Abstract
The aim of this paper was to propose key steps for community pharmacist integration into a patient care pathway for chronic obstructive pulmonary disease (COPD) management. A literature search was conducted to identify publications focusing on the role of the community pharmacist in identification and management of COPD. The literature search highlighted evidence supporting an important role for pharmacists at each of the four key steps in the patient care pathway for COPD management. Step 1 (primary prevention): pharmacists are ideally placed to provide information on disease awareness and risk prevention campaigns, and to encourage lifestyle interventions, including smoking cessation. Step 2 (early detection/case finding): pharmacists are often the first point of contact between the patient and the healthcare system and can therefore play an important role in the early identification of patients with COPD. Step 3 (management and ongoing support): pharmacists can assist patients by providing advice and education on dosage, inhaler technique, treatment expectations and the importance of adherence, and by supporting self‐management, including recognition and treatment of COPD exacerbations. Step 4 (review and follow‐up): pharmacists can play an important role in monitoring adherence and ongoing inhaler technique in patients with COPD. In summary, pharmacists are ideally positioned to play a vital role in all key stages of an integrated COPD patient care pathway from early disease detection to the support of management plans, including advice and counselling regarding medications, inhaler technique and treatment adherence. Areas requiring additional consideration include pharmacist training, increasing awareness of the pharmacist role, administration and reimbursement, and increasing physician–pharmacist collaboration.
Keywords: adherence, bronchodilators, community pharmacy, diagnosis, inhaler technique, integrated care pathway
Table of Links
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
As the fourth leading cause of mortality worldwide, chronic obstructive pulmonary disease (COPD) represents a major public health challenge 2. Indeed, in 2012, more than 3 million people worldwide died of COPD, equating to 6% of all deaths globally in that year 3. Although preventable and treatable 2, the burden of COPD is set to continue or worsen, with estimates from the World Health Organization (WHO) predicting that it will be the third leading cause of mortality worldwide by 2030 4, 5. In addition to its impact on patients' lives, COPD is associated with spiralling healthcare resource use and costs 2. For example, in the EU total COPD‐related expenses for outpatient and inpatient care are approximately €4.7 and €2.9 billion/year, respectively 5. In the UK, the costs associated with COPD are estimated to exceed £800 million 6. In the USA, more than 26 million people are estimated to have COPD but almost half of these are undiagnosed 7. Therefore, there remains much to do in terms of early detection and treatment of COPD, before patients reach symptomatic and costly disease stages 8.
The treatment of patients with COPD is complex and encompasses many different issues, such as early and correct diagnosis, primary and secondary prevention, and multiple pharmaceutical and nonpharmaceutical interventions. One of the most important issues, however, is motivating patients fully to participate in and adhere to their individualized management plan, including maintenance and self‐management aspects, such as lifestyle adaptation.
Most patients with COPD are seen in primary care but resource pressures within the healthcare service may limit physician–patient contact time 9. An integrated approach which utilizes contact time with other healthcare services, including community pharmacists, may therefore be beneficial for the efficient delivery of COPD management plans. Given their skill set, frequent contact with patients and expertise regarding available treatments, pharmacists may be well placed to have an input into the management and motivation of patients with COPD. Added to this is the convenience of the community pharmacy to patients, in terms of location, opening times and ‘open door’ consultation opportunities 10.
The present review proposes key steps of an integrated patient care pathway in which community pharmacists are best placed to help to optimize the diagnosis and management of COPD (Figure 1). In doing so, the evidence for the role of the pharmacist in primary prevention, screening and early diagnosis, management and follow‐up/review stages of the pathway is summarized.
Figure 1.
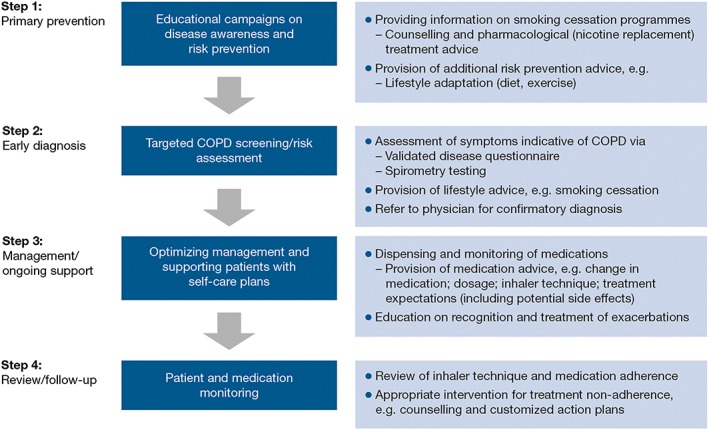
Role of the community pharmacist in an integrated patient care pathway for chronic obstructive pulmonary disease (COPD)
Methods
A literature review was conducted to find publications focusing on the role of the community pharmacist in identifying, managing and following up patients with COPD, as well as on the pharmacological treatments used in COPD and potential challenges associated with community pharmacist care.
PubMed searches were used to identify relevant English‐language papers over the past 10 years. Key search terms included, but were not limited to, chronic obstructive pulmonary disease, COPD, management, role of pharmacist, community pharmacist, spirometry, and inhaler techniques. Further articles were identified by cross‐referencing; key guidelines/health reports were also consulted. Appropriate information and data are included here, and the majority of the references cited were published within the last 5 years.
Results
Background: what do we mean by an integrated patient care pathway?
Integrated patient care pathways, also referred to as coordinated care pathways or care maps, may be defined as ‘structured multidisciplinary care plans’, detailing key steps in the care of a patient with a specific illness 11. Integrated patient care pathways were introduced to aid the translation of national guidelines into local management protocols and subsequent implementation into daily clinical practice. As well as promoting more focused patient care, this approach encourages multidisciplinary communication and care planning, which might ultimately translate into improved patient outcomes, e.g. quality of life, reduced complications or hospital admissions, better patient satisfaction and improved treatment adherence 11, 12, 13. In general, the steps constituting a patient care pathway include, but are not limited to: (i) primary prevention; (ii) screening and diagnosis; (iii) management of disease; and (iv) follow‐up and review of care. The following discusses the potential role that can be played by the community pharmacist for each of these four steps in a patient care pathway for COPD.
Step 1: primary prevention
Cigarette smoking is the leading cause of COPD in the Western world (85% of all COPD cases are attributed to smoking) 2, 14, 15. Compared with nonsmokers, cigarette smokers have a higher prevalence of respiratory symptoms/lung function abnormalities, a greater annual decline in their forced expiratory volume in 1 s (FEV1) profile, and are at greater risk of death from COPD 2, 16. Community pharmacists play a vital role in providing information on available smoking cessation programmes.
Local pharmacies are ideal venues for disease awareness and risk prevention campaigns 10, 14. In general, the level of awareness of COPD, its symptoms and its association with cigarette smoking is inadequate, with many believing that dyspnoea and limitations in performing daily activities or exercise are a simple part of the ageing process 14. As such, an important aspect of disease education is communicating the association between cigarette smoking and COPD, and the impact of COPD symptoms on daily activities and overall quality of life. Community pharmacists can assist by providing behavioural support to help with smoking cessation and by advising individuals on the correct use of nicotine replacement therapies 2, 17. Both pharmacological treatment and counselling have been shown to be effective strategies in smoking cessation 2, 17, 18. In a recent meta‐analysis of five studies involving 1426 smokers, pharmacist‐based interventions were associated with improved abstinence rates compared with a control group (relative risk 2.21, 95% confidence interval [CI] 1.49, 3.29) 19.
While smoking cessation is one of the key elements in COPD risk prevention, pharmacist‐directed disease education (in the form of awareness and risk prevention campaigns or motivating self‐management/medication adherence) may serve as an ideal portal to emphasize simultaneously the benefits of general lifestyle interventions. Healthy lifestyle options, including diet/nutrition, weight management and physical activity 20, 21, 22, can help to prevent additional complications arising from any chronic disease and reduce the risk of mortality 23. There are few data reporting lifestyle outcomes in patients with COPD as a result of pharmacist counselling. One study showed that patients with COPD who received structured intervention (including disease and medication education, inhaler technique, exercise and relaxation techniques) by a clinical pharmacist had no significant difference in their body mass index compared with patients who received usual outpatient care 20. A more recent study in India, however, demonstrated an improvement in all subscales of a questionnaire (St George's Respiratory Questionnaire [SGRQ]) that assessed quality of life (i.e. symptoms, activity and impact) in patients receiving pharmacist intervention, which included an emphasis on medication compliance, smoking cessation, simple exercise and the correct use of inhaler devices, compared with control patients who received standard hospital care 24. Further research focusing specifically on pharmacist‐led lifestyle counselling in COPD and associated outcomes is therefore of interest.
Step 2: early detection/case finding
Timely COPD diagnosis is important because it can result in earlier intervention (pharmacological or nonpharmacological) that may lead to improved quality of life and reduced healthcare burden 8, 14. Community pharmacists can play an important role in the early diagnosis of COPD, as pharmacists are often the first point of contact between the patient and the healthcare system. Based on a patient's needs when presenting to a pharmacist (e.g. advice for breathlessness) or medication needs (e.g. a cough remedy or nicotine replacement therapy), the community pharmacist can screen for COPD via completion of a validated disease risk assessment questionnaire (e.g. COPD Population Screener) 25 and spirometry testing; if appropriate, the pharmacist may offer lifestyle advice and refer that patient to their physician for confirmatory diagnosis and treatment 10, 26, 27. Studies showing that trained community pharmacists are able to perform accurate and reproducible spirometry screenings 28 are supported by a recent evidence‐based review demonstrating acceptable repeatability of spirometry testing (based on the American Thoracic Society/European Respiratory Society guideline standards) by pharmacists in all eight studies included in the analysis 29. Moreover, two recent publications showed that trained community pharmacists are able to detect individuals at high risk of COPD effectively via COPD case‐finding services 26, 27. Indeed, UK findings revealed that targeted screening could identify one patient with moderate COPD for every two individuals screened, and that early identification led to cost savings 26. In the Spanish case‐finding study, whereby community pharmacists received 16 h of COPD and spirometry training, 63.4% (N = 1456) of programme participants were found to be at a high risk of COPD [high risk was defined as a ‘yes’ answer to ≥3 questions relating to age, smoking exposure and symptoms on a five‐item Global Initiative for Chronic Obstructive Lung Disease (GOLD) screening questionnaire]; of these, 282 (19.8%) participants had prebronchodilator airflow limitation (FEV1/forced vital capacity <0.70), as confirmed by spirometry, and were requested to contact their primary care physician 27. In this particular study, the need for improvement in collaboration between primary care physicians and the community pharmacies was emphasized.
Step 3: management and ongoing support
Once a confirmatory diagnosis of COPD has been obtained and the patient has been prescribed appropriate treatment by their physician, the pharmacist can assist patients with disease management, not only by dispensing medications, but also by providing advice and education on dosage, inhaler technique, treatment expectations and the importance of adherence, and by supporting self‐management 10, 14, 15. Patients with long‐term conditions such as COPD are also encouraged to receive a yearly flu vaccination 30. Pharmacists can be key drivers in achieving flu vaccination targets, especially in these patient groups who tend to visit pharmacies more frequently. However, a recent report of a UK (London)‐based pharmacy initiative that compared annual influenza vaccine uptake from before pharmacy vaccination was introduced (from 2011) to after its introduction (2013–2015) showed no significant change in uptake for any of the risk groups studied, including chronic respiratory disease; nevertheless, economic benefits were incurred compared with general practitioner (GP) vaccine delivery 31.
Important elements of an optimal pharmacotherapeutic regimen
What constitutes an effective treatment? Simply, this is a therapy that a patient takes as prescribed over the long term because it is easy to administer and effectively reduces day‐to‐day symptoms, and because patients are aware of the potential long‐term benefits in terms of reductions in exacerbation risk (with exacerbation defined as an acute event characterized by worsening respiratory symptoms beyond normal day‐to‐day variations, leading to a change in medication) and lung function decline. Of course, this simple answer constitutes a realm of complex and interweaving components.
Overview of pharmacotherapy
Over recent years, significant advances have been made in COPD treatment, with numerous pharmacological options available that serve to provide individualized treatment plans. The GOLD Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease document provides pharmacological treatment recommendations for patients based on a combination of symptomatic assessment, spirometric classification and/or risk of exacerbations 2. Based on this combined assessment, patient groups are categorized as ‘low risk, fewer symptoms’ (Group A), ‘low risk, more symptoms’ (Group B), ‘high risk, fewer symptoms’ (Group C) and ‘high risk, more symptoms’ (Group D). Typically, low‐risk patients are those with mild or moderate airflow limitation and/or 0–1 exacerbation/year and no hospitalization for exacerbations, whereas high‐risk patients are those with severe or very severe airflow limitation and/or ≥2 exacerbations/year or ≥1 exacerbation necessitating hospitalization.
Most low‐risk COPD patient groups (i.e. GOLD Groups A and B) can be managed with long‐acting, inhaled bronchodilators as a first‐choice or alternative‐choice maintenance treatment, i.e. long‐acting β2‐agonists (LABAs; e.g. salmeterol, formoterol and indacaterol) or long‐acting muscarinic antagonists (LAMAs; e.g. tiotropium, glycopyrronium and umeclidinium) 2. Owing to their reported effects in terms of improvement in lung function, patient‐reported outcomes and exacerbation rates, LABAs and LAMAs are central to the management of COPD 2. While there is evidence suggesting that some of the newer LABAs (e.g. indacaterol) may provide greater improvements over LAMAs (e.g. tiotropium) in terms of symptoms 32, and that LAMAs (e.g. tiotropium) may provide greater improvements over LABAs (e.g. salmeterol or indacaterol) in terms of exacerbation rates 33, 34, the choice of treatment will depend on availability and individual patient response 2.
Treatment can then be intensified according to GOLD patient group by adding a second bronchodilator agent of a different class (e.g. LABA/LAMA fixed‐dose combinations), the addition of an inhaled corticosteroid (ICS) and by the use of rescue medication (as required) to allow patients to manage occurrences of worsening symptoms. For example, in patients categorized as high risk (GOLD Groups C and D), ICS/LABA and/or a LAMA are recommended as first‐choice therapies 2. LABA/LAMA combinations are associated with potent bronchodilation, irrespective of whether the patient is considered to be low‐ or high risk 35, 36, 37. Individual LABA/LAMAs have also been shown significantly to improve symptoms (indacaterol/glycopyrronium 35 and tiotropium/olodaterol 38), health status (indacaterol/glycopyrronium 35, tiotropium/olodaterol 38, and umeclidinium/vilanterol 39) and exacerbation rates (indacaterol glycopyrronium 40), compared with single agents. Further, indacaterol/glycopyrronium has been shown to be superior to ICS/LABA in terms of lung function and symptoms 41, 42, and in higher‐risk patients in terms of exacerbations and health status 2, 43.
In spite of GOLD recommendations concerning ICS use in high‐risk populations only 2, and their association with a heightened risk of pneumonia in COPD patients 44, ICSs are prescribed across all severities of COPD, independent of exacerbation risk 45. Nevertheless, data are emerging that show that it is possible to withdraw ICSs from treatment regimens in low‐risk patients in whom they are not indicated 46 and in high‐risk patients in whom there may be concerns about the risk of pneumonia 47, without having a negative impact on exacerbation risk, provided that adequate bronchodilation is in place.
Additional maintenance treatment options in COPD include the phosphodiesterase‐4 inhibitors (e.g. roflumilast) and the mucolytics (e.g. carbocysteine or N‐acetylcysteine), both recommended by GOLD for patients with severe‐to‐very‐severe airflow limitation and frequent exacerbations not adequately controlled by long‐acting bronchodilators 2. Management options for people suffering an exacerbation are short‐acting bronchodilators, oral or intravenous corticosteroids, and/or antibiotics when there are signs of bacterial infection during such episodes 2.
Inhaler characteristics
Another important factor to consider when discussing optimal drug therapy is the inhaler used to administer the medication, as incorrect use of an inhaler can lead to suboptimal drug delivery to the lungs and reduced disease control 48, 49. Different inhaler types have their own advantages and disadvantages. For example, one of the key benefits of dry‐powder inhalers (DPIs) compared with pressurized metered‐dose inhalers is that they are breath actuated and therefore overcome the potential problems of coordination of actuation and inhalation; however, breath‐actuated inhalers require patients to generate sufficient inspiratory effort to overcome the internal resistance of the inhaler 48. Key features affecting patients' preference for a specific inhaler device include perceived device efficacy, ease of use and its convenience 50. Various studies have assessed the relative patient preference for different DPIs, with variable results 48, 51, 52. Irrespective of how well designed an inhalation device might be, it is important to recognize that initial appropriate inhaler technique may be lost over time 53. This reinforces the need for continued education/monitoring of inhaler use and represents an important area in which community pharmacists can assist in COPD management.
Pharmacist role in supporting a management plan.
Inhaler technique
Pharmacists can play a vital role in supporting patients with their COPD management plan, including monitoring to ensure the appropriate and effective use of medications to minimize exacerbation/hospitalization risk 54. For many patients with COPD, mastering inhaler technique is a major challenge, which can impede effective treatment 14. Some patients require multiple inhalers, each with a different method of use, as well as other medications for comorbid diseases. Pharmacists can help by instructing patients on how to use their inhaler(s) properly, regularly checking technique and intervening where necessary 55. Moreover, by reviewing the number of daily doses and monitoring unnecessary device changes, a pharmacist can potentially make recommendations for medication changes, e.g. to a fixed‐dose combination product (several such products are now approved) 14.
In the Pharmaceutical care for patients with COPD (PHARMACOP) 3‐month study, conducted in 170 community pharmacies in Belgium, patients with COPD were randomized to a protocol‐based pharmacist intervention (n = 371), which focused on inhalation technique and adherence to maintenance therapy, or a control (usual pharmacist care) group (n = 363) 54. A protocol‐based pharmacist intervention was associated with significant improvements in inhaler technique (a 13.5% increase in the percentage of correct steps performed compared with the control group, P < 0.0001), adherence to maintenance medication, as assessed by the Medication Refill Adherence score (treatment difference of 8.51, P < 0.0001) and hospitalization rate (nine events with the pharmacist intervention vs. 35 events in the control group; estimated rate ratio 0.28, P = 0.003). In a separate cost‐effectiveness analysis, the PHARMACOP intervention resulted in overall costs per patient of €2221 within a 1‐year timeframe, compared with €2448 for usual care, leading to a cost saving per patient of €227 56.
Self‐management/action plans
Community pharmacists also support patients in the context of ‘self‐management’ plans, helping to empower those with COPD to take control of their condition 57. One definition of self‐management is ‘… manage the symptoms, treatment, physical and psychosocial consequences and life style changes inherent in living with a chronic condition ... [the] ability to monitor one's condition and to effect the cognitive, behavioural and emotional responses necessary to maintain a satisfactory quality of life’ 58.
In COPD, self‐care usually refers to the management of acute exacerbations but also includes lifestyle interventions (e.g. smoking cessation, exercise, nutrition and stress management) 57. COPD exacerbations have a negative impact on patient quality of life and are associated with increased hospital admission rates, accelerated lung function decline and significant mortality in cases requiring hospital admission 2, 56, 59, 60, 61. As such, action plans that assist patients with recognizing exacerbation onset and encourage prompt management can be of considerable benefit to the patient and the healthcare system (Figure 2 shows an example from the UK) 57. In this regard, pharmacists in the UK can refer patients to their GP to obtain a COPD rescue pack that contains home supplies of antibiotics and corticosteroids to be taken in the event of an exacerbation 62, 63. Pharmacists are also well placed to assist patients in agreeing an action plan, advising on how to recognize an acute exacerbation and when/how to use the rescue pack, as well as monitoring use of both maintenance and rescue medication 54, 57.
Figure 2.
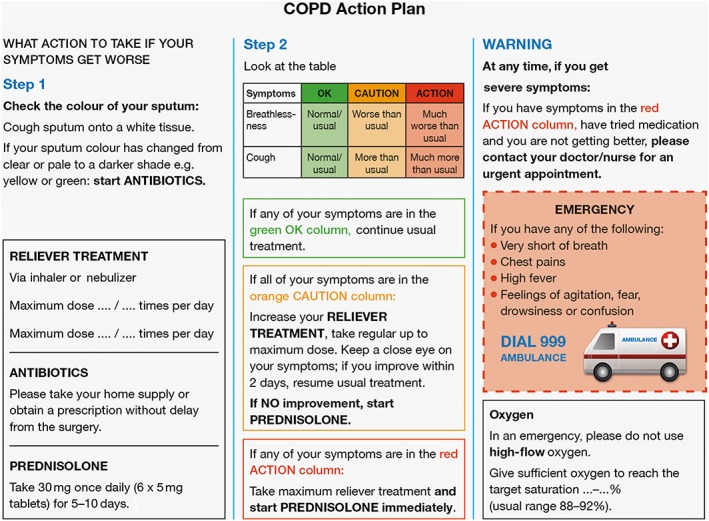
Example of a typical UK‐based chronic obstructive pulmonary disease (COPD) action plan (example action plan reproduced with the permission of Dr Rupert Jones 57)
The impact of pharmacy‐based intervention and self‐management has been investigated in patients with COPD. Studies have demonstrated benefits with regard to improved quality of life, increased medication adherence, and reductions in doctor visits and hospitalization rates 15, 20, 63. Furthermore, an update of the Cochrane systematic review of self‐management for patients with COPD (comprising 23 studies and 3189 participants) demonstrated, that compared with usual care, self‐management interventions were associated with improvements in health‐related quality of life (HRQoL), as assessed by SGRQ total score (mean treatment difference − 3.51; 95% CI −5.37, −1.65), and breathlessness, as measured by the modified Medical Research Council scale (mean treatment difference − 0.83; 95% CI −1.36, −0.30), and a lower probability of respiratory hospitalizations (odds ratio 0.57; 95% CI 0.43, 0.75) 57. In this review, no significant effect of self‐management vs. usual care was reported for all‐cause hospitalizations or mortality 64.
Step 4: review and follow‐up
Challenge of treatment adherence
Central to a community pharmacist's role in the framework of an integrated COPD care service is monitoring follow‐up, which may coincide with the collection of prescriptions 63. In the UK, annual systematic reviews of both inhaler technique (discussed above) and medications is recommended 63, 65. One aspect of the medication review relates to treatment adherence, which is a key area for community pharmacist involvement 14, 21.
For many chronic illnesses, including COPD, non‐adherence is a major challenge (adherence to treatment averages 50%) 14, 21. Non‐adherence is associated with adverse clinical/economic outcomes 66 and may arise for many reasons, including: complex treatment/dosing regimens, difficulties with using devices (e.g. inhalers), adverse treatment effects, concerns about drug dependency, and denial of illness severity 14, 67. The fact that community pharmacists are in a position to monitor long‐term dispensing data provides them with the necessary tools to identify patients who show signs of non‐adherence and intervene as necessary 21.
Optimizing management
The Medication Monitoring and Optimization (MeMO) programme in the Netherlands is a targeted pharmacist programme for improving adherence to long‐term therapy for the management of osteoporosis, cardiovascular disease and asthma/COPD 21. The initial phase of the programme encompasses structured counselling sessions with patients and is followed by monthly monitoring of treatment adherence via the pharmacy dispensing database. Results from the MeMO asthma/COPD programme showed a significant decrease in the frequency of exacerbations (−0.82 exacerbations/patient/year) as well as a decrease in total healthcare costs of €333 per patient, but minimal or no changes in terms of adherence or quality of life 68. The latter findings were similar to those of a US study in patients with COPD or asthma that compared pharmacist care, usual care and a peak expiratory flow rate monitoring control group 69. In this particular study, which assessed patients at 6 months and 12 months, there were no between‐group differences in treatment compliance or HRQoL, although a significant difference in treatment satisfaction was recorded in the pharmacist care group. Notably, for patients with osteoporosis, the MeMO programme resulted in a significant reduction in treatment discontinuation (from 31.7% to 16.1%, P < 0.001) 21. Furthermore, in a recent study of 154 respiratory patients (aged 18–65 years) that examined the effects on treatment satisfaction and adherence of patient counselling at discharge and follow‐up by a pharmacist, significant differences were reported in favour of pharmacist counselling 70. The results showed a 42.9% increase in medication adherence in the study group compared with the control group, as well as a 33.5% increase in treatment satisfaction. As discussed above, the PHARMACOP study also demonstrated a significant improvement in medication adherence following a protocol‐based pharmacist intervention 54.
In the UK, pharmacy services, including the Medicines Use Review (MUR) 71 and Prescription Intervention Service and the New Medicine Service (NMS) 72, aim to improve safety and treatment adherence, and reduce medicine wastage by supporting patients with long‐term conditions. The MUR service consists of pharmacists who conduct structured adherence‐focused reviews with patients on multiple medications for long‐term conditions 71. The NMS, which has been shown significantly to improve adherence, supports patients who have been prescribed a medicine for the first time for certain conditions, including COPD, and comprises three patient–pharmacist appointments, conducted fortnightly 72, 73.
Given that even a brief counselling session with patients on the importance of taking medications can result in significant improvements in adherence, more intensive, individually tailored, pharmacist‐delivered interventions, such as those described above, should result in beneficial outcomes. Indeed, one study reported positive results with individualized, pharmacist–patient educational sessions on COPD, medications and inhaler techniques, and the construction of customized action plans 20.
Are there any limitations associated with community pharmacist intervention?
Ensuring the continuity of healthcare quality
While pharmacist care/intervention helps to provide benefits in terms of medication adherence, patient satisfaction with treatment and various clinical advantages, such as a reduced number of hospitalizations or improvements in aspects of HRQoL among patients with COPD 22, there may be a number of areas in the context of expanding community pharmacy services that require further focus and planning. For example, in the aforementioned UK pilot flu vaccination programme 31, online surveys among participating GPs and pharmacists revealed a discordance in opinions related to support for the pharmacy initiative, with a 50/50 split among GPs on whether pharmacy intervention increased or decreased administrative burden. Moreover, 61% of GPs voiced concern with respect to loss of patient data resulting from incomplete reporting by pharmacists. Additionally, GPs were concerned about the potential for reduced quality of healthcare for their patients, safety issues and personal financial loss 31. As such, there is need to ensure: (i) adequate provision of additional pharmacist training; (ii) the introduction of mechanisms for reimbursement; (iii) the education of other healthcare professionals/the general public in relation to the additional services offered by community pharmacists; and (iv) that improvements are made with respect to various aspects of administration, including standardization of documentation and health information technology integration across health systems 14, 31.
Global feasibility
It is important to recognize that in some low‐ and middle‐income nations, adoption of an expanded role of the community pharmacist may not be feasible due to factors such as healthcare infrastructure, disparities in the establishment, the availability and accessibility of healthcare facilities, the shortage of qualified pharmacists and associated training 74, 75, and, in many developing countries, the perceived unimportance of a pharmacist's role by the public/community and other healthcare providers 76. However, pharmacy practice is evolving in some countries, such as China and India 76, 77. For example, in China, reform will enable community pharmacies to have increased responsibility in primary healthcare over and above the traditional role of dispensing and selling medicines 77. Although legislation/professional licensing (i.e. regulations that limit the provision of services based on specific government‐established criteria) differs between countries in terms of what a pharmacist can/cannot do 78, international recognition of the value of an extended role for pharmacists as healthcare providers is accumulating 74.
Conclusions
COPD represents a significant personal and societal burden. Lack of disease awareness, incorrect inhaler technique, adherence and lifestyle practices may hamper any success in the management of COPD, despite the availability of effective and well‐tolerated treatments and easy‐to‐use inhalers. In this regard, pharmacists are well positioned to play a vital role in all key stages of an integrated COPD patient care pathway, from early detection/case finding and support/monitoring of management plans to the provision of advice and counselling regarding medications, inhaler technique and treatment adherence. Nevertheless, current data are limited and this will necessitate further exploration. In addition, more focused, global considerations relating to improved pharmacist training in COPD, reimbursement issues, increasing awareness of the pharmacist's role, and optimization of physician–pharmacist collaboration are needed.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). Thys van der Molen discloses the following for the last 5 years. Advisory board membership: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, and Teva. Consultancy: Almirall, AstraZeneca, Boehringer Ingelheim, Certe, Chiesi, GlaxoSmithKline, MSD, Mundipharma, Novartis, Nycomed and Teva. Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis and Teva. Payment for the development of educational materials: Mundipharma and Teva. Payment for travel/accommodation/meeting expenses: Astrazeneca, Boehringer Ingelheim, Mundipharma, Novartis and Teva. Patents (planned, pending or issued): Clinical COPD Questionnaire (CCQ) copyrights, Inhaled Corticosteroids Questionnaire (ICQ) copyrights, Bronchial Hyperresponsiveness Questionnaire (BHQ) copyrights. In the last 5 years his research department at the University of Groningen has received grants (or has grants pending) and support for research in respiratory disease from: The Lung Foundation Netherlands, Stichting bestrijding Astma, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis, Nycomed. Job van Boven has no competing interests to disclose. Terence Maguire is an ad hoc advisor on community pharmacy issues and has received payments in this capacity from AstraZeneca, GlaxoSmithKline, Pfizer and Reckitt Benckiser. Pankaj Goyal is an employee of Novartis Pharma AG. Pablo Altman is an employee of Novartis Pharma AG.
The authors were assisted in the preparation of the manuscript by Lietta Nicolaides (CircleScience, an Ashfield Company, part of UDG Healthcare plc) and Sharon Smalley (contracted to CircleScience, an Ashfield Company, part of UDG Healthcare plc). Medical writing support was funded by Novartis Pharmaceuticals.
van der Molen, T. , van Boven, J. F. M. , Maguire, T. , Goyal, P. , and Altman, P. (2017) Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol, 83: 192–201. doi: 10.1111/bcp.13087.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2016[online]. Available at: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/ (last accessed 5 February 2016).
- 3. World Health Organization (WHO) . Chronic obstructive pulmonary disease (COPD). Fact sheet 315. Updated January 2015. [online]. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/ (last accessed 22 September 2015).
- 4. World Health Organization (WHO) . Burden of COPD [online]. Available at: http://www.who.int/respiratory/copd/burden/en/ (last accessed 29 October 2014).
- 5. European COPD Coalition (ECC) . Key facts; 2015. [online]. Available at: http://www.copdcoalition.eu/about-copd/key-facts (last accessed 22 September 2015).
- 6. NHS Medical Directorate . COPD commissioning toolkit; 2012. [online]. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/212876/chronic-obstructive-pulmonary-disease-COPD-commissioning-toolkit.pdf (last accessed 22 September 2015).
- 7. National Heart Lung and Blood Institute (NHLBI) NIoHN . Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases; 2012. [online]. Available at: https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf (last accessed 25 September 2015).
- 8. Lyngsø AM, Backer V, Gottlieb V, Nybo B, Østergaard MS, Frølich A. Early detection of COPD in primary care – the Copenhagen COPD Screening Project. BMC Public Health 2010; 10: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NHS England . Community pharmacy – helping provide better quality and resilient urgent care. Version 2; November 2014. [online]. Available at: https://www.england.nhs.uk/wp-content/uploads/2014/11/comm-pharm-better-quality-resilient-urgent-care.pdf (last accessed 10 July 2015).
- 10. Fathima M, Naik‐Panvelkar P, Saini B, Armour CL. The role of community pharmacists in screening and subsequent management of chronic respiratory diseases: a systematic review. Pharm Pract (Granada) 2013; 11: 228–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell H, Hotchkiss R, Bradshaw N, Porteous M. Integrated care pathways. BMJ 1998; 316: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Casas A, Troosters T, Garcia‐Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J 2006; 28: 123–130. [DOI] [PubMed] [Google Scholar]
- 13. Seemungal TA, Wedzicha JA. Integrated care: a new model for COPD management? Eur Respir J 2006; 28: 4–6. [DOI] [PubMed] [Google Scholar]
- 14. American Pharmacists Association F . White paper on expanding the role of pharmacists in chronic obstructive pulmonary disease: American Pharmacists Association Foundation. J Am Pharm Assoc (2003) 2011; 51: 203–211. [DOI] [PubMed] [Google Scholar]
- 15. Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharmacol 2012; 34: 53–62. [DOI] [PubMed] [Google Scholar]
- 16. Kohansal R, Martinez‐Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 2009; 180: 3–10. [DOI] [PubMed] [Google Scholar]
- 17. Sinclair HK, Bond CM, Stead LF. Community pharmacy personnel interventions for smoking cessation. Cochrane Database Syst Rev 2004; 1: Cd003698. [DOI] [PubMed] [Google Scholar]
- 18. Mdege ND, Chindove S. Effectiveness of tobacco use cessation interventions delivered by pharmacy personnel: a systematic review. Res Social Adm Pharm 2014; 10: 21–44. [DOI] [PubMed] [Google Scholar]
- 19. Saba M, Diep J, Saini B, Dhippayom T. Meta‐analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther 2014; 39: 240–247. [DOI] [PubMed] [Google Scholar]
- 20. Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy‐led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009; 68: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Boven JF, Stuurman‐Bieze AG, Hiddink EG, Postma MJ, Vegter S. Medication monitoring and optimization: a targeted pharmacist program for effective and cost‐effective improvement of chronic therapy adherence. J Manag Care Spec Pharm 2014; 20: 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhong H, Ni XJ, Cui M, Liu XY. Evaluation of pharmacist care for patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Int J Clin Pharm 2014; 36: 1230–1240. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . Chronic disease prevention and health promotion. Last updated 23 February 2016. [online]. Available at: http://www.cdc.gov/chronicdisease/overview/index.htm (last accessed 28 June 2016).
- 24. Suhaj A, Manu MK, Unnikrishnan MK, Vijayanarayana K, Mallikarjuna RC. Effectiveness of clinical pharmacist intervention on health‐related quality of life in chronic obstructive pulmonary disorder patients – a randomized controlled study. J Clin Pharm Ther 2016; 41: 78–83. [DOI] [PubMed] [Google Scholar]
- 25. Martinez FJ, Raczek AE, Seifer FD, Conoscenti CS, Curtice TG, D'Eletto T, et al. Development and initial validation of a self‐scored COPD Population Screener Questionnaire (COPD‐PS). COPD 2008; 5: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wright D, Twigg M, Thornley T. Chronic obstructive pulmonary disease case finding by community pharmacists: a potential cost‐effective public health intervention. Int J Pharm Pract 2015; 23: 83–85. [DOI] [PubMed] [Google Scholar]
- 27. Castillo D, Burgos F, Guayta R, Giner J, Lozano P, Estrada M, et al. Airflow obstruction case finding in community‐pharmacies: a novel strategy to reduce COPD underdiagnosis. Respir Med 2015; 109: 475–482. [DOI] [PubMed] [Google Scholar]
- 28. Fuller L, Conrad WF, Heaton PC, Panos R, Eschenbacher W, Frede SM. Pharmacist‐managed chronic obstructive pulmonary disease screening in a community setting. J Am Pharm Assoc (2003) 2012; 52: e59–e66. [DOI] [PubMed] [Google Scholar]
- 29. Cawley MJ, Warning WJ. Pharmacists performing quality spirometry testing: an evidence based review. Int J Clin Pharmacol 2015; 37: 726–733. [DOI] [PubMed] [Google Scholar]
- 30. NHS . Chronic obstructive pulmonary disease: living with COPD. Updated July 2015. [online]. Available at: http://www.nhs.uk/Conditions/Chronic-obstructive-pulmonary-disease/Pages/living-with.aspx (last accessed 29 October 2015).
- 31. Atkins K, van Hoek AJ, Watson C, Baguelin M, Choga L, Patel A, et al. Seasonal influenza vaccination delivery through community pharmacists in England: evaluation of the London pilot. BMJ Open 2016; 6: e009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010; 182: 155–162. [DOI] [PubMed] [Google Scholar]
- 33. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten‐van Mölken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 34. Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once‐daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel‐group study. Lancet Respir Med 2013; 1: 524–533. [DOI] [PubMed] [Google Scholar]
- 35. Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013; 42: 1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donohue JF, Maleki‐Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once‐daily umeclidinium/vilanterol 62.5/25 mg in COPD. Respir Med 2013; 107: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 37. Beeh KM, Westerman J, Kirsten AM, Hébert J, Grönke L, Hamilton A, et al. The 24‐h lung‐function profile of once‐daily tiotropium and olodaterol fixed‐dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2015; 32: 53–59. [DOI] [PubMed] [Google Scholar]
- 38. Buhl R, Maltais F, Abrahams R, Bjermer L, Derom E, Ferguson G, et al. Tiotropium and olodaterol fixed‐dose combination versus mono‐components in COPD (GOLD 2–4). Eur Respir J 2015; 45: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maleki‐Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: Results of a 24‐week, randomized, controlled trial. Respir Med 2014; 108: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 40. Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstöm T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double‐blind, parallel‐group study. Lancet Respir Med 2013; 1: 199–209. [DOI] [PubMed] [Google Scholar]
- 41. Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, et al. Efficacy and safety of once‐daily QVA149 compared with twice‐daily salmeterol‐fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double‐blind, parallel group study. Lancet Respir Med 2013; 1: 51–60. [DOI] [PubMed] [Google Scholar]
- 42. Zhong N, Wang C, Zhou X, Zhang N, Humphries M, Wang C, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J COPD 2015; 10: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol‐glycopyrronium versus salmeterol‐fluticasone for COPD. N Engl J Med 2016; 374: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 44. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3: CD010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price D, West D, Brusselle G, Gruffydd‐Jones K, Jones R, Miravitlles M, et al. Management of COPD in the UK primary‐care setting: an analysis of real‐life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014; 9: 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rossi A, van der Molen T, del Olmo R, Papi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J 2014; 44: 1548–1556. [DOI] [PubMed] [Google Scholar]
- 47. Magnussen H, Disse B, Rodriguez‐Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 48. Chapman KR, Fogarty CM, Peckitt C, Lassen C, Jadayel D, Dederichs J, et al. Delivery characteristics and patients' handling of two single‐dose dry‐powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis 2011; 6: 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; 105: 930–938. [DOI] [PubMed] [Google Scholar]
- 50. Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler. Int J Chron Obstruct Pulmon Dis 2009; 4: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Komase Y, Asako A, Kobayashi A, Sharma R. Ease‐of‐use preference for the ELLIPTA® dry powder inhaler over a commonly used single‐dose capsule dry powder inhaler by inhalation device‐naive Japanese volunteers aged 40 years or older. Int J Chron Obstruct Pulmon Dis 2014; 9: 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pascual S, Feimer J, De Soyza A, Sauleda Roig J, Haughney J, Padulles L, et al. Preference, satisfaction and critical errors with Genuair and Breezhaler inhalers in patients with COPD: a randomised, cross‐over, multicentre study. NPJ Prim Care Respir Med 2015; 25: 15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van der Palen J, Eijsvogel MM, Kuipers BF, Schipper M, Vermue NA. Comparison of the Diskus inhaler and the Handihaler regarding preference and ease of use. J Aerosol Med 2007; 20: 38–44. [DOI] [PubMed] [Google Scholar]
- 54. Tommelein E, Mehuys E, Van Hees T, Adriaens E, Van Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): a randomized controlled trial. Br J Clin Pharmacol 2014; 77: 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehuys E, Boussery K, Adriaens E, Van Bortel L, De Bolle L, Van Tongelen I, et al. COPD management in primary care: an observational, community pharmacy‐based study. Ann Pharmacother 2010; 44: 257–266. [DOI] [PubMed] [Google Scholar]
- 56. van Boven JF, Tommelein E, Boussery K, Mehuys E, Vegter S, Brusselle GG, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost‐effectiveness analysis. Respir Res 2014; 15: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jones R. Self management of chronic obstructive pulmonary disease (COPD) in primary care. Primary Care Respiratory Society, UK – Opinion No. 11. Revised June 2010 [online]. Available at: https://pcrs-uk.org/sites/pcrs-uk.org/files/os11_copd_self_man.pdf (last accessed 15 July 2015).
- 58. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self‐management approaches for people with chronic conditions: a review. Patient Educ Couns 2002; 48: 177–187. [DOI] [PubMed] [Google Scholar]
- 59. Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis 2014; 5: 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex‐smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001; 164: 358–364. [DOI] [PubMed] [Google Scholar]
- 61. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. National Institute for Health and Care Excellence (NICE) . Services for people with chronic obstructive pulmonary disease. 2011. Available at: https://www.nice.org.uk/Guidance/cg101 (last accessed 22 September 2015).
- 63. Wright D, Twigg M, Barton G, Thornley T, Kerr C. An evaluation of a multi‐site community pharmacy‐based chronic obstructive pulmonary disease support service. Int J Pharm Pract 2015; 23: 36–43. [DOI] [PubMed] [Google Scholar]
- 64. Zwerink M, Brusse‐Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3: Cd002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. NHS . NHS Improvement – Lung. Managing COPD as a long term condition: emerging learning from the National Improvement Projects [online]. Available at: http://www.slideshare.net/NHSImprovement/managing-copd-as-a-long-term-condition-emerging-learning-from-the-national-improvement-projects (last accessed 22 September 2015).
- 66. van Boven JF, Chavannes NH, van der Molen T, Rutten‐van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non‐adherence in COPD: a systematic review. Respir Med 2014; 108: 103–113. [DOI] [PubMed] [Google Scholar]
- 67. Bryant J, McDonald VM, Boyes A, Sanson‐Fisher R, Paul C, Melville J. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir Res 2013; 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Boven JF, Stuurman‐Bieze AG, Hiddink EG, Postma MJ. Effects of targeting disease and medication management interventions towards patients with COPD. Curr Med Res Opin 2015; 32: 229–239. [DOI] [PubMed] [Google Scholar]
- 69. Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, et al. Effectiveness of pharmacist care for patients with reactive airways disease: a randomized controlled trial. JAMA 2002; 288: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 70. Sanii Y, Torkamandi H, Gholami K, Hadavand N, Javadi M. Role of pharmacist counseling in pharmacotherapy quality improvement. J Res Pharm Pract 2016; 5: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pharmaceutical Services Negotiating Committee (PSNC) . New Medicine Service (NMS) [online]. Available at: http://psnc.org.uk/services-commissioning/advanced-services/nms/ (last accessed 16 July 2015).
- 72. NHS . Pharmacy services. Q&A about the New Medicine Service (NMS). Updated November 2013. [online]. Available at http://www.nhs.uk/NHSEngland/AboutNHSservices/pharmacists/Pages/medicine-service-qa.aspx (last accessed 9 October 2015).
- 73. Elliott RA, Boyd MJ, Salema NE, Davies J, Barber N, Mehta RL, et al. Supporting adherence for people starting a new medication for a long‐term condition through community pharmacies: a pragmatic randomised controlled trial of the New Medicine Service. BMJ Qual Saf 2016; 25: 747–758. [DOI] [PubMed] [Google Scholar]
- 74. International Pharmaceutical Federation (FIP) . 2013. FIP Ed Global Education Report [online]. Available at https://www.fip.org/static/fipeducation/2013/2013‐FIPEd‐GlobalEducationReport/ (last accessed 28 June 2016).
- 75. World Health Organization (WHO) . Global surveillance, prevention and control of chronic respiratory diseases. A comprehensive approach. Updated 2007 [online]. Bousquet J, Khaltaev N (eds). Available at http://www.who.int/gard/publications/GARD_Manual/en/ (last accessed 24 March 2014).
- 76. Sachan A, Sachan AK, Gangwar SS. Pharmacy education in India and its neighbouring countries. Int Curr Pharm J 2012; 1: 294–301. [Google Scholar]
- 77. Fang Y, Yang S, Zhou S, Jiang M, Liu J. Community pharmacy practice in China: past, present and future. Int J Clin Pharmacol 2013; 35: 520–528. [DOI] [PubMed] [Google Scholar]
- 78. Philipsen NJ. Regulation of pharmacists: a comparative law and economics analysis. Eur J Comparative Econ 2013; 10: 224–241. [Google Scholar]


