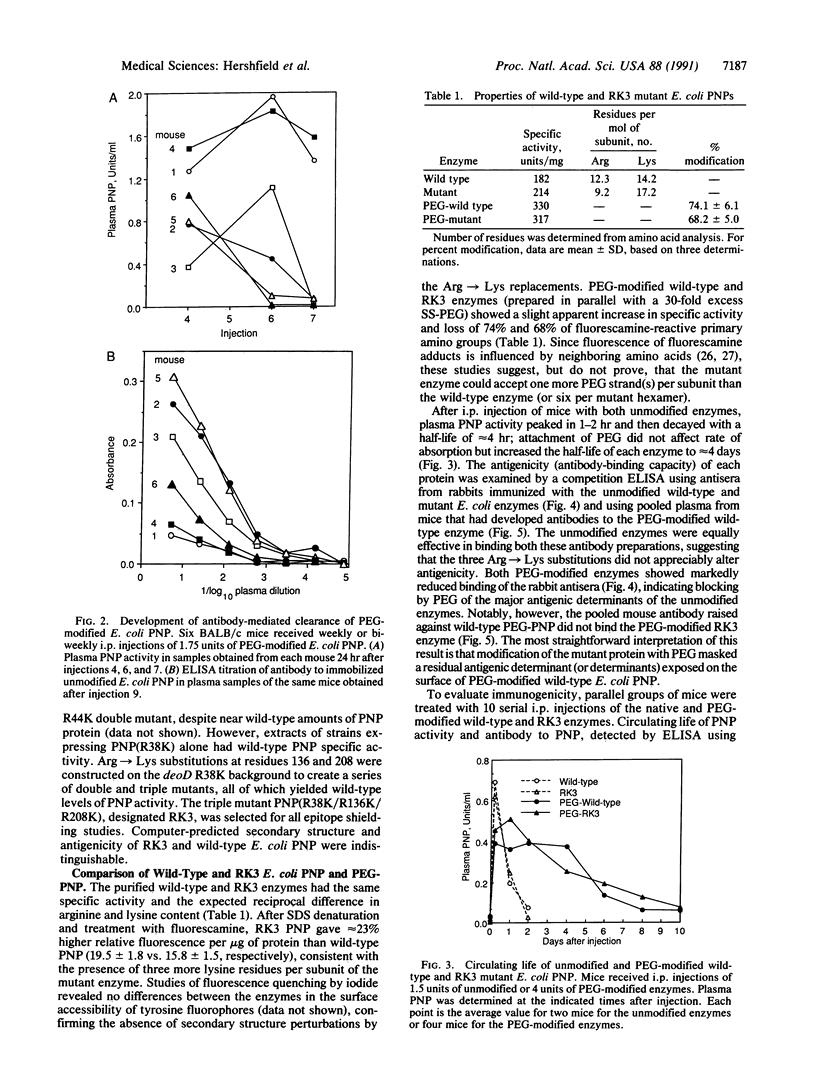
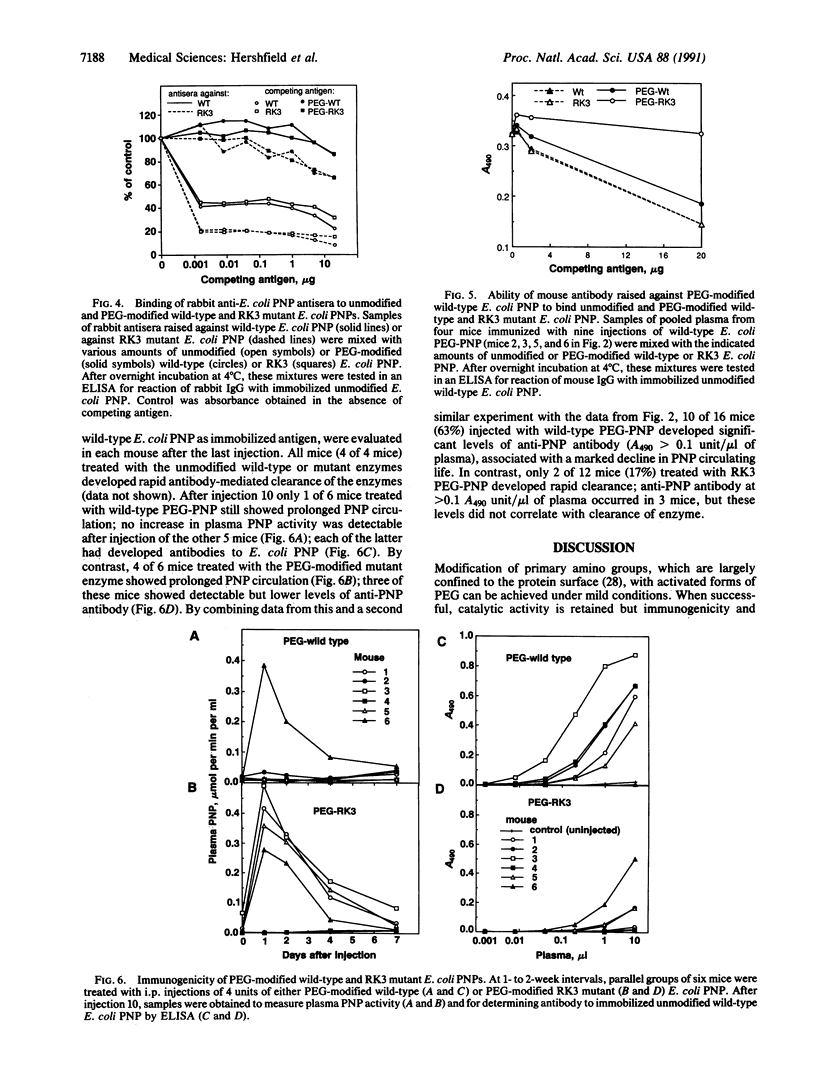
Abstract
Modification by covalent attachment of polyethylene glycol (PEG) can reduce the immunogenicity and prolong the circulating life of proteins, but the utility of this approach for any protein is restricted by the number and distribution of PEG attachment sites (e.g., epsilon-amino groups of lysine residues). We have developed a strategy for introducing additional sites for PEG attachment by using site-directed mutagenesis to selectively replace arginine with lysine codons and tested it with purine nucleoside phosphorylase (PNP) from Escherichia coli, an extremely stable but immunogenic enzyme, that could potentially be used to treat an inherited deficiency of PNP. A triple mutant, RK3, possessing three Arg----Lys substitutions was constructed that increased the number of lysines per PNP subunit from 14 to 17, providing an additional 18 potential PEG attachment sites per hexameric enzyme molecule. The wild-type and RK3 enzymes had similar catalytic activity, antigenicity, and immunogenicity. After PEG modification, both enzymes retained catalytic activity, the plasma half-life of both enzymes in mice increased from approximately 4 hr to 4 days, and the binding of both enzymes by antisera raised against each unmodified enzyme was markedly diminished. However, antibody raised against wild-type PEG-PNP did not bind the PEG-RK3 enzyme. PEG-RK3 PNP was also substantially less immunogenic than wild-type PEG-PNP. Accelerated antibody-mediated clearance of PEG-PNP occurred in 2 of 12 mice treated with PEG-RK3 PNP, compared with 10 of 16 mice treated with the modified wild-type enzyme. This combined use of directed mutagenesis and PEG modification is aimed at permitting the widest choice of proteins, including products of genetic and chemical "engineering," to be used for therapy of inherited and acquired disorders.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., Kazo G. M., Verhoest C. R., Jr, Van Es T., Kafkewitz D., Nucci M. L., Viau A. T., Davis F. F. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984 Jun;7(2):175–186. [PubMed] [Google Scholar]
- Abuchowski A., McCoy J. R., Palczuk N. C., van Es T., Davis F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977 Jun 10;252(11):3582–3586. [PubMed] [Google Scholar]
- Abuchowski A., van Es T., Palczuk N. C., Davis F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977 Jun 10;252(11):3578–3581. [PubMed] [Google Scholar]
- Barton N. W., Furbish F. S., Murray G. J., Garfield M., Brady R. O. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1913–1916. doi: 10.1073/pnas.87.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Fuerst T. R., Moss B. A soluble recombinant polypeptide comprising the amino-terminal half of the extracellular region of the CD4 molecule contains an active binding site for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2357–2361. doi: 10.1073/pnas.85.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealick S. E., Rule S. A., Carter D. C., Greenhough T. J., Babu Y. S., Cook W. J., Habash J., Helliwell J. R., Stoeckler J. D., Parks R. E., Jr Three-dimensional structure of human erythrocytic purine nucleoside phosphorylase at 3.2 A resolution. J Biol Chem. 1990 Jan 25;265(3):1812–1820. doi: 10.2210/pdb2pnp/pdb. [DOI] [PubMed] [Google Scholar]
- Fischer M., Short S. A. The cloning of the Escherichia coli K-12 deoxyribonucleoside operon. Gene. 1982 Mar;17(3):291–298. doi: 10.1016/0378-1119(82)90145-7. [DOI] [PubMed] [Google Scholar]
- Haber E., Quertermous T., Matsueda G. R., Runge M. S. Innovative approaches to plasminogen activator therapy. Science. 1989 Jan 6;243(4887):51–56. doi: 10.1126/science.2492113. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Buckley R. H., Greenberg M. L., Melton A. L., Schiff R., Hatem C., Kurtzberg J., Markert M. L., Kobayashi R. H., Kobayashi A. L. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med. 1987 Mar 5;316(10):589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Nygaard P. Purine nucleoside phosphorylase from Escherichia coli and Salmonella typhimurium. Purification and some properties. Eur J Biochem. 1975 Feb 3;51(1):253–265. doi: 10.1111/j.1432-1033.1975.tb03925.x. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Koszalka G. W., Tuttle J. V. Purine nucleoside synthesis, an efficient method employing nucleoside phosphorylases. Biochemistry. 1981 Jun 9;20(12):3615–3621. doi: 10.1021/bi00515a048. [DOI] [PubMed] [Google Scholar]
- Landgraf B., Cohen F. E., Smith K. A., Gadski R., Ciardelli T. L. Structural significance of the C-terminal amphiphilic helix of interleukin-2. J Biol Chem. 1989 Jan 15;264(2):816–822. [PubMed] [Google Scholar]
- Mably E. R., Fung E., Snyder F. F. Genetic deficiency of purine nucleoside phosphorylase in the mouse. Characterization of partially and severely enzyme deficient mutants. Genome. 1989 Dec;32(6):1026–1032. doi: 10.1139/g89-547. [DOI] [PubMed] [Google Scholar]
- McMillan C. W., Shapiro S. S., Whitehurst D., Hoyer L. W., Rao A. V., Lazerson J. The natural history of factor VIII:C inhibitors in patients with hemophilia A: a national cooperative study. II. Observations on the initial development of factor VIII:C inhibitors. Blood. 1988 Feb;71(2):344–348. [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Abildgaard C. F., Aledort L. M., Arkin S., Bloom A. L., Brackmann H. H., Brettler D. B., Fukui H., Hilgartner M. W., Inwood M. J. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. recombinant Factor VIII Study Group. N Engl J Med. 1990 Dec 27;323(26):1800–1805. doi: 10.1056/NEJM199012273232604. [DOI] [PubMed] [Google Scholar]
- Stocks S. J., Jones A. J., Ramey C. W., Brooks D. E. A fluorometric assay of the degree of modification of protein primary amines with polyethylene glycol. Anal Biochem. 1986 Apr;154(1):232–234. doi: 10.1016/0003-2697(86)90520-8. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Goddard J. M., Martin D. W., Jr Human purine nucleoside phosphorylase cDNA sequence and genomic clone characterization. Nucleic Acids Res. 1984 Jul 25;12(14):5779–5787. doi: 10.1093/nar/12.14.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]



