ABSTRACT
Lotus japonicus THIC is expressed in all organs, and the encoded protein catalyzes thiamine biosynthesis. Loss of function produces chlorosis, a typical thiamine-deficiency phenotype, and mortality. To investigate thiamine's role in symbiosis, we focused on THI1, a thiamine-biosynthesis gene expressed in roots, nodules, and seeds. The thi1 mutant had green leaves, but formed small nodules and immature seeds. These phenotypes were rescued by THI1 complementation and by exogenous thiamine. Thus, THI1 is required for nodule enlargement and seed maturation. On the other hand, colonization by arbuscular mycorrhiza (AM) fungus Rhizophagus irregularis was not affected in the thi1 mutant or by exogenous thiamine. However, spores of R. irregularis stored more thiamine than the source (host plants), despite lacking thiamine biosynthesis genes. Therefore, disturbance of the thiamine supply would affect progeny phenotypes such as spore formation and hyphal growth. Further investigation will be required to elucidate thiamine's effect on AM.
KEYWORDS: Arbuscular mycorrhiza, Lotus japonicus, root nodule symbiosis, thiamine
Abbreviation
- AM
Arbuscular mycorrhiza
- RN
Root nodule
Legumes establish two typical mutualistic plant–microbe interactions: root nodule (RN) symbiosis with rhizobia and arbuscular mycorrhiza (AM) with AM fungi. Rhizobia and AM fungi, respectively, provide host plants with nitrogen generated by fixation of atmospheric nitrogen and mineral nutrients such as phosphorus.1,2 Many symbiosis-related genes have been isolated and their functions have been described. Researchers have found both functions common to RN and AM symbioses, as well as RN-specific and AM-specific functions.1,2 In previous research, we clarified that one of the symbiosis-related genes, the thiamine biosynthesis gene THI1, promoted nodule enlargement but did not affect AM.3
Thiamine (vitamin B1) is essential for living organisms. Unlike animals, plants can synthesize thiamine through a multiple-step pathway.4,5 Thiamine is assembled from pyrimidine and thiazole moieties. THI1 and THI2 (a THI1 paralog) catalyze the biosynthesis of the thiazole moiety, and THIC catalyzes the biosynthesis of the pyrimidine moiety.4,5 Fig. 1 shows the phenotypes of thiamine-deficient mutants of L. japonicus. THIC is a single copy gene in L. japonicus and is expressed in all tissues, including leaves.3,6 Therefore, the thiC mutant displayed chlorosis, which is a typical and lethal thiamine-deficiency phenotype in plants.3 THIC was also expressed in nodules, and the nodule number in the thiC mutant was reduced.3 However, it is not clear whether the nodulation defect in the thiC mutant is due to the loss of THIC function or to the consequences of chlorosis. In L. japonicus, THI1 is expressed mainly in roots, nodules, and seeds, whereas THI2 is expressed in shoots.3,6 Although THI2-knockdown plants displayed chlorosis similar to that in the thiC mutant, the thi1 mutant had green leaves and no significant growth defect.3 By using this mutant, we successfully demonstrated that thiamine affects nodule development in roots by excluding the effects of chlorosis and the growth defect in shoots.3 Plants treated with exogenous thiamine had enlarged nodules, suggesting that thiamine promotes nodulation.3 In addition, exogenous thiamine treatment rescued the small nodules and immature seeds of the thi1 mutant.3 These results indicated that THI1 is involved in both nodule development in roots and seed maturation in shoots (Fig. 1).
Figure 1.
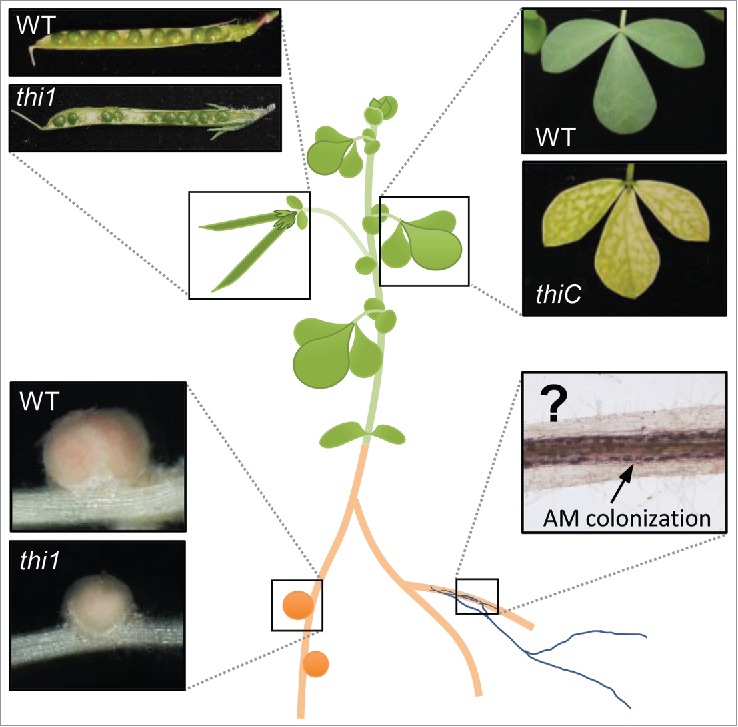
Phenotypes of thiamine-deficient mutants of Lotus japonicus. Photographs show the pods, leaves, and nodules of the wild-type (WT) plant and the thiamine-deficient thi1 and thiC mutants. The thi1 mutant showed abnormal phenotypes in pods and nodules, and the thiC mutant showed chlorosis. Although thiamine did not affect AM in our previous study, 3 further investigations will be required to understand its function in AM.
Unlike RN symbiosis, AM colonization was not affected by exogenous thiamine treatment, and AM colonization of the thi1 mutant root was normal (Fig. 1).3 However, this result did not rule out the possibility that thiamine contributes to the AM symbiosis. In the thi1 mutant, the root thiamine content decreased slightly but significantly,3 and this small decrease might cause no detectable morphological or quantitative changes in AM colonization. In addition, it has been reported that the AM fungus Rhizophagus irregularis lacks a thiamine biosynthesis pathway.7 This suggests that if thiamine is an essential nutrient for this AM fungus, it must be obtained from the host's roots.
To investigate thiamine accumulation in AM fungi, we measured the thiamine content in their spores (Fig. 2A). The thiamine content in the AM spores was comparable to that in wheat bran, which is a nutrient-rich tissue that contains more thiamine than other plant tissues.8 For example, the content in the L. japonicus roots was smaller than that of AM spores (Fig. 2A). Despite the lack of thiamine biosynthesis genes in R. irregularis, the high accumulation of thiamine in its spores suggests that AM fungi positively absorb thiamine from the host plant's roots during the AM symbiosis.
Figure 2.
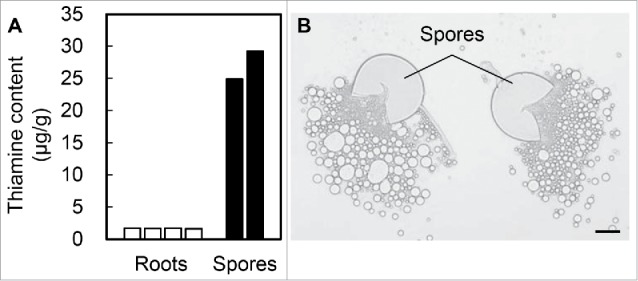
Thiamine content and lipids in the spores of the AM fungus Rhizophagus irregularis. (A) Thiamine content of L. japonicus roots and AM spores was determined using a VitaFast Vitamin B1 kit (Biopharm) that detects thiamine and its phosphate esters. Three-week-old L. japonicus roots and AM spores (DAOM197198; Premier Tech; ca. 40 000 spores) were homogenized and incubated in 71.4 mM citric acid buffer (20 mL; pH 4.5) in the presence of 300 mg Taka-Diastase (Sigma) for 1 h at 37°C, and then for 30 min at 95°C; the solution was then quickly cooled to 30°C and centrifuged at 9200× g for 10 min. The supernatants were passed through 0.22-μm mixed cellulose ester filters. Bioassay was performed according to the manufacturer's instructions. Bar plots represent independent biological replicates. (B) Ruptured AM spores releasing lipids. Scale bar, 50 μm.
Sequencing the genome of R. irregularis also revealed a lack of some genes for fatty acid biosynthesis.7,9 As in the case of thiamine, it has been reported that large amounts of fatty acids are stored in the spores,10,11 and we observed this in the form of lipid drops released from the ruptured spores (Fig. 2B). These observations demonstrate that the AM fungus accumulates various nutrients, which they cannot synthesize by themselves, in the spores. These nutrients would be required to sustain the germinating spores until they can reach their next host (i.e., the next source of these nutrients) during their non-symbiotic state, from spore germination through hyphal elongation in the soil and infection of the host root to arbuscule formation. Therefore, the supply of thiamine from host plants during the symbiosis state should be a crucial process for them. Our previous evaluation of AM phenotypes in the thi1 mutant was limited to infection of the host plant (morphological and quantitative analysis of hyphal and arbuscular colonization).3 However, the later life cycle stages, namely spore formation and growth of spore progeny, may be influenced by a deficiency of key nutrients such as thiamine. Therefore, further investigations will be required to elucidate the effect of thiamine on AM symbiosis and AM fungi.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the National Institute for Basic Biology Core Research Facilities and Bioresource Center for measurement of thiamine content.
Funding
This work was supported by the Japan Society for the Promotion of Science (No. 24770050, 15KT0122), and Science and Technology Research Promotion Program for agriculture, forestry, fisheries and food industry (No. 250023A).
References
- 1.Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 2011; 45:119-44; PMID:21838550; http://dx.doi.org/ 10.1146/annurev-genet-110410-132549 [DOI] [PubMed] [Google Scholar]
- 2.Gutjahr C, Parniske M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 2013; 29:593-617; PMID:24099088; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122413 [DOI] [PubMed] [Google Scholar]
- 3.Nagae M Parniske M, Kawaguchi M Takeda N. The thiamine biosynthesis THI1 promotes nodule growth and seed maturation. Plant Physiol 2016; 172:2033-2043; PMID:27702844; http://dx.doi.org/ 10.1104/pp.16.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurgenson CT, Begley TP, Ealick SE. The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 2009; 78:569-603; PMID:19348578; http://dx.doi.org/ 10.1146/annurev.biochem.78.072407.102340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyer A. Thiamine in plants: Aspects of its metabolism and functions. Phytochemistry 2010; 71:1615-24; PMID:20655074; http://dx.doi.org/ 10.1016/j.phytochem.2010.06.022 [DOI] [PubMed] [Google Scholar]
- 6.Verdier J, Torres-Jerez I, Wang M, Andriankaja A, Allen SN, He J, Tang Y, Murray JD, Udvardi MK. Establishment of the Lotus japonicus Gene Expression Atlas (LjGEA) and its use to explore legume seed maturation. Plant J 2013; 74:351-62; PMID:23452239; http://dx.doi.org/ 10.1111/tpj.12119 [DOI] [PubMed] [Google Scholar]
- 7.Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frei dit Frey N, Gianinazzi-Pearson V, et al.. Genome of arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci USA 2013; 110:20117-22; PMID:24277808; http://dx.doi.org/ 10.1073/pnas.1313452110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaupa M, Scazzina F, Dall'Asta M, Calani L, Del Rio D, Bianchi MA, Melegari C, De Albertis P, Tribuzio G, Pellegrini N, et al.. In vitro bioaccessibility of phenolics and vitamins from durum wheat aleurone fractions. J Agric Food Chem 2014; 62:1543-9; PMID:24450764; http://dx.doi.org/ 10.1021/jf404522a [DOI] [PubMed] [Google Scholar]
- 9.Wewer V, Brands M, Dörmann P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J 2014; 79:398-412; PMID:24888347; http://dx.doi.org/ 10.1111/tpj.12566 [DOI] [PubMed] [Google Scholar]
- 10.Bonfante P, Balestrini R, Mendgen K. Storage and secretion processes in the spore of Gigaspora margarita Becker & Hall as revealed by high-pressure freezing and freeze substitution. New Phytol 1994; 128:93-101; http://dx.doi.org/ 10.1111/j.1469-8137.1994.tb03991.x. [DOI] [PubMed] [Google Scholar]
- 11.Needelman BA. What are soils? Nature Education Knowledge 2013; 4:2; http://www.nature.com/scitable/knowledge/library/what-are-soils-67647639. [Google Scholar]


