarcinoma epithelial components, and the other one had squamous cells carcinoma epithelial components. Strong positive ALK rearrangement was observed in both the carcinomatous and sarcomatoid components of all five tumors (Figures 1 and 2). ALK rearrangement was found to be mutually exclusive with mutations in EGFR and KRAS.
Table 2.
Clinical-pathological characteristics of ALK arrangement-positive PSC
| Patient no. | Gender | Age | Smoking history | TNM | Pathology | Epithelial component type (%) | Sarcomatous component type (%) | EGFR | KRAS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 30 | No | pT2bN0M0 | CCS | Adenocarcinoma (30%) | Fibrosarcoma (70%) | Wild-type | Wild-type |
| 2 | Male | 52 | No | pT4N2M0 | CCS | Adenocarcinoma (40%) | Fibrosarcoma (60%) | Wild-type | Wild-type |
| 3 | Female | 40 | No | cT4N1M1a | PPC | Adenocarcinoma (20%) | Spindle cells (80%) | Wild-type | Wild-type |
| 4 | Male | 63 | No | pT1aN0M0 | PPC | Adenocarcinoma (40%) | Spindle cells (60%) | Wild-type | Wild-type |
| 5 | Male | 66 | Yes | pT2N0M0 | PPC | Squamous cell carcinoma (70%) |
Giant cells (30%) | Wild-type | Wild-type |
CCS, carcinosarcoma; PPC, pleomorphic carcinoma.
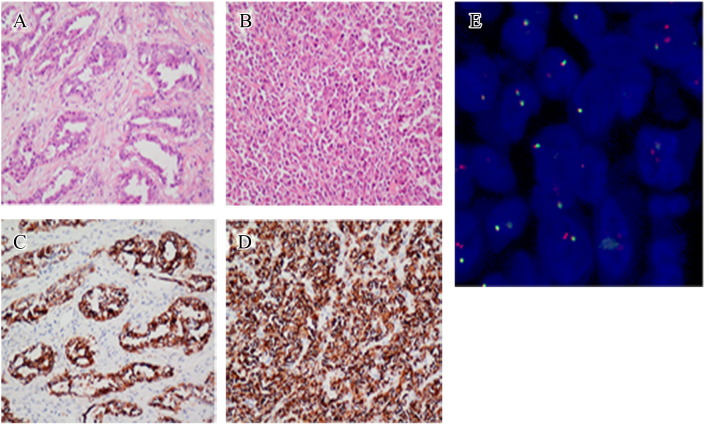
Figure 1.
IHC and FISH images showing an ALK rearrangement-positive pulmonary carcinosarcoma in a 30-year-old never-smoked man. Hematoxylin and eosin (H&E) staining images (magnification: ×20) showing a moderately differentiated adenocarcinoma component (A), and a fibrosarcomatous component (B). Ventana anti-ALK IHC staining showing strong positive ALK rearrangement in both the carcinomatous component (C) and the sarcomatous component (D). FISH image showing ALK rearrangement in 45% of the tumor cells (E).
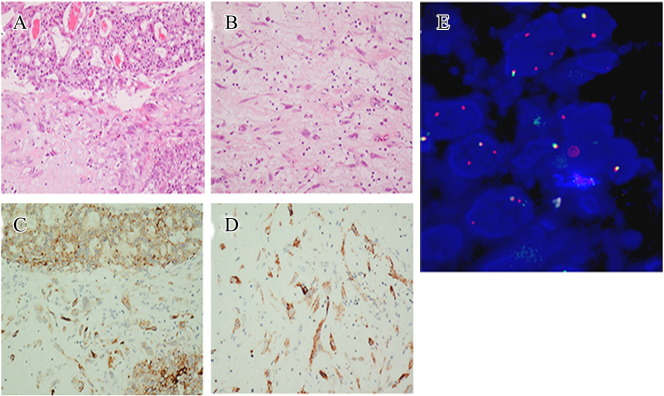
Figure 2.
IHC and FISH images showing an ALK rearrangement-positive PPC in a 40-year-old never-smoked woman. H&E staining images (magnification: ×20) showing a poorly differentiated adenocarcinoma component (A) and a spindle cell component (B). Ventana anti-ALK IHC staining showing strong positive ALK rearrangement in both the carcinomatous component (C) and the sarcomatous component (D). FISH image showing ALK rearrangement in 70% of the tumor cells (E).
The characteristics of the ALK rearrangement-positive and the ALK rearrangement-negative PSCs were compared in Table 3. The median age of ALK-positive patients was younger than that of ALK-negative patients (52 years vs. 57 years). PSCs in never-smokers were more likely to harbor ALK rearrangement than those in former or current smokers (P = .046). There was no significant difference in the incidence rates of ALK rearrangement among different histological subtypes of PSC.
Table 3.
Comparison of the clinical-pathologic characteristics of ALK arrangement-positive and-negative PSCs
| Characteristics | ALK+ (n = 5) | ALK− (n = 136) | P value |
|---|---|---|---|
| Median age | 52 (30–66) | 57 (28–84) | |
| Sex | .500 | ||
| Male | 4 | 119 | |
| Female | 1 | 17 | |
| Smoking status | .046 | ||
| Never-smoker | 4 | 44 | |
| Former/current smoker | 1 | 92 | |
| Stage | 1.000 | ||
| I-II | 3 | 80 | |
| III-IV | 2 | 56 | |
| Histological subtypes | |||
| Carcinosarcoma | 2 | 19 | .160 |
| Pleomorphic carcinoma | 3 | 93 | .654 |
Dramatic Response to Crizotinib in a Patient With Advanced PSC and ALK Rearrangement
A 40-year-old woman (case 3 in Table 2) without tobacco exposure visited our hospital because she had a persistent cough. A chest computed tomography screen found irregular lesions in the left hilar and the pleura. Subsequent fluorodeoxyglucose positron emission tomography (FDG-PET) showed abnormally high uptake in those lesions, and no brain metastasis was detected by brain magnetic resonance imaging (MRI). In transbronchial biopsy specimens, sarcomatoid spindle cells and polygonal epithelioid cells proliferated without distinct architectural features. IHC demonstrated positive for pancytokeratin and vimentin and negative for thyroid transcription factor-1 (TTF-1) and other mesenchymal markers such as S-100, desmin, bcl-2, and CD34. Thus, the disease was diagnosed as stage IV (T4N1M1a) pleomorphic carcinomas. Expanded molecular testing revealed ALK rearrangement in the tumor, and no EGFR/KRAS mutations, ROS1 rearrangement, and c-Met amplification were detected. Therefore, the patient started crizotinib 250 mg orally twice daily. She achieved an excellent radiographic partial response (−32%) after 3 months of treatment (Figure 3). At the time of this report (11 months), the patient is continuing on crizotinib without evidence of disease progression and without any severe side effects.
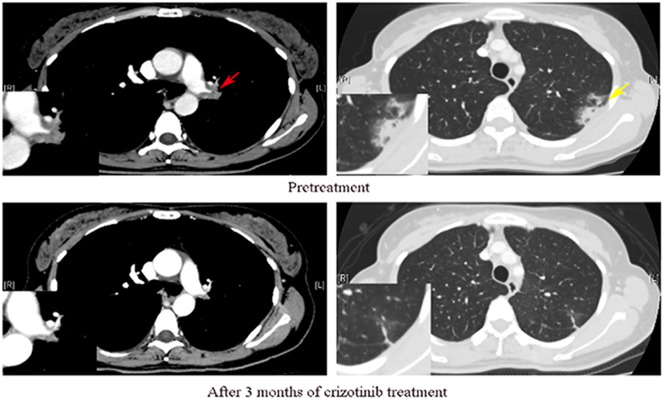
Figure 3.
Representative pre-crizotinib (upper row) and post-crizotinib (lower row) treatment CT images in a 40-year-old never-smoked woman with PSC harboring ALK rearrangement. After 3 months of crizotinib treatment, lesions involving the left hilar (red arrow) and the pleura (yellow arrow) became markedly reduced compared with those in the pre-crizotinib treatment CT images.
Discussion
There are currently few therapeutic options for patients with PSC, and there are few new insights into the EGFR and KRAS mutations in PSC, and there has been fairly little research conducted on the aspect of ALK rearrangement [8], [9], [15], [16]. Therefore, we carried out this large sample size, multi-center study, and we identified ALK rearrangement in 3.5% of PSC. We also found that PSCs in younger never-smokers were likely to harbor ALK rearrangement. An excellent partial response to the ALK inhibitor crizotinib in a woman with advanced PSC and ALK rearrangement provides clinical validation and translatability of ALK-targeted therapy in PSC.
The incidence rates of ALK rearrangement in PSC remain unknown. Previously, almost all of the studies using a small sample size have reported that no ALK rearrangement occurred in PSC [10], [11]. Recently, a few cases of ALK rearrangement-positive PSC have been reported in Japan [12], [13], [14]. In our large sample study, the incidence rate of ALK rearrangement in PSC was 3.5%, which is similar to the frequency in typical NSCLC. The previous controversy of the incidence rates may partly be a result of the bias caused by the small sample sizes. Notably, until now, almost all reported ALK rearrangement-positive PSCs were from studies based on the East Asian population. Therefore, we also speculate that PSCs in the East Asian population are more likely to harbor ALK rearrangement.
The clinical profile of patients with ALK-rearranged PSC is similar to that of typical ALK-rearranged NSCLC. Both previous reports and our present study revealed that ALK rearrangement is more likely to be detected in PSCs with adenocarcinoma components of younger never-smoked patients [12], [13], [14]. Another intriguing observation we found involves two patients with carcinosarcoma harboring ALK rearrangement. To the best of our knowledge, no ALK-rearranged pulmonary carcinosarcoma has been reported previously. Pulmonary carcinosarcoma has a predilection to occur in older males and is also well associated with heavy smoking [17], [18]. However, both of the two patients with ALK-rearranged PSC in our study were younger never-smoked men. Identifying these clinical and histological characteristics will help us to find out patients most likely to harbor ALK-rearrangement and to benefit from ALK-targeted therapy.
We described a patient with ALK-rearranged PSC who showed a partial response to crizotinib treatment by 3 months, and this benefit was still seen on the follow-up CT images at 8 months. Murakami et al. treated a 50-year-old never-smoked man who had pleomorphic carcinomas harboring ALK rearrangement with crizotinib, and achieved a partial response by 1 month [13]. However, the disease progressed after 3 months due to new liver and brain metastases. The exact reason for this discrepancy is not known. Therefore, further clinical trials are needed to verify the efficiency of ALK-targeted therapy in patients with ALK-rearranged PSC.
In summary, the incidence rates of ALK rearrangement in PSC in the Chinese population are similar to those of other subtypes of NSCLC. PSCs in younger never-smokers are more likely to harbor ALK rearrangement. ALK inhibitors may serve as an effective treatment for ALK-rearranged PSC.
Conflict of Interest
None.
Acknowledgements
None.
Footnotes
Funding: This work was supported by the Natural Science Foundation of Guangdong Province [Grant numbers 2014A030310053, X. L.].
Contributor Information
Xuewen Liu, Email: avein_liu@hotmail.com.
Likun Chen, Email: chenlk@sysucc.org.cn.
References
- 1.Terzi A, Gorla A, Piubello Q, Tomezzoli A, Furlan G. Biphasic sarcomatoid carcinoma of the lung: report of 5 cases and review of the literature. Eur J Surg Oncol. 1997;23:457. doi: 10.1016/s0748-7983(97)93733-1. [DOI] [PubMed] [Google Scholar]
- 2.Venissac N, Pop D, Lassalle S, Berthier F, Hofman P, Mouroux J. Sarcomatoid lung cancer (spindle/giant cells): an aggressive disease? J Thorac Cardiovasc Surg. 2007;134:619–623. doi: 10.1016/j.jtcvs.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Yendamuri S, Caty L, Pine M, Adem S, Bogner P, Miller A, Demmy TL, Groman A, Reid M. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152:397–402. doi: 10.1016/j.surg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Park JS, Lee Y, Han J, Kim HK, Choi YS, Kim J, Shim YM, Kim K. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81:206–213. doi: 10.1159/000333095. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 6.Bae HM, Min HS, Lee SH, Kim DW, Chung DH, Lee JS, Kim YW, Heo DS. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer. 2007;58:112–115. doi: 10.1016/j.lungcan.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiano A, Cortot AB, Ilie M, Martel-Planche G, Fabas T, Pop D, Mouroux J, Hofman V, Hofman P, Pedeutour F. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy. Int J Cancer. 2009;125:2479–2482. doi: 10.1002/ijc.24610. [DOI] [PubMed] [Google Scholar]
- 9.Chang YL, Wu CT, Shih JY, Lee YC. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol. 2011;18:2952–2960. doi: 10.1245/s10434-011-1621-7. [DOI] [PubMed] [Google Scholar]
- 10.Pelosi G, Gasparini P, Cavazza A, Rossi G, Graziano P, Barbareschi M, Perrone F, Barberis M, Takagi M, Kunimura T. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer. 2012;77:507–514. doi: 10.1016/j.lungcan.2012.05.093. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Jia Y, Stoopler MB, Shen Y, Cheng H, Chen J, Mansukhani M, Koul S, Halmos B, Borczuk AC. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol. 2016;34:794–802. doi: 10.1200/JCO.2015.62.0674. [DOI] [PubMed] [Google Scholar]
- 12.Shiroyama T, Tanaka A, Tamiya M, Hamaguchi M, Osa A, Takeoka S, Tani E, Azuma Y, Morishita N, Suzuki H. A Rare Case of Pleomorphic Carcinoma of the Lung Harboring an Anaplastic Lymphoma Kinase (ALK) Rearrangement. Intern Med. 2015;54:2741–2743. doi: 10.2169/internalmedicine.54.4474. [DOI] [PubMed] [Google Scholar]
- 13.Murakami Y, Saka H, Oki M. Response to Crizotinib and Clinical Outcome in ALK-Rearranged Pulmonary Pleomorphic Carcinoma. J Thorac Oncol. 2015;10:e28–e29. doi: 10.1097/JTO.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama R, Matsumura F, Shibata Y, Takahashi H, Okabayashi H, Kosai S, Honda I, Ohkawara S, Sugimoto M. Detection of ALK rearrangement in an octogenarian patient with pleomorphic carcinoma of the lung. Gen Thorac Cardiovasc Surg. 2016;64:167–169. doi: 10.1007/s11748-014-0428-4. [DOI] [PubMed] [Google Scholar]
- 15.Kaira K, Horie Y, Ayabe E, Murakami H, Takahashi T, Tsuya A, Nakamura Y, Naito T, Endo M, Kondo H. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol. 2010;5:460–465. doi: 10.1097/JTO.0b013e3181ce3e3c. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Kim Y, Sun JM, Choi YL, Kim JG, Shim YM, Park YH, Ahn JS, Park K, Han JH. Molecular profiles of EGFR, K-ras, c-met, and FGFR in pulmonary pleomorphic carcinoma, a rare lung malignancy. J Cancer Res Clin Oncol. 2011;137:1203–1211. doi: 10.1007/s00432-011-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis MP, Eagan RT, Weiland LH, Pairolero PC. Carcinosarcoma of the lung: Mayo Clinic experience and response to chemotherapy. Mayo Clin Proc. 1984;59:598–603. doi: 10.1016/s0025-6196(12)62410-0. [DOI] [PubMed] [Google Scholar]
- 18.Olobatoke AO, David D, Hafeez W, Van T, Saleh HA. Pulmonary carcinosarcoma initially presenting as invasive aspergillosis: a case report of previously unreported combination. Diagn Pathol. 2010;5:11. doi: 10.1186/1746-1596-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]