SUMMARY
This retrospective, observer blinded case-control study aims to compare the prevalence of neurovascular conflicts (NVCs) of the vestibulocochlear nerve and the anterior inferior cerebellar artery (AICA) in patients presenting with clinical signs of acute vestibular neuritis with and without subsequent objective vestibular function loss (VFL). 58 acute cases of clinically suspected acute vestibular neuritis were investigated with same day cranial MRI at a tertiary referral centre and compared to 61 asymptomatic controls. The prevalence of NVCs in cases with objective VFL were also compared to cases without VFL. Radiologists described the NVC as "no contact" (Grade 0), "contact < 2 mm" (Grade 1), "contact > 2 mm" (Grade 2) and "vascular loop presence" (Grade 3) without knowledge of neurotological data. Neurotological data was collected without knowledge of MRI findings. Vestibular function was tested by bithermic caloric irrigation. 26 cases (45%) showed caloric VFL (Group A), whereas 32 (55%) exhibited no VFL (Group B). Group A included 13 cases with NVCs (50%), Group B included 26 NVC cases (82%) (p = 0.012) and the control group included 16 individuals (26%) (p < 0.001 for comparison of all 3 groups). Group B had a significantly higher NVC-Grading than Group A (p = 0.009). There was no statistically significant association between NVCs and either SNHL or tinnitus (p > 0.05). Our results suggest that patients presenting with clinical signs of acute vestibular neuritis who show symmetrical caloric vestibular function test results have a significantly higher NVC prevalence in the cerebellopontine angle.
KEY WORDS: Neurovascular conflict, Vestibular neuritis, Vestibular function loss, SNHL, Tinnitus
RIASSUNTO
Il presente studio retrospettivo a singolo cieco si pone come obbiettivo quello di valutare in che percentuale di casi di pazienti che si presentano con sintomatologia compatibile con neurite vestibolare acuta, con e senza perdita oggettiva della funzione vestibulare (VFL), sia presente un conflitto neurovascolare fra il nervo vestibolococleare e la arteria cerebellare anteroinferiore (AICA). 58 pazienti con sintomatologia suggestiva per neurite vestibolare acuta, valutati con RMN presso un centro di terzo livello, sono stati confrontati con 61 pazienti asintomatici. I radiologi hanno dato valutato la presenza di conflitto neurovascolare, in assenza di dati clinici, conferendo ai rilievi oggettivi una valutazione in una scala da 0 a 3 a seconda che il contatto fosse: nesuno; inferiore a 2 mm; superiore ai 2 mm; presenza di vacular loop. I reperti neurootologici sono stati quindi raccolti all'oscuro del risultato dell'imaging. La funzione vestibolare è stata testata con prova calorica bitermica. Alla prova calorica 26 casi (45%) hanno mostrato segni oggettivi di deficit vestibolare (Gruppo A), 32 casi (55%) non hanno invece mostrato alcun deficit labirintico (Gruppo B). Il gruppo A ha incluso 13 casi (50%) con evidenza di conflitto neurovascolare (NVC), il gruppo B ha incluso 26 casi con NVC (82%) (p = 0.012) mentre i controlli hanno incluso 16 casi con NVC (26%). La differenza fra i tre gruppi ha mostrato significatività statistica (p<0.001). Il Gruppo B ha mostrati un associazione con un grading di conflitto piu elevato rispetto al Gruppo A (p = 0.009). La presenza di NVC non ha avuto un associazione statisticamente significativa ne con la presenza di SNHL ne con la presenza di acufene (p > 0.05). I nostri dati indicano che la presenza di conflitti neurovascolari a livello dell'angolo pontocerebellare è superiore in quei pazienti che in presenza di una sintomatologia compatibile con neurite vestibolare acuta abbiano una funzionalità simmetrica alla prova calorica.
Introduction
The phenomenon of neurovascular conflicts (NVCs) and compression syndromes of the head and neck has been controversially discussed for over three decades. Primarily entities such as trigeminal neuralgia and hemifacial spasm have been accepted to have a pathogenetic and clinically relevant connection to a neurovascular conflict 1. In neurotology, a variety of symptom combinations have been studied to analyse the relevance of neurovascular conflicts within the cerebellopontine angle. Primarily, sensorineural hearing loss, tinnitus and recurrent paroxysmal vertigo have been studied. Neurovascular surgical intervention for intractable symptoms, mainly vertigo, have repeatedly been described since 1975 2 3. Additionally, Esfahani et al. studied the efficiency of CT-cisternography in the analyses of patients with recurrent vertigo episodes in the late 1980s 4. With the development of MRI techniques, the focus on vessel loops, neurovascular compression and neurovascular conflicts has intensified 5-7. After having been described over 30 years ago, vestibular paroxysmia has subsequently become a well-known differential diagnosis in patients with recurrent paroxysmal vertigo, characterised by short intense recurrent vertigo attacks 8.
However, it is not yet clear whether NVCs might be connected to acute vestibular neuropathy itself or if NVCs may mimic the clinical signs of acute vestibular neuritis in certain cases. For instance, although suffering from vestibular paroxysmia and responding well to medical treatment, it has been shown that patients suffer from a slow progressive reduction in peripheral vestibular function over time 9. It has also been shown that a NVC does alter the functionality of the 8th cranial nerve as such and influences the compound action potential in audiometric brainstem response (ABR) studies 10. To the best of our knowledge, it is not yet evident that a NVC might trigger a prolonged and intense vertigo attack such as is seen in acute vestibular neuritis.
Therefore, the present study aimed to scrutinise the prevalence of the neurovascular conflict in patients presenting with clinical signs of acute vestibular neuritis, such as massive primary onset vertigo with a duration of several hours, vomiting and spontaneous horizontal nystagmus, without signs of central nervous system pathology. Our study especially aimed to identify a difference in the frequency of neurovascular contact, and the extent of contact between nerve and blood vessel, in patients with subsequent normal vestibular function compared to those with reduced objective vestibular activity.
Materials and methods
Ethical Considerations
Due to the anonymous and retrospective and completely non-invasive nature of this study, there were no ethical concerns with this study. Where applicable, the instructions in the Declaration of Helsinki were followed.
Overall, data from 119 MRI patients admitted to our institution for clinically suspected acute vestibular neuritis were retrospectively analysed. 58 patients with clinically suspected vestibular neuritis (acute vertigo, spontaneous nystagmus, nausea, vomiting) and 61 controls (who had presented with tinnitus without vestibular signs) were included in the study.
Firstly, age and sex were noted before results of audiometric and vestibular testing were documented. All patients included had a pathological clinical head thrust test in the plane of the lateral semicircular canal, suggesting superior vestibular neuritis. Further objective vestibular testing was done by binaural caloric water irrigation according to Fitzgerald and Hallpike (44°C and 30°C) 3-5 days after symptom onset before the Jongkees formula was used to determine vestibular loss. Patients with a caloric asymmetry of >30% were grouped as objective VFL. Total bilateral vestibular areflexia was deemed bilateral VFL. Posturography and vestibular evoked myogenic potential testing was not available at our institution during the time frame patients presented to our clinic. Central signs such as ataxia and skew deviation were excluded via neurological consult. All patients underwent pure tone audiometry. No patient with low-frequency hearing loss was included in the study, thereby eliminating Meniere's disease from cohort. Cases with a positive history of Meniere's disease and/or recurrent vertigo attacks were also excluded. Patients with benign paroxysmal positional vertigo were not included. Cases of acoustic neurinoma, cerebellar infarction, multiple sclerosis or other central nervous system pathologies were excluded.
Secondly, radiologists independently re-evaluated all images for the presence of neurovascular contact between the anterior inferior cerebellar artery (AICA) and the vestibulocochlear nerve (VIII). This neurovascular contact was graded as "no contact" (Grade 0), "contact up to 2 mm" (Grade 1), "contact larger than 2 mm" (Grade 2) and "vascular loop presence" (3). The grading system was based on the work of Siricki et al. 11
The study was conducted with bilateral observer blinding. In essence, the radiologist did all analyses without knowledge of otologic pathologies, while the otologic findings were also documented without knowledge of radiologic results.
The standard 1.5 TESLA-MRI protocol consisted of T2 turbo spin echo (TSE) transversal with 5 mm section thickness, diffusion weighted imaging transversal (DWI), T2 constructive interference steady state (CISS) transversal with 1 mm section thickness across the cerebellopontine angle and T1 multiplanar reconstruction (MPR) coronal 3 mm sections with contrast.
Statistical analyses
Statistical analyses were independently done by the Centre for Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna. Frequencies of binary variables were compared between groups using the Chi-square test. The ordinal scaled grade of neurovascular contact (grades 0 to 3) was compared between groups using the nonparametric Wilcoxon rank sum test (2-group comparisons) and the nonparametric Kruskal-Wallis test (3-group comparisons), respectively. P values < 0.05 were considered as indicating statistical significance. The SAS software (SAS 9.3; 2002-2010, SAS Institute Inc., Cary, NC, USA) was used for statistical calculations.
Results
In total 119 patients were included in the study. 58 patients with clinical signs of vestibular neuritis had an average age of 54.2 years (± 14.1 years) with 33 males and 25 females in the cohort, without significant differences between sexes. Of these, 58 cases 26 had objective caloric vestibular loss (Group A), whereas 32 had no objective caloric weakness (Group B). The control group of patients without signs of vestibular neuritis (Group C) had an average age of 55.1 years (± 11.2 years) with 24 males and 37 females.
There was no statistically significant correlation between neurovascular conflict and tinnitus or sensorineural hearing loss with no significant differences between the groups (p > 0.05).
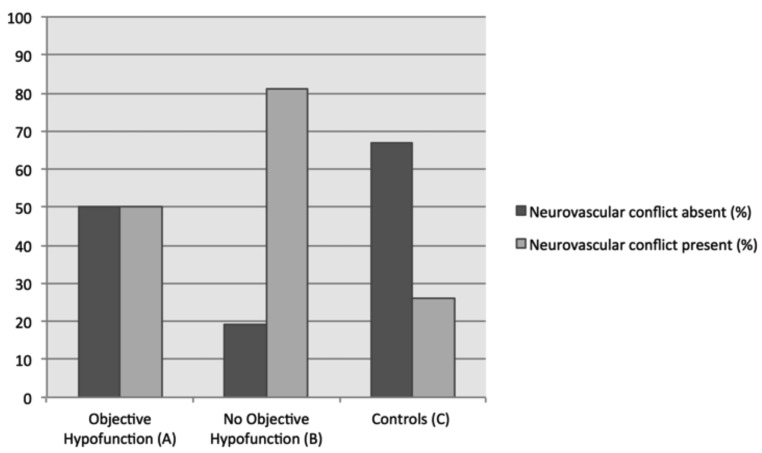
In cases of suspected vestibular neuritis with pathological caloric irrigation results (Group A, Fig. 1, n = 26), neurovascular contact could only be seen in 50% of cases. In patients presenting with initial clinical signs of acute vestibulopathy, who consequently showed symmetrical caloric results, without objective peripheral vestibular loss (Group B, n = 32) a significantly higher presence of neurovascular contact was seen (n = 26/32 = 82%; p = 0.012) (Fig. 1).
Fig.1.
13 of 26 cases (50%) without caloric vestibular loss (Group A; left) presented NVCs. In Group B, i.e. cases without vestibular loss, a significantly higher amount NVCs could be identified, namely in 26/32 cases (82%) compared to 7 (20%) cases without NVCs (p = 0.012) and controls (p < 0.001).
In Group C (asymptomatic controls), 16/61 cases with NVCs could be identified (26%). When compared to controls (Group C), patients with signs of vestibular neuritis (Group A and B) had significantly more NVCs (p < 0.001). When compared separately, a significantly higher prevalence of NVCs could be identified in Group A (with objective loss) than in controls (Group C, p = 0.039) (Table I).
Table I.
Absolute number of cases analysed. Detailed analyses showed an equal amount of patients with neurovascular conflicts (NVCs) in patients with signs of vestibular neuritis with subsequent objective vestibular loss (Group A). Group B (patients with signs of vestibular neuritis without objective vestibular loss) had significantly more NVC's than controls (p < 0.001), as well as significantly more than Group A (p = 0.012).
| NVC | Group A (n) |
Group B (n) |
Group C (Controls) (n) |
Total (n) |
|---|---|---|---|---|
| No | 13 | 6 | 45 | 64 |
| Yes | 13 | 26 | 16 | 55 |
| Total (n) | 26 | 32 | 61 | 119 |
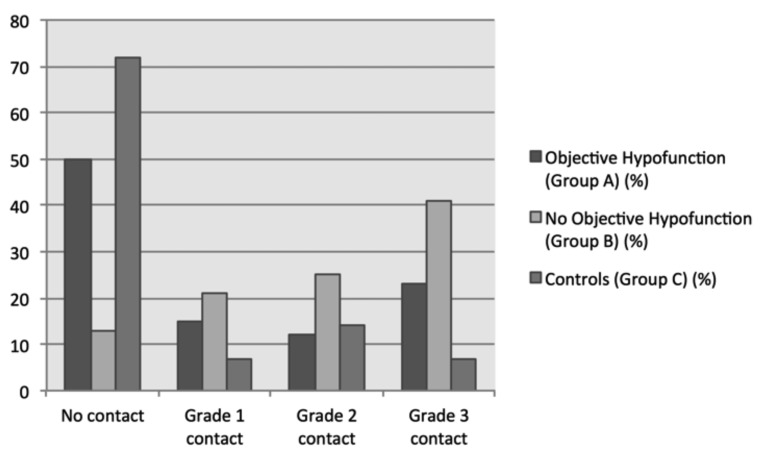
Additionally, the grading of the NVC was significantly higher in patients with no objective vestibular loss compared to patients with vestibular loss and controls (p < 0.001, Fig. 2). Group A (n = 26) showed 13 cases (50%) without NVC, while grade 1 NVC could be identified in 4 (15%), grade 2 in 3 (12%) and grade 3 in 6 (23%) cases. In comparison, patients with acute vestibular clinical signs without objective VFL in Group B exhibited no contact in 4 (13%), grade 1 in 7 (21%), grade 2 in 8 (25%) and grade 3 in 13 (41%) cases. Group C (control group) revealed no contact in 44 (72%), grade 1 in 4 (7%), grade 2 in 9 (15%) and grade 3 in 4 (7%) cases. The absolute number of cases per grade is summarised in Table II. Group B (patients with signs of vestibular neuritis without objective vestibular loss) had significantly higher NVC grading than Group A (patients with signs of vestibular neuritis with subsequent objective vestibular loss) (p = 0.009).
Fig. 2.
Distribution of NVC grading depicted as percentage of cases per NVC Grade. In cases with caloric vestibular loss, significantly fewer NVC's could be described ("no contact") and the higher the NVCs grading became, the more cases with normal calorics were identified (p < 0.0001).
Table II.
Grading of NVCs per group. The grading of groups A and B where significantly higher than the control group C (p < 0.001). Group B (patients with signs of vestibular neuritis without objective vestibular loss) had significantly higher NVC grading than Group A (patients with signs of vestibular neuritis with subsequent objective vestibular loss) (p = 0.009).
| Grade of NVC | Group A (n) |
Group B (n) |
Group C (Controls) (n) |
Total (n) |
|---|---|---|---|---|
| 0 | 13 | 4 | 44 | 61 |
| 1 | 4 | 7 | 4 | 15 |
| 2 | 3 | 8 | 9 | 20 |
| 3 | 6 | 13 | 4 | 23 |
| Total (n) | 26 | 32 | 61 | 119 |
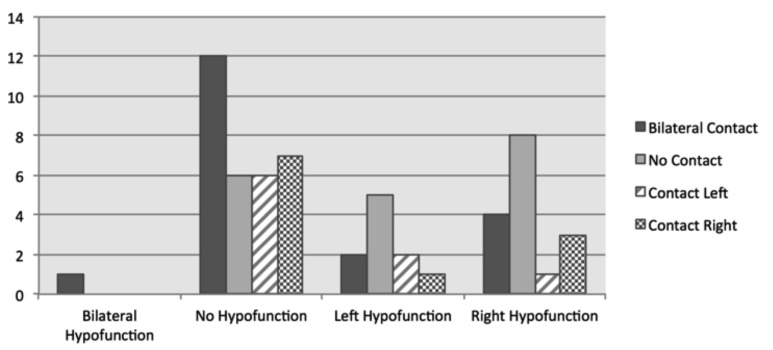
Interestingly, more bilateral NVC's could be identified in group B, while unilateral contact was evenly spread between right and left (Fig. 3). In patients with objective VFL (Group A), the side of the NVC did not correlate significantly with the acute objective vestibular loss.
Fig. 3.
One case showed bilateral contact and bilateral vestibular loss, whereas in 12 symptomatic cases bilateral NVCs with no vestibular loss could be identified. In addition, noticeably more cases of unilateral hypofunction could be identified in patients without NVC's ("no contact") than patients with objective vestibular loss.
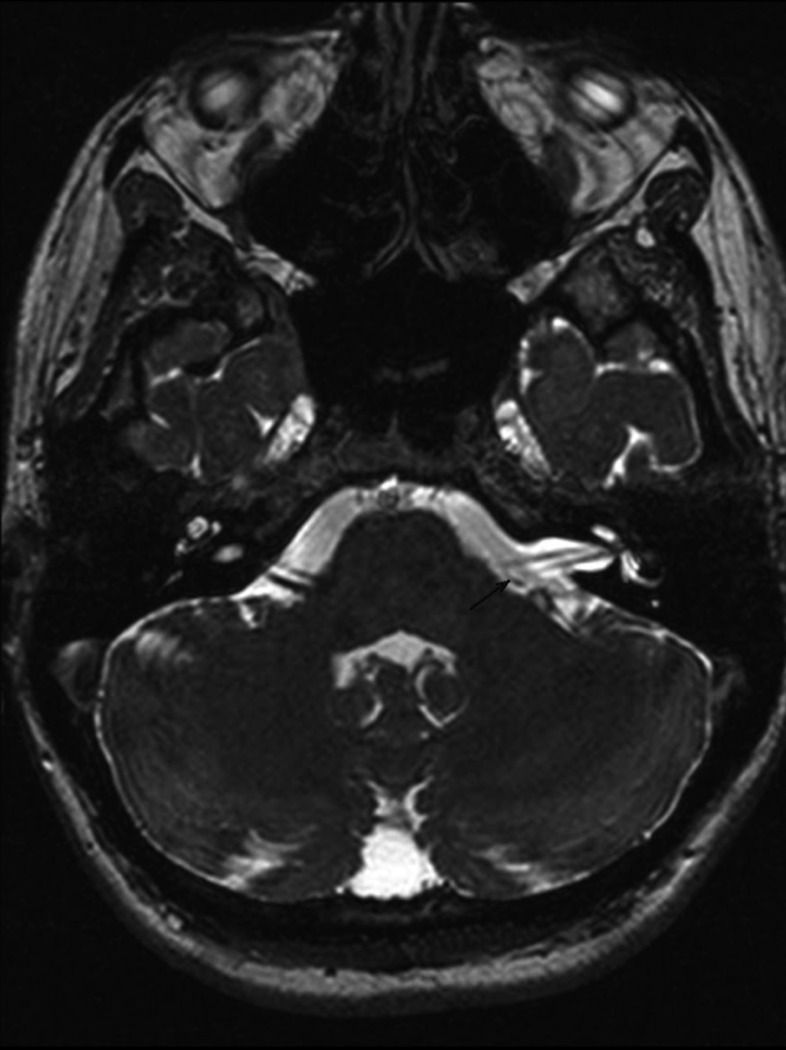
Fig. 4.
Vessel loop formation (Grade 3 NVC) on the left in a patient with clinically suspected acute vestibular neuritis without objective caloric weakness. It can be noted that the blood vessel does not enter the internal auditory meatus.
Discussion
New findings
The present study showed a significantly higher number of NVCs in patients presenting with symptoms of acute peripheral vestibulopathy without objective vestibular function loss (Group B) compared to patients with VFL and controls. Secondly, Group B had a statistically significantly higher NVC grading than Group A and controls (Fig. 2). Thirdly, a noticeably high ratio of bilateral NVC's could be found in Group B (Fig. 3).
Comparison to previously published data:
It has been reported previously that caloric weakness does not correlate to the severity of subjective symptom perception and symptom intensity in peripheral vestibular disorders 12. This also seems to be a relevant consideration when scrutinising the relevance of vessel loop presence in the inner ear canal. Interestingly, Applebaum et al. described normal calorics in 50% of a small cohort of vessel loop cases (n = 10) with sensorineural hearing loss as identified with CT, where the looping occurred within the auditory canal 13. A second manuscript by the same research group also identified pathological vestibular function results in a case series of 15 patients, with spontaneous nystagmus in all but one case, without having vertigo as a primary presenting symptom 14. As in our study, the low frequency caloric test was used. In the data presented here, the clinical head thrust test (which is a high frequency test) complemented the caloric findings. In our MRIbased collective, similar results to the first patient collective was found with 50% of vestibular loss patients having NVCs, yet all our cases presented with acute clinical signs of vestibular neuritis such as spontaneous nystagmus, persistent rotatory vertigo of sudden onset and vomiting. Additionally, in this study it was not a prerequisite, as it was in the cited study, that the NVC was located within the internal auditory canal (IAC) but merely in the entry zone of the vestibulocochlear nerve into the cerebellopontine angle. Our findings for vertigo patients seem to be analogous to the findings of van der Steenstraten, who reported no correlation between the extension of the AICA into the IAC and hearing loss cases 15. This is in turn analogous to the work of Sirikci, who classified NVCs similarly to the grading system used in this study, while adding on a class 4 ("indentation"), which could not be implemented using our methodology 11.
It has been accepted that the mere chronic contact between the nerve and blood vessel seems to be vital in the pathogenesis of vestibular paroxysmia. This vertigo entity typically presents with short, sharp spells of vertigo, periodic (but not compulsory) nausea and has a recurrent nature. In up to 60% of paroxysmia cases vertigo spells might be induced by certain head movement 9. Over time, patients seem to develop a caloric vestibular function loss 9 14. Despite evidence of vestibular loss developing over time in patients with vestibular paroxysmia, it is still unclear whether a neurovascular conflict can mimic the acute symptoms of vestibular neuritis. This stands in contrast to vestibular "pseudoneuritis" or acute vestibular syndrome of vascular origin, which can easily be mistaken for acute peripheral vestibulopathy and has been widely accepted as important differential diagnoses to acute vestibular neuropathy 16.
The actual pathogenesis of acute vestibulopathy (i.e. "vestibular neuritis") has to date not been proven beyond a doubt. Among others, strong arguments have been made for the reactivation of herpes simplex virus type 1 17. Recurrence of vestibular neuritis is deemed as rare as 1.9% and mostly occurs contralaterally, although varying numbers and hypotheses on the cause of recurrence have been published 18 19. However, objective recovery of vestibular function occurs over months to years and not over days 20. In our patient cohort, we identified a remarkably high number of cases with presenting signs of vestibular pathology, where caloric irrigation values were symmetrical and within the normal range within days (Group B, Fig. 1). With the exclusion of Meniere's disease (through the absence of recurrent attacks in the patient history and no low-frequency hearing loss found in the pure tone audiometry), migraine and other central nervous system pathologies via neurological consult and imaging from Groups A and B, the focus fell on why patients showed non-pathological caloric irrigation results within days of symptom onset. The only remarkable finding in Group B (Fig. 1) was the high prevalence of NVCs within this group (n = 28 / 32 = 88%, Fig. 1). No remarkable findings could be found in the remaining 7 cases of Group B reported herein.
Clinical aspects and hypotheses
Although our data cannot objectively prove beyond a doubt that the neurovascular conflict of the cerebellopontine angle is to blame for the results of the cases of Group B, it does suggest a possible connection between NVCs and acute vestibular system dysfunction. Further study is necessary to prove or disprove the suspicions raised by our patient groups before treatment options similar to those implemented in vestibular paroxysmia could be explored.
Hypothetically, two pathological mechanisms might be suspected and considered for further study: 1) neuronal excitation due to direct contact between blood vessel and nerve; 2) progressive neuronal damage. On the one hand, the nerve-vessel conflict may stimulate the activity of the vestibular nerve pathologically in analogous fashion to vestibular paroxysmia, albeit with a vertigo attack of longer duration and a greater intensity of the attack as a result. On the other hand, repeated and continual contact of nerve and blood vessel might result in a slow and progressive loss of function until an attack is precipitated. This seems possible (although speculative at best), because it has previously been shown that vestibular paroxysmia patients slowly lose vestibular activity over time 9. To the best of our knowledge, there have been no reports on AICA – vestibular nerve conflicts resulting in acute vestibular neuritis symptoms to date. Conversely, in cases of bilateral NVC, a combination of excitation and damage might be a relevant factor. However, the data available to us at present does not allow for a conclusive explanation of the pathogenesis and physiological proof behind our observations.
Conclusions
To the best of our knowledge, the clinical data that patients presenting with classical signs of vestibular neuritis, who show normal caloric irrigation results, have a high presence of NVCs between the 8th cranial nerve and the AICA compared to patients with objective vestibular functional loss, is unique.
Acknowledgements
The authors would like to thank Tanja Hojka and Tina Lehner for their logistical assistance.
References
- 1.Jannetta PJ. Trigeminal neuralgia and hemifacial spasmetiology and definitive treatment. Trans Am Neurol Assoc. 1975;100:89–91. [PubMed] [Google Scholar]
- 2.Jannetta PJ. Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surg Forum. 1975;26:467–469. [PubMed] [Google Scholar]
- 3.Reuter W, Fetter M, Albert FK. Vestibular paroxysmia. A rare but important differential diagnosis. HNO. 2008;56:421–424. doi: 10.1007/s00106-006-1535-z. [DOI] [PubMed] [Google Scholar]
- 4.Esfahani F, Dolan KD. Air CT cisternography in the diagnosis of vascular loop causing vestibular nerve dysfunction. AJNR Am J Neuroradiol. 1989;10:1045–1049. [PMC free article] [PubMed] [Google Scholar]
- 5.Papanagiotou P, Grunwald IQ, Politi M, et al. Vascular anomalies of the cerebellopontine angle. Radiologe. 2006;46:216–222. doi: 10.1007/s00117-005-1327-6. [DOI] [PubMed] [Google Scholar]
- 6.Parnes LS, Shimotakahara SG, Pelz D, et al. Vascular relationships of the vestibulocochlear nerve on magnetic resonance imaging. Am J Otol. 1990;11:278–281. [PubMed] [Google Scholar]
- 7.Nowé V, Heyning P, Parizel PM. MRI in patients with otovestibular complaints of unknown origin. B-ENT. 2007;3:27–35. [PubMed] [Google Scholar]
- 8.Lempert T. Recurrent spontaneous attacks of dizziness. Continuum (Minneapolis Minn) 2012;18(5 Neuro-otology):1086–1101. doi: 10.1212/01.CON.0000421620.10783.ac. [DOI] [PubMed] [Google Scholar]
- 9.Hüfner K, Barresi D, Glaser M, et al. Vestibular paroxysmia: diagnostic features and medical treatment. Neurology. 2008;71:1006–1014. doi: 10.1212/01.wnl.0000326594.91291.f8. [DOI] [PubMed] [Google Scholar]
- 10.Ryu H, Yamamoto S, Sugiyama K, et al. Neurovascular compression syndrome of the eighth cranial nerve. Can the site of compression explain the symptoms? Acta Neurochir (Wien) 1999;141:495–501. doi: 10.1007/s007010050330. [DOI] [PubMed] [Google Scholar]
- 11.Sirikci A, Bayazit Y, Ozer E, et al. Magnetic resonance imaging based classification of anatomic relationship between the cochleovestibular nerve and anterior inferior cerebellar artery in patients with non-specific neuro-otologic symptoms. Surg Radiol Anat. 2005;27:531–535. doi: 10.1007/s00276-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 12.Møller MB. Vascular compression of the eighth cranial nerve as a cause of vertigo. Keio J Med. 1991;40:146–150. doi: 10.2302/kjm.40.146. [DOI] [PubMed] [Google Scholar]
- 13.Applebaum EL, Valvassori GE. Auditory and vestibular system findings in patients with vascular loops in the internal auditory canal. Ann Otol Rhinol Laryngol Suppl. 1984;112:63–70. doi: 10.1177/00034894840930s412. [DOI] [PubMed] [Google Scholar]
- 14.Applebaum EL, Valvasorri G. Internal auditory canal vascular loops: audiometric and vestibular system findings. Am J Otol. 1985:110–113. [PubMed] [Google Scholar]
- 15.Steenstraten F, Ru JA, Witkamp TD. Is microvascular compression of the vestibulocochlear nerve a cause of unilateral hearing loss? Ann Otol Rhinol Laryngol. 2007;116:248–252. doi: 10.1177/000348940711600404. [DOI] [PubMed] [Google Scholar]
- 16.Kim H-A, Lee H. Recent advances in central acute vestibular syndrome of a vascular cause. J Neurol Sci. 2012;321:17–22. doi: 10.1016/j.jns.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Arbusow V, Strupp M, Wasicky R, et al. Detection of herpes simplex virus type 1 in human vestibular nuclei. Neurology. 2000;55:880–882. doi: 10.1212/wnl.55.6.880. [DOI] [PubMed] [Google Scholar]
- 18.Huppert D, Strupp M, Theil D, et al. Low recurrence rate of vestibular neuritis: a long-term follow-up. Neurology. 2006;67:1870–1871. doi: 10.1212/01.wnl.0000244473.84246.76. [DOI] [PubMed] [Google Scholar]
- 19.Leeuwen RB, Bruintjes TD. Recurrent vestibulopathy: natural course and prognostic factors. J Laryngol Otol. 2010;124:19–22. doi: 10.1017/S0022215109991009. [DOI] [PubMed] [Google Scholar]
- 20.Silvoniemi P. Vestibular neuronitis. An otoneurological evaluation. Acta Otolaryngol Suppl. 1988;453:1–72. doi: 10.3109/00016488809098974. [DOI] [PubMed] [Google Scholar]