Abstract
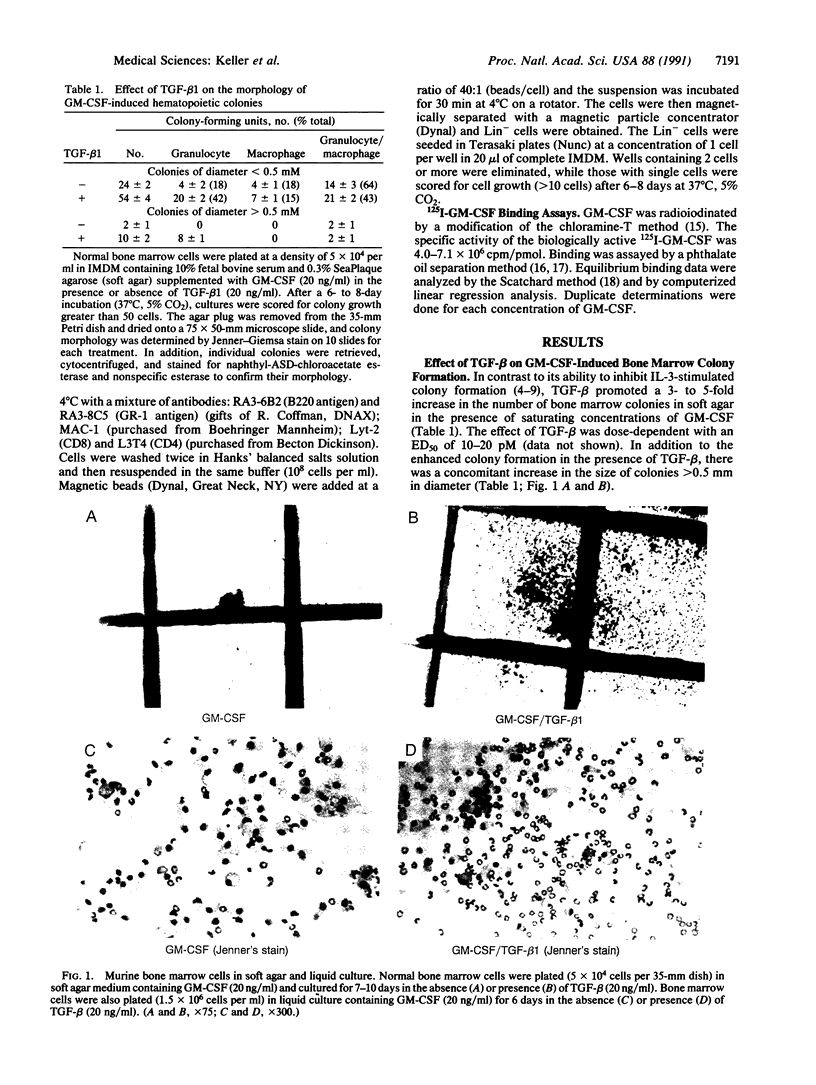
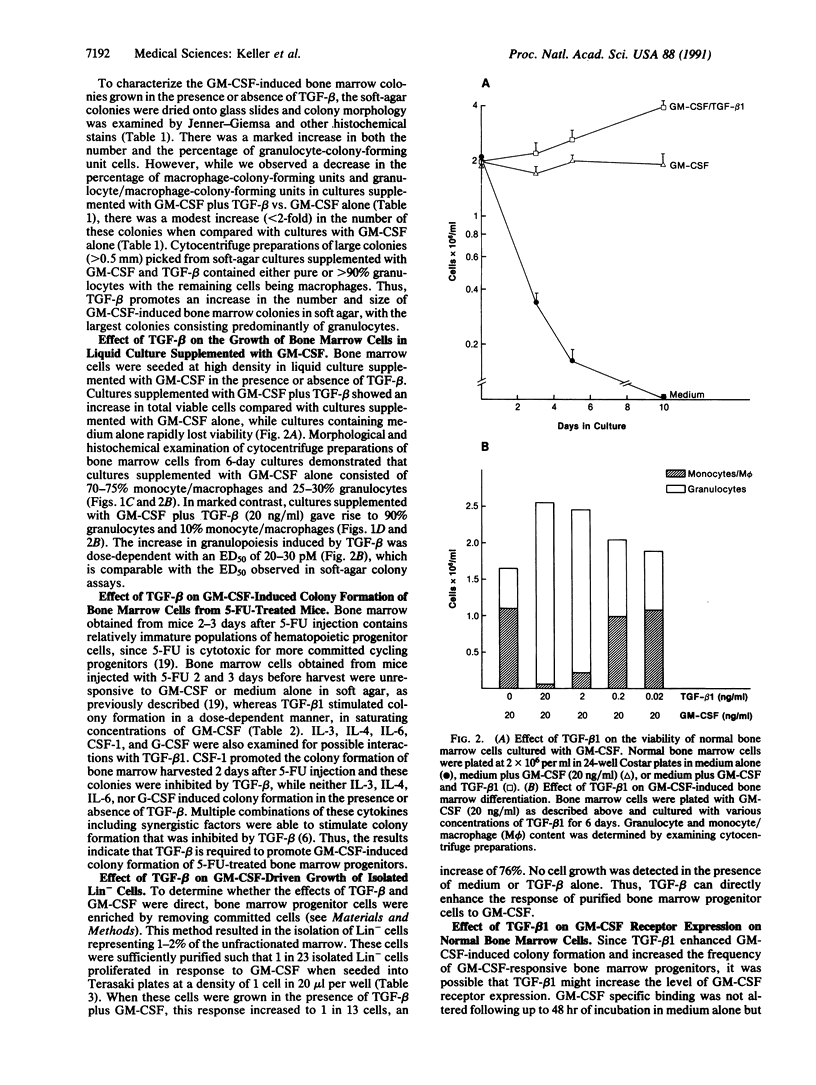
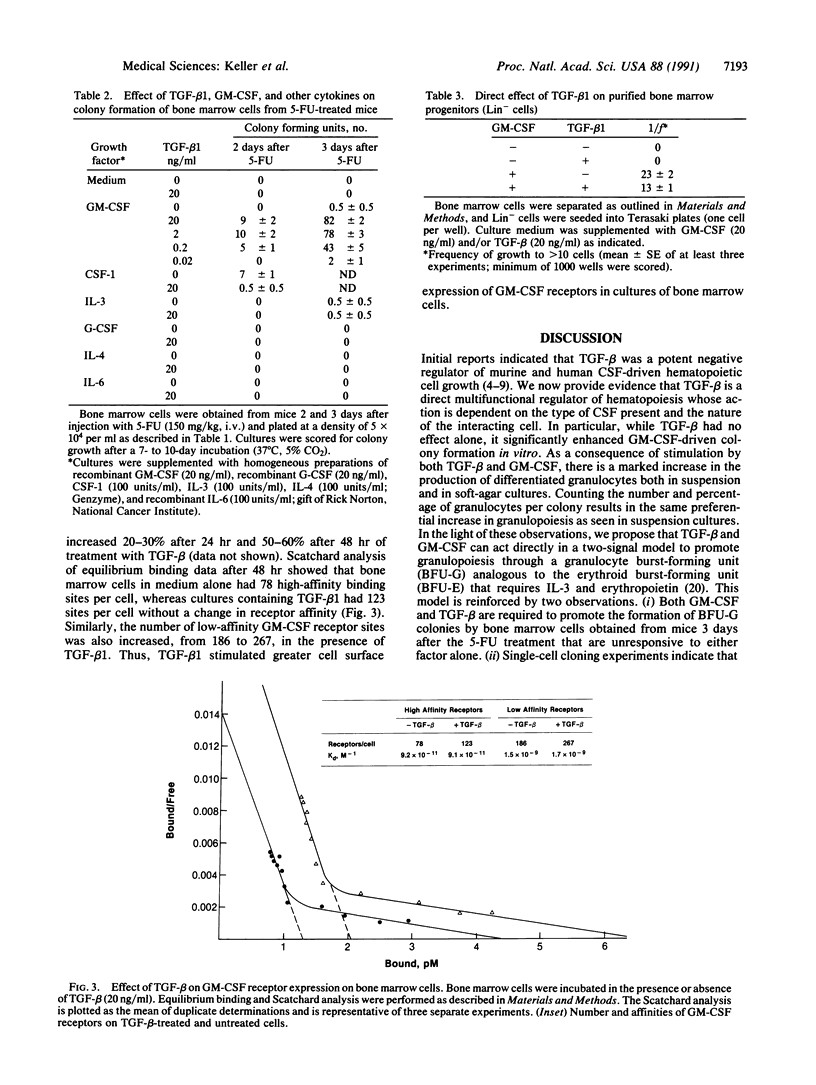
Transforming growth factor beta 1 (TGF-beta 1) is known to inhibit the growth of immature hematopoietic progenitor cells, whereas more mature, lineage-restricted progenitors are not inhibited. In contrast, in the presence of saturating concentrations of granulocyte/macrophage-colony-stimulating factor (GM-CSF), TGF-beta promoted a 3- to 5-fold increase in the number and size (greater than 0.5 mm) of bone marrow colonies in a dose-dependent manner with an ED50 of 10-20 pM; TGF-beta 1 alone had no effect. Morphological examination showed an increase in granulocyte colonies. In suspension cultures, TGF-beta 1 and GM-CSF stimulated an increase in total viable cells with markedly enhanced neutrophilic differentiation and a concomitant decrease in the number of monocytes/macrophages by day 6 in culture. Limiting dilution analysis demonstrated a 2- to 5-fold increase in the frequency of progenitor cells that responded to GM-CSF plus TGF-beta 1 vs. GM-CSF alone. Bone marrow progenitors obtained from mice 3 days after treatment with 5-fluorouracil responded to a combination of GM-CSF and TGF-beta 1, whereas either factor alone had no effect. A single-cell assay identified a progenitor cell that directly responded to TGF-beta and GM-CSF. TGF-beta increased the number of GM-CSF receptors on bone marrow cells. Thus, TGF-beta 1 can act as a bifunctional mediator of hematopoietic cell growth, and TGF-beta 1 and GM-CSF act together to stimulate granulopoiesis as measured by large granulocyte colony formation; the progenitor cell is tentatively designated granulocyte burst-forming unit.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S., Kriegler A. B., McNiece I. K. Generation of CFU-S13 in vitro. Prog Clin Biol Res. 1985;184:39–56. [PubMed] [Google Scholar]
- Dower S. K., Ozato K., Segal D. M. The interaction of monoclonal antibodies with MHC class I antigens on mouse spleen cells. I. Analysis of the mechanism of binding. J Immunol. 1984 Feb;132(2):751–758. [PubMed] [Google Scholar]
- Ellingsworth L. R., Brennan J. E., Fok K., Rosen D. M., Bentz H., Piez K. A., Seyedin S. M. Antibodies to the N-terminal portion of cartilage-inducing factor A and transforming growth factor beta. Immunohistochemical localization and association with differentiating cells. J Biol Chem. 1986 Sep 15;261(26):12362–12367. [PubMed] [Google Scholar]
- Goey H., Keller J. R., Back T., Longo D. L., Ruscetti F. W., Wiltrout R. H. Inhibition of early murine hemopoietic progenitor cell proliferation after in vivo locoregional administration of transforming growth factor-beta 1. J Immunol. 1989 Aug 1;143(3):877–880. [PubMed] [Google Scholar]
- Hill D. J., Strain A. J., Elstow S. F., Swenne I., Milner R. D. Bi-functional action of transforming growth factor-beta on DNA synthesis in early passage human fetal fibroblasts. J Cell Physiol. 1986 Aug;128(2):322–328. doi: 10.1002/jcp.1041280226. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Miller S. L., Burstein S. A. Type beta transforming growth factor is a potent inhibitor of murine megakaryocytopoiesis in vitro. Blood. 1987 Jun;69(6):1737–1741. [PubMed] [Google Scholar]
- Jacobsen S. E., Ruscetti F. W., Dubois C. M., Lee J., Boone T. C., Keller J. R. Transforming growth factor-beta trans-modulates the expression of colony stimulating factor receptors on murine hematopoietic progenitor cell lines. Blood. 1991 Apr 15;77(8):1706–1716. [PubMed] [Google Scholar]
- Keller J. R., Mantel C., Sing G. K., Ellingsworth L. R., Ruscetti S. K., Ruscetti F. W. Transforming growth factor beta 1 selectively regulates early murine hematopoietic progenitors and inhibits the growth of IL-3-dependent myeloid leukemia cell lines. J Exp Med. 1988 Aug 1;168(2):737–750. doi: 10.1084/jem.168.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. R., Mcniece I. K., Sill K. T., Ellingsworth L. R., Quesenberry P. J., Sing G. K., Ruscetti F. W. Transforming growth factor beta directly regulates primitive murine hematopoietic cell proliferation. Blood. 1990 Feb 1;75(3):596–602. [PubMed] [Google Scholar]
- McNiece I. K., Bertoncello I., Kriegler A. B., Quesenberry P. J. Colony-forming cells with high proliferative potential (HPP-CFC). Int J Cell Cloning. 1990 May;8(3):146–160. doi: 10.1002/stem.5530080302. [DOI] [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Ottmann O. G., Pelus L. M. Differential proliferative effects of transforming growth factor-beta on human hematopoietic progenitor cells. J Immunol. 1988 Apr 15;140(8):2661–2665. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Flanders K. C., Kondaiah P., Thompson N. L., Van Obberghen-Schilling E., Wakefield L., Rossi P., de Crombrugghe B., Heine U., Sporn M. B. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res. 1988;44:157–197. doi: 10.1016/b978-0-12-571144-9.50010-7. [DOI] [PubMed] [Google Scholar]
- Sing G. K., Keller J. R., Ellingsworth L. R., Ruscetti F. W. Transforming growth factor beta selectively inhibits normal and leukemic human bone marrow cell growth in vitro. Blood. 1988 Nov;72(5):1504–1511. [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D., Maritz J. S., Yeo G. F. Standardized bioassay for bone marrow colony stimulating factor in human urine: levels in normal man. J Lab Clin Med. 1972 Apr;79(4):657–668. [PubMed] [Google Scholar]







