Abstract
Febrile infection-related epilepsy syndrome (FIRES) is a devastating epileptic encephalopathy with limited treatment options and an unclear etiology. Anakinra is a recombinant version of the human interleukin-1 receptor antagonist used to treat autoinflammatory disorders. This is the first report of anakinra for treatment of a child with super-refractory status epilepticus secondary to FIRES. Anakinra was well-tolerated and effective. Cerebral spinal fluid analysis revealed elevated levels of proinflammatory cytokines before treatment that normalized on anakinra, suggesting a potential pathogenic role for neuroinflammation in FIRES. Further studies are required to assess anakinra efficacy and dosing, and to further delineate disease etiology.
INTRODUCTION
Febrile infection-related epilepsy syndrome (FIRES) is a rare but devastating epileptic encephalopathy typically affecting school-aged children, characterized by a preceding febrile illness, followed by multifocal seizures and often refractory status epilepticus (RSE)1. With the exception of anaesthetic coma, which is not sustainable, and the ketogenic diet in some patients2, 3, treatment that effectively controls seizures is lacking in FIRES, and outcomes are generally poor1.
While an inflammatory etiology is suspected, informative biomarkers of inflammation have yet to be elucidated. Interleukin (IL)-1β is a prototypical proinflammatory cytokine implicated in a variety of autoinflammatory disorders4. IL-1β has ictogenic properties in various seizure models and contributes to the generation of febrile seizures in immature rodents5, 6. Anakinra is a recombinant version of the human IL-1 receptor antagonist, the selective endogenous antagonist of the IL-1 receptor type 1 (IL-1R1), which inhibits the biological actions of IL-1β4. Anakinra is currently approved for treating autoinflammatory diseases, including in neonates and children7,8, and has shown powerful anticonvulsant properties in animal models9.
We describe the use of anakinra to successfully treat super-RSE in a child with FIRES and maintain this seizure control over the subsequent twelve months. Together with cytokine analysis from serum and cerebral spinal fluid (CSF) before and after anakinra treatment, these findings suggest a pathologic role for neuroinflammation.
PATIENT AND METHODS
This study was performed with approval from the Mayo Clinic IRB (#08-007810) with a waiver for informed consent to use residual patient specimens for research purposes.
FIRES diagnosis
A developmentally normal 32 month old girl presented with her first generalized tonic-clonic seizure following a week-long febrile respiratory infection. She progressed into super-RSE requiring intubation and multiple antiepileptic medications. Her daily EEG seizure burden and treatment regimen during the first 65 days of ICU admission are outlined (Fig 1). Continuous video-EEG (cEEG) monitoring confirmed migrating, frequent repetitive, multifocal clinical and subclinical seizures arising independently from both hemispheres (Fig 2). Her daily EEG seizure burden was quantified as follows: 1) total number of seizures; and 2) maximum hourly seizure burden (maximal number of minutes per hour with seizures).
Figure 1.
Daily EEG seizure burden and treatment regimen (days 1-65 of pediatric ICU admission). Antiseizure medications were uptitrated rapidly to the following maximum daily doses: phenobarbital (20 mg/kg bolus, then 5 mg/kg/d); lacosamide (15 mg/kg/d); methylprednisolone (mp) (30 mg/kg/d); topiramate (15 mg/kg/d); propofol (60 mcg/kg/min); felbamate (83 mg/kg/d); and ketamine (3 mg/kg/h). The ketogenic diet was rapidly escalated to a 4:1 ratio (sustained ketosis was not achieved until day 47) and successfully weaned on day 98 (not shown). A midazolam infusion commenced on day 1 (daily infusion rate ranged from 0.01 mg/kg/h to 3 mg/kg/h), with a slow wean beginning on day 59 until it was discontinued on day 88 (not shown). Medications not shown include brief unsuccessful trials of levetiracetam (days 1-5) (max 63 mg/kg/d), fosphenytoin (days 1-2) (max 20 mg/kg/d), and ketamine (day 25) (1 mg/kg/h). Propofol was used briefly to break clusters of seizures (days 2, 4, 8). Clonazepam was added day 59 to help wean midazolam. DRESS = drug reaction with eosinophilia and systemic symptoms. cEEG = continuous electroencephalogram.
Figure 2.
Refractory subclinical status epilepticus depicted on a quantitative EEG trends panel displaying a 4 hour epoch of continuous video-EEG monitoring and 2 raw EEG epochs. Numerous multifocal (mainly subclinical) seizures migrating independently over the right and left hemispheres (A). Left (1) and right (2) rhythmicity spectrograms; left (3) and right (4) FFT power spectrograms, asymmetry spectrogram (5) (red = right hemisphere, blue = left hemisphere), amplitude integrated EEG (6) (red=right hemisphere, blue=left hemisphere). Electrodes were applied according to the international 10-20 system (software XLTEK and Persyst 12). The red vertical line on the trends panel corresponds to a focal right posterior (max P4/O2) subclinical seizure (B). The blue vertical line on the trends panel corresponds to a focal left posterior (max P7/O1) subclinical seizure (C). Raw EEG (10 second epochs) displayed on a longitudinal bipolar montage (10μV, LFF 0.16, HFF 70 Hz, 60 Hz notch) (B & C). Throughout her ICU course, EEG background activity featured mainly reactive, diffuse polymorphic delta activity, without the need for prolonged burst suppression pattern.
Extensive infectious (including 17 pathogen respiratory panel, HIV, CMV, EBV, HHV-6 and CSF HSV) and metabolic investigations (including CSF lactate, amino acids, and neurotransmitters) were negative. A comprehensive epilepsy genetics panel (GeneDx) was negative, including for PCDH19, POLG, and SCN1A. An extensive serum and CSF autoimmune epilepsy evaluation was also negative, including for antibodies against NMDA-R, neuronal (V-G) K+ channel, GAD65, GABA-B-R, and AMPA-R (for complete list of antibodies tested see www.mayomedicallaboratories.com/test-catalog/Overview/61511). Neuroimaging was initially normal (3Tesla brain MRI/V/A) (Fig 3A and C). Despite numerous antiepileptic drugs and a 3-day course of high-dose methylprednisolone, she continued in super-RSE with a putative diagnosis of FIRES.
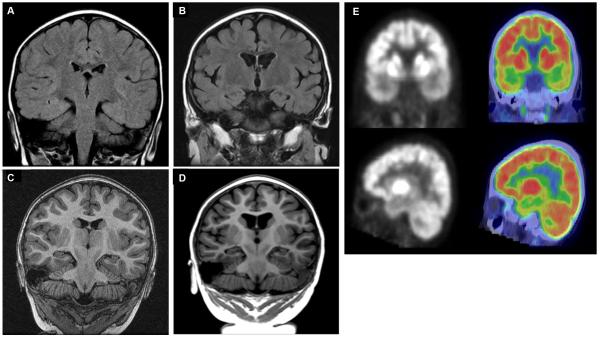
Figure 3.
Neuroimaging highlights MRI T2 FLAIR coronal sections performed on day 3 (A) and day 228 (B) of admission. MRI MPRage coronal sections performed on day 3 (C) and day 228 (D) of admission. While the initial acute imaging was normal (A&C), marked atrophy affecting both grey and white matter evolved to include the hippocampi, external capsules, corticospinal tracts, and corpus callosum (B&D). A F-18 FDG PET/CT scan performed on day 251 of admission (E). Images were obtained 30 minutes after injection with CT fusion imaging. Marked hypometabolism in the bilateral temporal lobes, insular cortex, and hippocampi are seen.
Anakinra treatment epoch #1 (day 6-23)
Anakinra commenced on day 6 at 5 mg/kg twice daily (total: 200 mg/day) via subcutaneous injections. After starting anakinra, the total number of daily seizures decreased from an average of 5.8 seizures/day (days 1-6) to 1.3 seizures/day (days 7-29) (P=0.009, Mann-Whitney test), and her maximum hourly seizure burden decreased from a mean of 10 min/hr (days 1-6) to 0.75 min/hr (days 7-29) (P<0.001, Mann-Whitney test). The ketogenic diet was also started on day 6, although ketosis was very difficult to achieve and her serum beta-hydroxybutyrate levels were not persistently over 2 mmol/L until day 47, despite aggressive efforts at maintaining a 4:1 ratio.
This inability to sustain ketosis was multifactorial but most attributable to developing a severe drug reaction with eosinophilia and systemic symptoms (DRESS) (day 22), during which time her survival became quite tenuous. Since the culprit was unclear, many medications were discontinued including anakinra and phenobarbital (day 23). She required prolonged ECMO support for severe bronchospasm before resuming conventional ventilation via her tracheostomy tube, and remained seizure-free when cEEG was discontinued (day 30).
Anakinra treatment epoch #2 (day 54-190)
On day 42, she developed paroxysmal clinical events featuring behavioral arrest and staring. CEEG was re-started and confirmed status epilepticus. It is unclear exactly when these seizures returned since she had been off cEEG. Anakinra was restarted on day 54. Her EEG seizure burden improved from an average of 8 seizures/day (days 42-53) to 0.17 seizures/day (days 54-65) (P=0.036, Mann-Whitney test) and her maximal hourly seizure burden declined from an average of 8.5 min/hr (days 42-53) to 0.08 min/hr (days 54-65) (P=0.066, Mann-Whitney test).
Comparing all EEG seizure data with and without anakinra across the first 65 ICU days, both the total number of seizures (P<0.001, two-way ANOVA) and the maximal hourly seizure burden (P=0.001, ANOVA) were significantly reduced. There was no statistically significant difference between anakinra treatment epochs 1 and 2 for both total seizures (P=0.820, two-way ANOVA powered at 0.946) and maximum hourly seizure burden (P=0.755, two-way ANOVA powered at 0.883), indicating that response to anakinra did not depend on treatment epoch.
CEEG (day 78) and the midazolam infusion (day 88) were discontinued in the context of occasional brief focal subclinical seizures and steadily improving encephalopathy. The ketogenic diet was successfully weaned (day 98), due to perceived inefficacy and concerns related to compliance feasibility.
Anakinra treatment epoch #3 (day 191-current)
We attempted to slowly wean anakinra (day 191) by removing one morning dose each week. During week seven of the wean (receiving once daily dosing) she began experiencing frequent clinical seizures. Full dose anakinra was reinitiated and she remained without clinical seizures for the next six weeks.
Repeat neuroimaging (day 228) showed diffuse cortical and subcortical volume loss (Fig 3B and D). A PET/CT scan (day 251) showed diffuse hypometabolism maximal over her bitemporal regions (Fig 3E). At outpatient follow-up, twelve months after her initial admission, her motor, verbal, and social development were within normal limits for age based on clinical exam alone. She continued to experience rare isolated focal clinical seizures on treatment with anakinra, felbamate, and levetiracetam.
Inflammatory cytokine analysis
Interleukin-8, IL-6, IL-1β, IL-10, IL-12p70, TNF-α, CCL2, CCL5, CXCL9, and CXCL10 were measured in the patient’s serum and CSF prior to starting anakinra (day 3) and repeated during anakinra treatment epoch #3 (day 254). Of these factors, IL-10, IL-12p70, and TNF-α were not detected and the chemokine measurements did not differ from healthy control levels. Using a flow cytometric bead array assay, IL-8 measured 4523 pg/mL in CSF (8% CV) before treatment and normalized to 15 pg/mL (3% CV) on anakinra (BD Bioscience human chemokine kit #552990, verified with the human inflammatory cytokine kit #551811; interassay variability was <10%). Normal pressure hydrocephalus and non-epilepsy autoimmune control CSF IL-8 levels ranged from 15-54 pg/mL (n=8). Serum IL-8 levels were 12 pg/mL (18% CV) before treatment and 20 pg/mL (19% CV) with anakinra. Healthy control serum IL-8 levels ranged from 8-21 pg/mL (n=7).
Using an enhanced sensitivity cytometric bead assay (BD Bioscience #561512), CSF IL-6 measured 252 pg/mL (21% CV) prior to anakinra, and 4 pg/mL (23% CV) during therapy. Normal pressure hydrocephalus CSF levels ranged from 2-7 pg/mL (n=4). Serum IL-6 levels were 2 pg/mL (25% CV) prior to therapy and 2 pg/mL (24% CV) during anakinra, with control serum levels ranging from 1-3 pg/mL (n=3).
Finally, IL-1β levels were undetectable using either a standard cytometric bead array or ELISA. Application of an enhanced sensitivity cytometric bead assay (BD Bioscience #561509) indicated 218 fg/mL IL-1β in CSF before therapy and 90 fg/mL during anakinra. Serum levels were 40 fg/mL before treatment and 434 fg/mL during treatment. However, the IL-1β measurements had very high variability (~40% CV) and signals were just above threshold of detection in the assay, limiting confidence.
DISCUSSION
Our patient has safely tolerated elevated doses of anakinra for over twelve months. During three separate treatment epochs, starting anakinra (epochs 1 & 2), or re-introducing it at full doses (epoch 3), was associated with improved seizure control. Twelve months after initial presentation, she continues to exceed her pre-illness developmental and cognitive levels but experiences rare focal seizures.
While good outcomes in children with FIRES are described, the vast majority develop profound neurological sequelae, refractory epilepsy, and many die or remain in a persistent vegetative state1. It remains to be seen whether our patient will experience language, behavior and memory difficulties later in childhood, as described in FIRES children with similar neuroimaging10.
Consistent with previous FIRES cohort studies, we did not identify neuronal autoantibodies11, or an infantile epilepsy genetic mutation12. However, case reports have suggested that children with an autoimmune epileptic encephalopathy and certain genetic epilepsies can mimic FIRES13,14. Our patient’s neuroimaging is consistent with that of many FIRES patients including her normal initial brain MRI gradually progressing to diffuse atrophy1, and diffuse predominantly bi-temporal hypometabolism10.
As commonly seen in encephalopathic critically-ill children, the majority of her seizures lacked any clinical signs and required cEEG to confirm their presence and assess treatment response15. The multifocal, migratory nature to her seizures is typical of FIRES patients1. Anakinra’s putative efficacy allowed us to avoid prolonged EEG burst suppression to control seizures, potentially contributing to her positive outcome16.
While the etiology of FIRES remains unclear, early disease overproduction of intrathecal proinflammatory cytokines and chemokines such as IL-6 and IL-8, and the effect of anakinra, an antiinflammatory drug that selective inhibits IL-1β actions by blocking IL-1R1, both implicate a pathogenic inflammatory process17. Our patient had high levels of IL-8 and IL-6 in her CSF at the time of presentation which anakinra likely helped to normalize. The normalization of IL-8 and IL-6 in CSF suggests that inhibition of the IL-1R1 by anakinra blocked a down-stream signaling cascade responsible for their central nervous system (CNS) production. Notably, high levels of IL-8 and IL-6 were not observed in her serum, suggesting local CNS production. Whether IL-8 or IL-6 in her CNS acted to recruit peripheral leukocytes, altered the function at the blood-brain barrier, or directly impacted microglia, astrocytes, or neurons functions to contribute to seizures remains to be determined. There is a complex relationship between inflammation and seizures which implicates a vicious cycle whereby seizures induce neuroinflammation which in turn can foster more seizures18. We cannot exclude therefore, that normalization of these inflammatory mediators is a consequence of seizure reduction.
Given the highly labile nature of IL-1β we are unable to conclude whether its levels were appreciably altered by treatment. Nonetheless, any effect mediated by anakinra is mediated by blockade of IL-1β biological actions. Although IL-1β plays a homeostatic role in normal brain physiology and in response to infection, prolonged tissue exposure to this cytokine contributes to pathologic processes like neurodegeneration, seizures and cognitive dysfunctions18. Accordingly, increased expression of IL-1β in microglia and astrocytes is a hallmark of pharmacoresistant epileptic foci in various symptomatic forms of epilepsy and related animal models18. Anakinra was demonstrated to reduce the duration and recurrence of seizures in rodents, and mice overexpressing the human recombinant protein in astrocytes are intrinsically resistant to seizures9, 19.
Anakinra is clinically available to treat several neonatal and pediatric autoinflammatory conditions7, 8. Children often require higher doses to achieve sustained remission20 and an effective steady-state concentration21. Doses ranging between 1-10 mg/kg/day (maximum typically100 mg/day) are reported, while our patient tolerated 5 mg/kg twice daily (200 mg/day). The duration of anakinra treatment in children with FIRES requires further study. After 6 months of seizure freedom we were unsuccessful in weaning to once daily dosing, but are preparing to try again. Some patients with autoinflammatory diseases require anakinra long-term and its longitudinal pediatric safety has been established22.
While promising, these results must be interpreted with caution. It is difficult to determine precisely which therapy resulted in seizure control at a given time. Increased electrographic seizures were at least in part controlled by escalating the midazolam infusion but these effects were temporary and seizures broke through despite unsustainable, extremely high doses of midazolam (Fig 1). Only with the addition of anakinra were we able to successfully decrease and wean the midazolam infusion (epochs 1 & 2). Further, seizure control was maintained for five months after midazolam and the ketogenic diet had been discontinued, and only recurred when we attempted to slowly wean anakinra (epoch 3).
While we showed dramatic seizure reduction on three separate occasions with anakinra, the diffuse brain atrophy often seen among patients with FIRES was not prevented, although it may have been modulated. Since the child had multiple severe seizures before starting anakinra treatment, earlier intervention might help to prevent the encephalopathic effect of seizures. While further studies are needed to assess anakinra efficacy, dosing and time of intervention, and to delineate FIRES’ etiology, our case offers care providers and families a relatively safe option for an often untreatable disease, and it provides insight into FIRES’ inflammatory pathophysiology.
ACKNOWLEDGEMENT
This work was supported by grants to C.L.H. from the National Institutes of Health (NS064571).
We thank Dr. Randy Cron, MD, PhD, Children’s Hospital of Alabama, Birmingham, AL for his input regarding the dosing of anakinra. Finally, we thank our patient and her family for allowing us to share their story and the innumerable care providers who contributed to her positive outcome.
Footnotes
AUTHOR CONTRIBUTIONS:
D.L.K., R.J.K., A.V., E.C.W., C.L.H., and E.T.P., contributed to conception and design of the study. All authors contributed to data acquisition or analysis of data, and helped to draft the text or prepare the figures.
POTENTIAL CONFLICTS OF INTEREST
The authors do not have any potential conflicts of interest.
REFERENCES
- 1.Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. 2011 Nov;52(11):1956–65. doi: 10.1111/j.1528-1167.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 2.Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010 Oct;51(10):2033–7. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 3.Singh RK, Joshi SM, Potter DM, Leber SM, Carlson MD, Shellhaas RA. Cognitive outcomes in febrile infection-related epilepsy syndrome treated with the ketogenic diet. Pediatrics. 2014 Nov;134(5):e1431–5. doi: 10.1542/peds.2013-3106. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature reviews Drug discovery. 2012 Aug;11(8):633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain, behavior, and immunity. 2011 Oct;25(7):1281–9. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Annals of neurology. 2005 Jan;57(1):152–5. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. The Journal of experimental medicine. 2005 May 2;201(9):1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ter Haar NM, Oswald M, Jeyaratnam J, et al. Recommendations for the management of autoinflammatory diseases. Annals of the rheumatic diseases. 2015 Sep;74(9):1636–44. doi: 10.1136/annrheumdis-2015-207546. [DOI] [PubMed] [Google Scholar]
- 9.Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proceedings of the National Academy of Sciences of the United States of America. 2000 Oct 10;97(21):11534–9. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzuca M, Jambaque I, Hertz-Pannier L, et al. 18F-FDG PET reveals frontotemporal dysfunction in children with fever-induced refractory epileptic encephalopathy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011 Jan;52(1):40–7. doi: 10.2967/jnumed.110.077214. [DOI] [PubMed] [Google Scholar]
- 11.van Baalen A, Hausler M, Plecko-Startinig B, et al. Febrile infection-related epilepsy syndrome without detectable autoantibodies and response to immunotherapy: a case series and discussion of epileptogenesis in FIRES. Neuropediatrics. 2012 Aug;43(4):209–16. doi: 10.1055/s-0032-1323848. [DOI] [PubMed] [Google Scholar]
- 12.Appenzeller S, Helbig I, Stephani U, et al. Febrile infection-related epilepsy syndrome (FIRES) is not caused by SCN1A, POLG, PCDH19 mutations or rare copy number variations. Developmental medicine and child neurology. 2012 Dec;54(12):1144–8. doi: 10.1111/j.1469-8749.2012.04435.x. [DOI] [PubMed] [Google Scholar]
- 13.Illingworth MA, Hanrahan D, Anderson CE, et al. Elevated VGKC-complex antibodies in a boy with fever-induced refractory epileptic encephalopathy in school-age children (FIRES) Developmental medicine and child neurology. 2011 Nov;53(11):1053–7. doi: 10.1111/j.1469-8749.2011.04008.x. [DOI] [PubMed] [Google Scholar]
- 14.Specchio N, Fusco L, Vigevano F. Acute-onset epilepsy triggered by fever mimicking FIRES (febrile infection-related epilepsy syndrome): the role of protocadherin 19 (PCDH19) gene mutation. Epilepsia. 2011 Nov;52(11):e172–5. doi: 10.1111/j.1528-1167.2011.03193.x. [DOI] [PubMed] [Google Scholar]
- 15.Payne ET, Hahn CD. Continuous electroencephalography for seizures and status epilepticus. Current opinion in pediatrics. 2014 Dec;26(6):675–81. doi: 10.1097/MOP.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 16.Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): does duration of anesthesia affect outcome? Epilepsia. 2011 Oct;52(Suppl 8):28–30. doi: 10.1111/j.1528-1167.2011.03230.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. Journal of neurology, neurosurgery, and psychiatry. 2015 Jul;86(7):820–2. doi: 10.1136/jnnp-2014-309388. [DOI] [PubMed] [Google Scholar]
- 18.Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Experimental neurology. 2013 Jun;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Vezzani A, Moneta D, Richichi C, et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl 5):30–5. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 20.Sibley CH, Plass N, Snow J, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis and rheumatism. 2012 Jul;64(7):2375–86. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urien S, Bardin C, Bader-Meunier B, et al. Anakinra pharmacokinetics in children and adolescents with systemic-onset juvenile idiopathic arthritis and autoinflammatory syndromes. BMC pharmacology & toxicology. 2013;14:40. doi: 10.1186/2050-6511-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullenberg T, Lofqvist M, Leinonen M, Goldbach-Mansky R, Olivecrona H. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology. 2016 May 3; doi: 10.1093/rheumatology/kew208. [DOI] [PMC free article] [PubMed] [Google Scholar]