Abstract
BACKGROUND
Innate immune system responses to damage-associated molecular patterns (DAMPs) are involved in hypertension. However, the mechanisms of this contribution are not well understood. Circulating mitochondrial DNA is a DAMP that activates Toll-like receptor (TLR) 9 and is elevated in spontaneously hypertensive rats (SHR). Therefore, we hypothesized that lysosomotropic agent chloroquine (CQ) would impair TLR9 signaling, as well as prevent the development of hypertension and immune cell recruitment to the vasculature, in SHR.
METHODS
Initially, adult SHR and Wistar–Kyoto (WKY) rats (12 weeks old), as well as a group of young SHR (5 weeks old), were treated with CQ (40mg/kg/day) or vehicle (saline) via intraperitoneal injections for 21 days and then TLR9–myeloid differentiation primary response protein (MyD88) signaling proteins were assessed in mesenteric resistance arteries (MRA) via western blot. Subsequently, young SHR and WKY were treated from 5–8 weeks of age and then were allowed to mature without further treatment. Blood pressure was measured pretreatment, posttreatment, and after maturation, and immune cell recruitment to the vasculature was measured via flow cytometry after maturation.
RESULTS
In MRA from adult SHR, CQ increased the expression of MyD88-dependent proteins, whereas young SHR MRA exhibited a decrease. This inhibition was subsequently associated with suppression of blood pressure, as well as decreased counts of circulating T cells and vascular infiltrating leukocytes in SHR, when CQ was administered during the prehypertensive phase.
CONCLUSIONS
These data bring into question the participation of TLRs during the maintenance phase of hypertension and promote the exploration of innate immune system therapy during the critical developmental phase.
Keywords: blood pressure, hypertension, immune system, Toll-like receptors.
Inappropriate immune system activation has been proposed as a unifying mechanism linking the 3 major organ systems that are dysregulated in the development and maintenance of hypertension—the cardiovascular system, the kidneys, and the autonomic nervous system.1,2 However, the exact mechanisms that initiate this deleterious response, thereby contributing to further increases in blood pressure, are not well understood.1,2 Although the participation of the adaptive immune system, and specifically T cells, in the pathogenesis of hypertension is not in question,3,4 there are still gaps surrounding how it is signaled for involvement in the first place.
Immune system recognition and response to danger is becoming apparent in a wide range of disease states, including cardiovascular diseases.5 In this paradigm, the immune system responds to stimuli that it perceives as indicative of danger.6 As such, molecules released from cell injury and death, called damage-associated molecular patterns (DAMPs), as well as pathogen-associated molecular patterns, signals to the immune system that a response is required. Normally, endogenous molecules are shielded from the immune system via compartmentalization within plasma membranes. However, when a cell dies or undergoes undue stress, these molecules can be released and are able to activate pattern recognition receptors on immune and nonimmune cells.5
Toll-like receptors (TLR) are a class of innate immune system pattern recognition receptors that recognize and respond to DAMPs.2,7 We have recently observed that mitochondrial DNA (mtDNA), a DAMP that activates TLR9, is increased in the circulation of spontaneously hypertensive rats (SHR).8 Specifically, TLR9 recognizes unmethylated cytosine–phosphate–guanine (CpG) dinucleotides and these are common in prokaryotic DNA and mtDNA, but not nuclear DNA.9 Upon recognition of unmethylated CpG dinucleotides in endosomes, TLR9 traffics from the endoplasmic reticulum to endolysosomal compartments.10 Inflammatory signaling is then initiated through myeloid differentiation primary response protein (MyD88) and proceeds through interleukin-1-receptor-activated kinase (IRAK) and tumor-necrosis factor receptor-associated factor 6 (TRAF6). This signaling leads to the activation of transcription factors activator protein 1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) resulting in the subsequent generation of proinflammatory cytokines and interferons.2
Previously, we observed that TLR9 was able to increase arterial blood pressure and cause endothelial dysfunction in rats with a normotensive background.8 Nonetheless, the link between the innate immune system and the subsequent recruitment of the adaptive immune system in hypertensive rats remains unclear. Therefore, in this investigation, we hypothesized that inhibition of TLR9 with the lysosomotropic agent chloroquine (CQ), which has long been used as an inhibitor of endosomal TLR signaling,11–13 would lower blood pressure and prevent the subsequent recruitment of immune cells to the vasculature in SHR. We treated SHR of 2 different ages (young and adult) to see if CQ treatment had differential effects on endosomal TLR-signaling during the developmental and maintenance phases of hypertension. We hypothesized that in adult SHR CQ would reduce TLR9 signaling proteins, whereas in young SHR it would prevent an age-dependent increase, similar to what has been reported for TLR4.14
METHODS
Animals
Male SHR and Wistar–Kyoto (WKY) rats were used for this investigation (Envigo, Indianapolis, IN). In accordance with the National Institutes of Health proposed gender equity policy, the justification for not using female rats was based on our previous publication observing no differences in circulating mtDNA expression in female WKY or SHR.8 Adult SHR and WKY were 12 weeks of age at the onset of treatment and young SHR and WKY were 5 weeks of age at the onset of treatment. The sample size indicated per experiment (see figures and figure legends) is the number of independent rats used for statistical analysis, respective of strain and treatment group.
All rats were maintained on a 12:12h light–dark cycle with both chow and water ad libitium. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee of Augusta University. All surgical procedures were undertaken on rats under isoflurane anesthesia, administered via nose cone (5% in 100% O2). Rats were killed by thoracotomy and exsanguination via cardiac puncture.
Treatment
Rats were randomly assigned to receive either 40mg/kg/day CQ (Sigma-Aldrich, St Louis, MO) or vehicle (Veh; saline), administered via intraperitoneal injections for 21 days. This dose of CQ was chosen based on the investigation by Long et al. 15 who observed beneficial effects on indices of pulmonary hypertension at 25 and 50mg/kg/day. To investigate the therapeutic potential of CQ to inhibit TLR signaling proteins in hypertension, adult (12 week old) SHR (Veh n = 13; CQ n = 13) and WKY (Veh n = 11; CQ n = 11) rats, as well as a group of young (5 week old) SHR (Veh n = 6; CQ n = 6), were killed at the conclusion of the 21-day treatment and mesenteric resistance arteries (MRA) were harvested (this is referred to as study A). In a follow-up investigation to further assess the preventive actions of CQ on hypertension, young SHR (Veh n = 6; CQ n = 6) and young WKY (Veh n = 4; CQ n = 4) were treated for 21 days, from 5 to 8 weeks of age. Then from 8 to 12 weeks of age, these rats were allowed to mature without any further treatment (this is referred to as study B) (Supplementary Figure 1).
Immunoblotting and protein expression
MRA were cleaned of perivascular adipose tissue, snap frozen in liquid nitrogen, and then homogenized in ice-cold tissue protein extraction reagent (Thermo Fisher Scientific, Waltham, MA), with protease inhibitors (sodium orthovanadate, phenylmethanesulfonylfluoride, and protease inhibitor cocktail) and phosphatase inhibitors (sodium fluoride and sodium pyrophosphate) (all Sigma-Aldrich). Equal amounts of protein (30–50 µg) were loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (8–12%) for western blotting. Polyvinylidene difluoride or nitrocellulose membranes were probed for the expression of endosomal TLR signaling proteins (Supplementary Table 1). Phosphorylated protein expression was normalized to total protein expression; all other proteins were normalized to β actin. Densitometric analysis was performed by Un-Scan-It software (version 6.1) (Silk Scientific, Orem, UT).
Blood pressure
Systolic blood pressure (SBP) was measured in conscious rats via tail cuff using a RTBP1001 blood pressure system (Kent Scientific Corporation, Torrington, CT). Young rats were treated with CQ or Veh starting at 5 weeks of age for 21 days and SBP was measured before treatment (day 0–BPpre) and 24 hours after the last intraperitoneal injection (day 22–BPpost). Subsequently, these rats were not subjected to any further treatment for 4 weeks. Blood pressure was then measured for a third time in these rats (BPadult) at the conclusion of this maturation period (12 weeks of age). An average of the SBP from 10 cycles was taken from each animal and then averaged within group.
Flow cytometry
Flow cytometry was used to evaluate whether CQ administered during the prehypertensive phase of SHR (5–8 weeks) would prophylactically inhibit vascular immune cell recruitment upon maturation to adulthood (12 weeks). Whole blood was collected in microhematocrit capillary tubes (Thermo Fisher Scientific) and thoracic aortas (cleaned of perivascular adipose tissue to reduce intra-assay variability) were homogenized with a Polytron (Kinematica, Bohemia, NY) and pushed through a 100 µm nylon cell strainer (Thermo Fisher Scientific) to create single cell suspensions. Aortic single cell suspensions were lysed of red blood cells with Ammonium–Chloride–Potassium (ACK) lysing buffer (Life Technologies-Thermo Fisher Scientific) and resuspended in 1X phosphate buffered saline.
Phenotypic and intracellular analyses of whole blood and aortic single cell suspensions were performed as described previously.16,17 Briefly, cells were incubated with antibodies for surface markers including CD45, CD3, and CD44 (BD Biosciences, San Jose, CA) for 15 minutes on ice in the dark. Cells were then washed, run through a 4-color flow cytometer (FACS Calibur, BD Biosciences), and data were collected using CellQuest software (BD Biosciences).
Statistical analysis
The statistical procedures used included Student’s t-test and 1-way and 2-way analysis of variance. Tukey’s post-hoc testing was used in all cases using a 1-way analysis of variance and the Bonferroni post-hoc was used in all cases using a 2-way analysis of variance. Statistical significance was set at α = 0.05 and P-values less than 0.05 were considered significant for all statistical tests. All analyses were performed using the data analysis software GraphPad Prism 5.0 (La Jolla, CA). The data are presented as mean ± SEM.
RESULTS
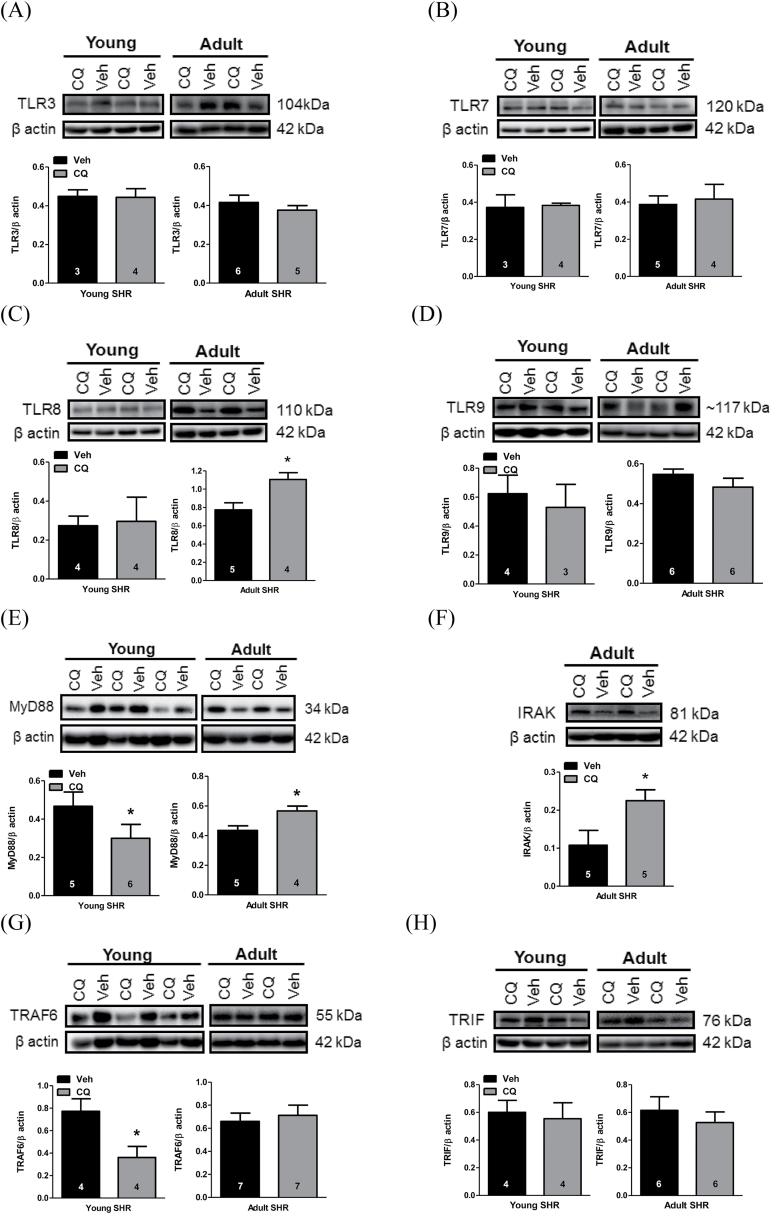
CQ has long been used as a pharmacological agent to disrupt endosomal TLRs,11–13 which include TLR3, TLR7, TLR8, and TLR9. With this knowledge, we hypothesized that CQ would decrease the expression of TLR signaling proteins in SHR (study A). Expression of endosomal TLRs and TLR signaling proteins were all unchanged in MRA isolated from normotensive WKY after CQ treatment (Supplementary Figure 2). Similarly, we did not observe protein expression changes for TLRs 3, 7, or 9 (Figures 1A and B and 2D) in adult or young SHR MRA. However, TLR8 expression increased in adult, but not young, SHR MRA (Figure 1C). In MRA from young SHR, CQ decreased expression of TLR signaling proteins MyD88 (Figure 1E) and TRAF6 (Figure 1G). On the other hand, in MRA from adult SHR, CQ increased expression of TLR signaling proteins MyD88 (Figure 1E) and IRAK (Figure 1F), and did not change TRAF6 (Figure 1G). Toll-interleukin-1 receptor (TIR)-domain-containing adaptor inducing interferon-β (TRIF), which is the adaptor molecule for TLR3, was unchanged after CQ treatment in young and adult SHR MRA (Figure 1H). Overall, these results indicate that CQ could inhibit vascular TLR signaling proteins in young SHR, but not adult SHR.
Figure 1.
CQ inhibited MyD88-dependent signaling proteins in young, but not adult, SHR MRA. MRA were isolated from SHR treated with CQ or Veh for 21 days starting at 12 weeks of age (adult) or 5 weeks of age (young). Protein expression analysis for (a) TLR3, (b) TLR7, (c) TLR8, (d) TLR9, (e) MyD88, (f) IRAK, (g) TRAF6, and (h) TRIF, all normalized for β actin, in young and adult SHR MRA. Above, representative images of immunoblots; below, densitometric analysis. Sample sizes for statistical analyses are presented within figure. Student’s t-test: *P < 0.05 vs. Veh. Abbreviations: CQ, chloroquine; IRAK, interleukin-1-receptor-activated kinase; MRA, mesenteric resistance arteries; MyD88, myeloid differentiation primary response protein; SHR, spontaneously hypertensive rats; TRAF6, tumor-necrosis factor receptor-associated factor 6; TRIF, Toll-interleukin-1 receptor-domain-containing adaptor inducing interferon-β; Veh, vehicle.
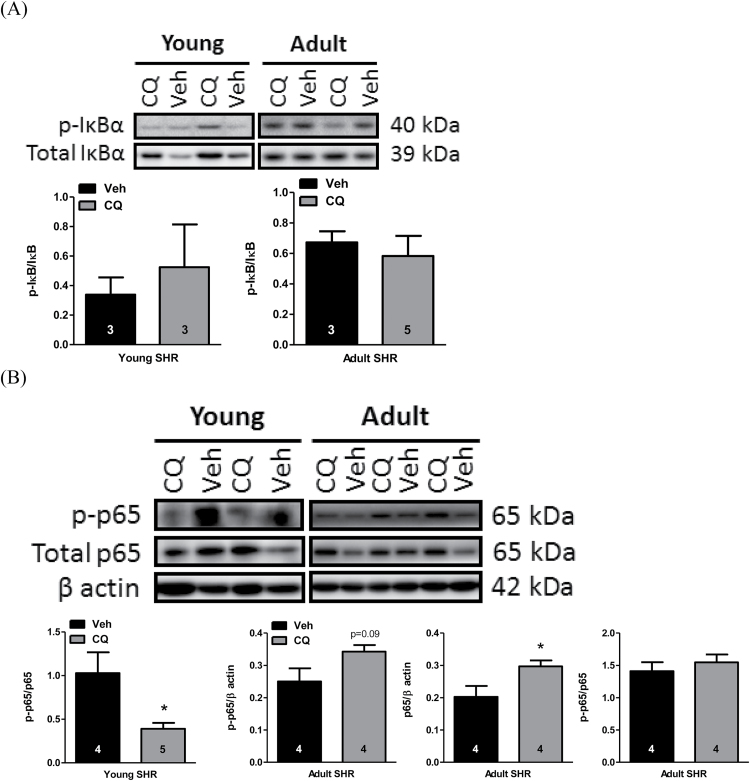
Figure 2.
CQ inhibited noncanonical NF-κB signaling in young, but not adult, SHR MRA. MRA were isolated from SHR treated with CQ or Veh for 21 days starting at 12 weeks of age (adult) or 5 weeks of age (young). Protein expression analysis for (a) phospho-IκB normalized for total IκB and (b) phospho-p65 and total p65 normalized for β actin, as well as phospho-p65 normalized for total p65, in young and adult SHR MRA. Above, representative images of immunoblots; below, densitometric analysis. Sample sizes for statistical analyses are presented within figure. Student’s t-test: *P < 0.05 vs. Veh. Abbreviations: CQ, chloroquine; IκB, inhibitor of κB; MRA, mesenteric resistance arteries; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; SHR, spontaneously hypertensive rats; Veh, vehicle.
To evaluate the downstream consequences of CQ treatment on MyD88-dependent signaling, we investigated NF-κB. Interestingly, canonical NF-κB signaling, as indicated by phosphorylation of inhibitor of κB (IκB)α, was not changed after CQ treatment in either age of SHR (Figure 2A). However, in MRA from young SHR, phosphorylation of NF-κB transcription factor p65 (RelA) was reduced after CQ treatment, indicative of less NF-κB-dependent transcription (Figure 2B). In MRA from adult SHR, there was no change in the phospho-to-total p65 expression ratio; however, total p65 protein expression was increased after CQ treatment (Figure 2B). These changes in NF-κB indicate that the contrasting effects of CQ treatment on MyD88-dependent signaling proteins in young and adult SHR has downstream consequences on inflammation via noncanonical NF-κB signaling.
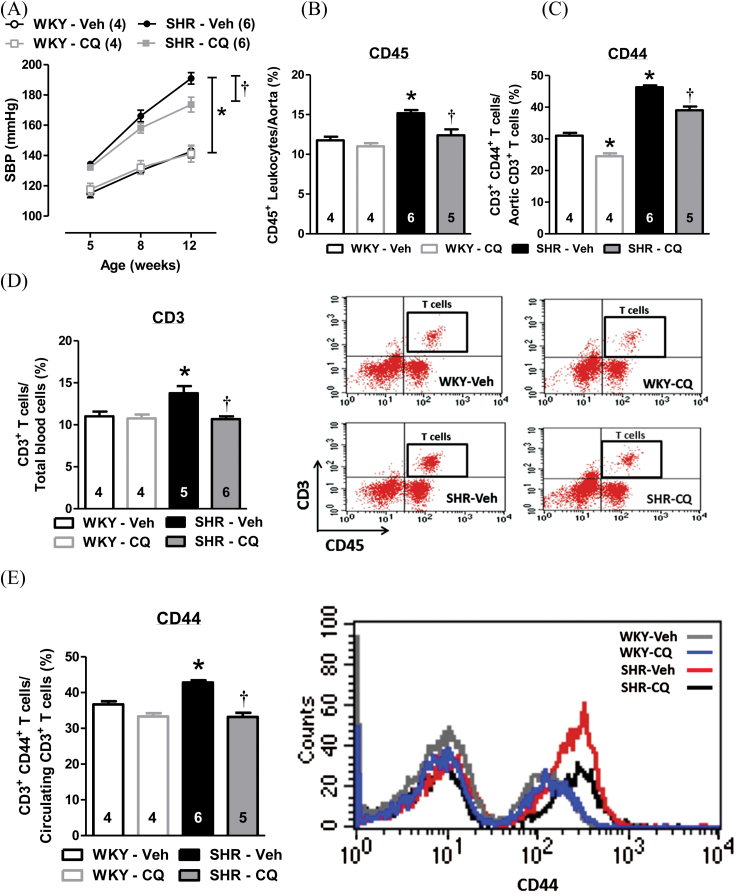
Given that TLR-signaling proteins from young SHR, but not adult SHR, were sensitive to inhibition by CQ, we hypothesized that inhibition of MyD88-dependent signaling during the critical prehypertensive phase of SHR would prophylactically prevent the development of hypertension and immune cell recruitment to the vasculature upon maturation to adulthood (study B). To test these, we treated young SHR from 5 to 8 weeks of age with CQ, as we did previously. We then allowed the SHR to mature to 12 weeks of age without any further treatment (Supplementary Figure 1). CQ did not affect WKY or SHR body mass across treatment (Supplementary Figure 3A), nor did it decrease total heart mass (Supplementary Figure 3B) or right ventricle mass (Supplementary Figure 3D). However, CQ did lower SHR LV mass (Supplementary Figure 3C) and wet-to-dry lung mass (Supplementary Figure 3E). Although we did not observe a decrease in the development of SBP in SHR immediately following CQ treatment at 8 weeks of age, SBP was significantly lower at 12 weeks of age compared to Veh-treated SHR (Figure 3A). We also observed that the inhibition of endosomal MyD88-dependent signaling proteins in young (prehypertensive) SHR was associated with decreased immune cell recruitment and infiltration into the vasculature. Specifically, CQ treatment during this critical period prevented aortic accumulation of CD45+ leukocytes (Figure 3B), attenuated the expression of cell adhesion protein CD44+ on CD3+ T cells from aorta (Figure 3C), reduced circulating counts of CD3+ T cells (Figure 3D), and decreased the expression of CD44+ on CD3+ T cells from whole blood (Figure 3E).
Figure 3.
Prophylactic treatment with CQ during the prehypertensive phase of SHR prevented the development of hypertension and immune cell recruitment to the vasculature upon maturation to adulthood. (a) The values of SBP before treatment (5 weeks old), concluding treatment (8 weeks old), and following maturation to adulthood (12 weeks old) were measured in Veh- or CQ-treated WKY and SHR. Immune cell recruitment analysis included: (b) aortic accumulation of leukocytes (CD45+), (c) CD44+ expression on T cells (CD3+) from aorta, (d) circulating T cell (CD3+) counts (left, quantification; right, dot plots representing live gating for T cells from whole blood cells), and (e) CD44+ expression on circulating T cells (CD3+) from whole blood cells (left, quantification; right, representative histogram based on live gating for T cells—Figure 3E dot plots). Sample sizes for statistical analyses are presented within figure. Two-way (treatment × age) ANOVA and 1-way ANOVA: *P < 0.05 vs. WKY-Veh; † P < 0.05 vs. SHR-Veh. Abbreviations: ANOVA, analysis of variance; CQ, chloroquine; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; Veh, vehicle; WKY, Wistar–Kyoto.
DISCUSSION
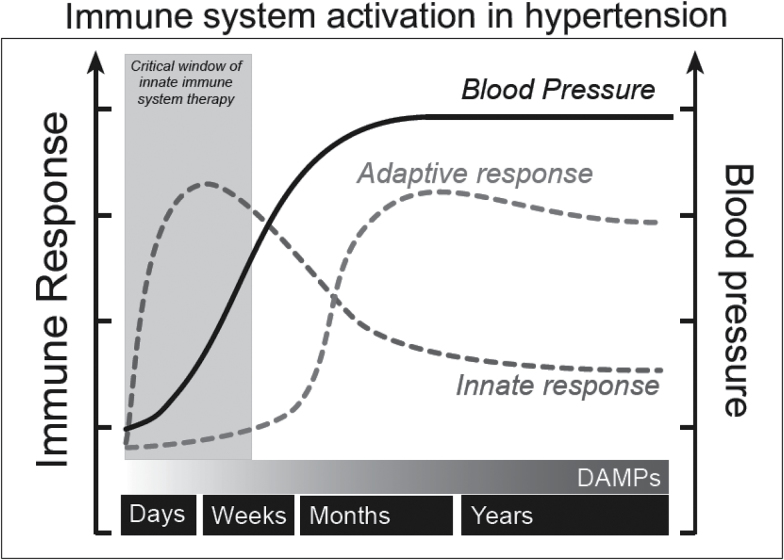
The first significant observation of the present investigation was that CQ treatment inhibited MyD88-dependent signaling proteins in young SHR, but not adult SHR. As a result of these age-specific responses, we went on to observe that CQ prevents the development of hypertension and modulates circulating and vascular infiltrating immune cells when administered prophylactically during the critical prehypertensive phase. Overall, these data bring into question whether TLRs are involved in the development and/or maintenance of hypertension and promotes the exploration of TLRs and the innate immune system during the developmental phase (Figure 4).
Figure 4.
Hypothesized schematic of how the innate and adaptive immune systems synergize during the development and maintenance of hypertension. After an inappropriate spike in blood pressure due to genetics or a prohypertensive factor such as angiotensin II, high salt, or chronic stress, the innate immune system initially responds. With the continued presence of prohypertensive factors and increasing blood pressure, the contribution of the innate immune system begins to wane and the adaptive immune system becomes increasingly engaged. In a feed-forward manner, chronic pressure- and ischemic-induced cell death and remodeling in hypertension fosters the escalating presence of DAMPs and the continued participation of the adaptive immune system during the maintenance phase of hypertension. Therefore, we propose a critical therapeutic window during the development of hypertension if the innate immune system is to going be targeted. Abbreviation: DAMPs, damage-associated molecular patterns.
Endosomal acidification and processing of CpG DNA precedes activation of NF-κB and AP-1 transcription factors.11–13 It was first observed by Ahmad-Nejad et al. 11 that CpG DNA and TLR9 are co-localized in endosomal compartments. Upon stimulation by CpG DNA, TLR9 recruits MyD88 and this can be blocked by the administration of CQ.11 It has also been proposed that CQ can act as a nucleic-acid binding compound, in conjunction with its action as acidification inhibitor. Therefore, CQ accumulates in endosomes and masks TLR ligand-binding epitopes, subsequently preventing TLR activation.18 Collectively, these studies demonstrate that CQ prevents TLR9–MyD88-dependent signaling in response to CpG DNA.
At the onset of this investigation, we wanted to use CQ as an inhibitor of TLR9–MyD88-dependent signaling in SHR. Our rationale for investigating TLR9 was based on knowledge that hypertension presents an exaggerated levels of cell death19 that could contribute to the release of DAMPs,2 and our subsequent observations that representative mtDNA genes have greater expression in the circulation of SHR compared to normotensive WKY.8 Mitochondrial DNA is particularly immunogenic because mitochondria were once a prokaryote that entered into eukaryotic cells to become an essential organelle (endosymbiosis).20 This ancestry of mitochondria means they still express evolutionarily conserved similarities to bacteria and thus predispose immune responses.21 Therefore, we hypothesized that treating SHR with CQ would abrogate the deleterious contribution of mtDNA on hypertension.8
Although our data support the notion that TLR9 contributes to hypertension in SHR, the use of CQ as our pharmacological inhibitor of choice did not exclude the possibility that other MyD88-dependent TLRs located on endosomes similarly contribute, namely TLR7 and TLR8. Interestingly, TLR7 and TLR9 have been reported to evoke the greatest proinflammatory responses to angiotensin (Ang) II when primed with their respective ligands in young SHR splenocytes.22 This report further supported our use of CQ as a pharmacological agent to ubiquitously inhibit endosomal TLRs. We observed that CQ impaired MyD88-dependent signaling proteins in young SHR MRA, and therefore suggest a disruption of TLR7, TLR8, and TLR9 signaling similarly. TLR7 and TLR8 are akin to TLR9 in that they all share MyD88 as a common adapter protein, and they use the same trafficking pathway from the endoplasmic reticulum upon recognition of their ligand, single stranded RNA, in endolysosomes.23 Interestingly, TRIF, the adaptor protein for TLR3 (and that also functions through TLR4), did not change after treatment with CQ in young SHR MRA. Although the TRIF adaptor protein has been observed to be important for the development of Ang II-dependent hypertension and cardiac hypertrophy in knockout mice,24 TLR4 inhibition only decreased MyD88 protein expression, and did not change TRIF, in SHR MRA.25
Although CQ did not decrease the expression of any endosomal TLR regardless of age or strain, it decreased the expression of signaling proteins MyD88 and TRAF6 in MRA from young SHR. On the other hand, in adult SHR MRA, CQ increased TLR8, MyD88, and IRAK and no effect was observed in WKY MRA (probably due to the fact that WKY, in the absence of infection, generally lack inflammation in which to inhibit). The reason for these age-dependent differences could be a result of the fact that naïve (untreated) young SHR MRA have changes in TLR signaling proteins that suggesting increased activity relative to both WKY and adult SHR (Supplementary Figure 4). Therefore, CQ functions as we initially hypothesized and inhibits this signaling. On the other hand, TLR signaling proteins in naïve adult SHR MRA were surprisingly not different from WKY, suggesting a possible desensitization of the signaling as the SHR aged. Therefore, we can extrapolate that the administration of CQ as therapeutic in adult SHR results in the compensatory upregulation of TLR signaling proteins to maintain innate immune defense. For example, cross-talk between effector pathways and feedback inhibition are vital aspects of normal molecular signaling that allows cellular responses to be dynamic and adaptable. However, when this signaling is blocked by an inhibitor (e.g., CQ), regulatory feedback loops are often disrupted, causing upregulation of pathway components and/or activation of parallel circuits.26 We hypothesize that these age-dependent differences in naïve adult SHR MRA, compared to young SHR MRA, are to prevent excessive inflammatory signaling via TLRs (Figure 4). It is interesting to note that these data surrounding the expression of TLR signaling proteins are in contrast with plasma membrane located TLR4, which is known to contribute to hypertension and vascular dysfunction in SHR.14 Specifically, TLR4 protein expression was augmented in MRA from adult SHR compared to normotensive Wistar rats, as well as young SHR (however, whether TLR4 expression was different between young SHR and Wistar was not clear).14
Our data demonstrate that inhibition of endosomal MyD88-dependent signaling proteins during the critical prehypertensive phase of young SHR can mitigate the blood pressure of adult SHR, and this is due in part to impaired immune cell recruitment directly into the vasculature. Although the perivascular adipose tissue can serve as significant source of infiltrating immune cells that can modulate vascular function,27 we dissected it off in the current study so we could measure immune cell infiltration into the vasculature and as a means of reducing intra-assay variability for our flow cytometry experiments. The participation of the adaptive immune system in the pathogenesis of hypertension has been known for a number of years,4 including that thymectomy of 4-week-old SHR delayed blood pressure elevation and produced significant reductions in lymphocyte counts.28 Nonetheless, the principal meditator of the adaptive immune response in hypertension and vascular dysfunction was not determined until recently. It was Guzik et al. who observed that mice lacking T cells are resistant to the development of both Ang II and deoxycorticosterone acetate–salt induced hypertension, and adoptive transfer of T cells, but not B cells, restored hypertension in these mice.3 Despite this investigation and others29,30 considerably improving our understanding of the adaptive immune system in the pathogenesis of hypertension and vascular dysfunction, there are still gaps in knowledge surrounding how it is signaled for involvement in the first place.
We proposed that MyD88-dependent signaling during the prehypertensive phase contributes to this inappropriate activation in SHR. CQ treatment during this critical phase reduced leukocytes in the aorta, suggesting reduced vascular injury31 and also decreased the number of circulating T cells, which is of importance because they are the lymphocyte subtype primarily responsible for increases in blood pressure.3 Finally, decreased expression of adhesion protein CD44 on circulating and aortic T cells is important because CD44 can mediate adhesion of T cells to endothelium,32 as well as stimulate the release of proinflammatory mediators.33 Previous studies have indicated that CQ (and its analogs) can be immunosuppressive by direct inhibition of T cell activation and proliferation.34,35 Therefore, in the current investigation, we cannot rule out the possibility that systemic CQ treatment has actions on the thymus that prevents T cell maturation and release in SHR, analogous to thymectomy.28
In conclusion, our investigation attempted to address the contribution of the innate immune system on the pathogenesis of hypertension, which is an area currently not well understood. We observed that lysosomotropic agent CQ, a well-established inhibitor of endosomal TLRs, inhibits MyD88-dependent signaling proteins in young SHR MRA, but not adult SHR MRA. Therefore, prophylactic treatment of young SHR with CQ subsequently prevented the development of high blood pressure and recruitment of immune cells to the vasculature. Overall, our results promote the exploration of innate immune system mechanisms in hypertension during the critical developmental phase of hypertension, as opposed to the maintenance phase, when the adaptive immune system may already be recruited.
Our understanding of the immune system in the pathogenesis of hypertension has expanded exponentially in the past several years, and as mentioned above, has provided general model for the involvement of T cells as the primary proinflammatory mediator.3,29,30 T cells also have a central role in the development of autoimmune disorders, and as a result, loss of immunological tolerance has been proposed as an underlying factor in hypertension.36 Interestingly, CQ and other antimalarial agents have long been used as therapeutic agents in the treatment of autoimmune conditions (e.g., rheumatoid arthritis and systemic lupus erythematosus37,38), even though their precise mechanism(s) of action are still not known.39 Our results indicate that CQ also has antihypertensive actions, possibly through regulation of TLR signaling, which may lower cardiovascular disease risk in autoimmune patients with hypertension.40
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURES
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This study was supported in part by the American Heart Association (#13PRE14080019, #14POST20490292, #13SDG17050056, #15GRNT25700451) and the National Institutes of Health (R01 HL071138, R01 DK083685).
REFERENCES
- 1. Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res 2014; 59:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol 2014; 306:H184–H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med 1967; 25:257–264. [PubMed] [Google Scholar]
- 5. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matzinger P. The danger model: a renewed sense of self. Science 2002; 296:301–305. [DOI] [PubMed] [Google Scholar]
- 7. Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res 2011; 108:1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, Matsumoto T, Webb RC. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res 2015; 107:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stacey KJ, Young GR, Clark F, Sester DP, Roberts TL, Naik S, Sweet MJ, Hume DA. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol 2003; 170:3614–3620. [DOI] [PubMed] [Google Scholar]
- 10. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004; 5:190–198. [DOI] [PubMed] [Google Scholar]
- 11. Ahmad-Nejad P, Häcker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol 2002; 32:1958–1968. [DOI] [PubMed] [Google Scholar]
- 12. Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol 1998; 160:4755–4761. [PubMed] [Google Scholar]
- 13. Häcker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J 1998; 17:6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012; 122:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 2013; 112:1159–1170. [DOI] [PubMed] [Google Scholar]
- 16. Baban B, Chandler PR, Johnson BA, 3rd, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. Physiologic control of IDO competence in splenic dendritic cells. J Immunol 2011; 187:2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 2009; 183:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011; 186:4794–4804. [DOI] [PubMed] [Google Scholar]
- 19. Gardner DL. Arteriolar necrosis and the prenecrotic phase of experimental hypertension. Q J Exp Physiol Cogn Med Sci 1963; 48:156–163. [DOI] [PubMed] [Google Scholar]
- 20. Sagan L. On the origin of mitosing cells. J Theor Biol 1967; 14:255–274. [DOI] [PubMed] [Google Scholar]
- 21. Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 2011; 32:157–164. [DOI] [PubMed] [Google Scholar]
- 22. Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 2012; 111:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brencicova E, Diebold SS. Nucleic acids and endosomal pattern recognition: how to tell friend from foe? Front Cell Infect Microbiol 2013; 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh MV, Cicha MZ, Meyerholz DK, Chapleau MW, Abboud FM. Dual activation of TRIF and MyD88 adaptor proteins by angiotensin II evokes opposing effects on pressure, cardiac hypertrophy, and inflammatory gene expression. Hypertension 2015; 66:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bomfim GF, Echem C, Martins CB, Costa TJ, Sartoretto SM, Dos Santos RA, Oliveira MA, Akamine EH, Fortes ZB, Tostes RC, Webb RC, Carvalho MH. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci 2015; 122:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 2011; 36:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 2013; 9:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khraibi AA, Smith TL, Hutchins PM, Lynch CD, Dusseau JW. Thymectomy delays the development of hypertension in Okamoto spontaneously hypertensive rats. J Hypertens 1987; 5:537–541. [DOI] [PubMed] [Google Scholar]
- 29. Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2010; 298:R1089–R1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 2010; 107:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 2001; 104:2228–2235. [DOI] [PubMed] [Google Scholar]
- 32. DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med 1996; 183:1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huet S, Groux H, Caillou B, Valentin H, Prieur AM, Bernard A. CD44 contributes to T cell activation. J Immunol 1989; 143:798–801. [PubMed] [Google Scholar]
- 34. Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood 2000; 95:3460–3466. [PubMed] [Google Scholar]
- 35. Xu JC, Peng YB, Wei MY, Wu YF, Guo D, Qin G, Ji G, Shen J, Liu QH. Chloroquine Inhibits Ca(2+) Signaling in Murine CD4(+) Thymocytes. Cell Physiol Biochem 2015; 36:133–140. [DOI] [PubMed] [Google Scholar]
- 36. Mathis KW, Broome HJ, Ryan MJ. Autoimmunity: an underlying factor in the pathogenesis of hypertension. Curr Hypertens Rep 2014; 16:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scherbel AL, Schuchter SL, Harrison JW. Comparison of effects of two antimalarial agents, hydroxychloroquine sulfate and chloroquine phosphate, in patients with rheumatoid arthitis. Cleve Clin Q 1957; 24:98–104. [DOI] [PubMed] [Google Scholar]
- 38. Tye MJ, White H, Appel B, Ansell HB. Lupus erythematosus treated with a combination of quinacrine, hydroxychloroquine and chloroquine. N Engl J Med 1959; 260:63–66. [DOI] [PubMed] [Google Scholar]
- 39. Wozniacka A, Carter A, McCauliffe DP. Antimalarials in cutaneous lupus erythematosus: mechanisms of therapeutic benefit. Lupus 2002; 11:71–81. [DOI] [PubMed] [Google Scholar]
- 40. Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus 2000; 9:166–169. [DOI] [PubMed] [Google Scholar]