Abstract
The cps5-138 fission yeast mutant shows an abnormal lemon-like morphology at 28°C in minimal medium and a lethal thermosensitive phenotype at 37°C. Cell growth is completely inhibited at 28°C in a Ca2+-free medium, in which the wild type is capable of growing normally. Under these conditions, actin patches become randomly distributed throughout the cell, and defects in septum formation and subsequent cytokinesis appear. The mutant cell is hypersensitive to the cell wall-digesting enzymatic complex Novozym234 even under permissive conditions. The gene SPBC31E1.02c, which complements all the mutant phenotypes described above, was cloned and codes for the Ca2+-ATPase homologue Pmr1p. The gene is not essential under optimal growth conditions but is required under conditions of low Ca2+ (<0.1 mM) or high temperature (>35°C). The green fluorescent protein-tagged Cps5 proteins, which are expressed under physiological conditions (an integrated single copy with its own promoter in the cps5Δ strain), display a localization pattern typical of endoplasmic reticulum proteins. Biochemical analyses show that 1,3-β-d-glucan synthase activity in the mutant is decreased to nearly half that of the wild type and that the mutant cell wall contains no detectable galactomannan when the cells are exposed to a Ca2+-free medium. The mutant acid phosphatase has an increased electrophoretic mobility, suggesting that incomplete protein glycosylation takes place in the mutant cells. These results indicate that S. pombe Pmr1p is essential for the maintenance of cell wall integrity and cytokinesis, possibly by allowing protein glycosylation and the polarized actin distribution to take place normally. Disruption and complementation analyses suggest that Pmr1p shares its function with a vacuolar Ca2+-ATPase homologue, Pmc1p (SPAPB2B4.04c), to prevent lethal activation of calcineurin for cell growth.
A transient increase in the intracellular Ca2+ concentration ([Ca2+]) plays a key role in transmitting signals that regulate a variety of cellular functions in eukaryotes. A substantial body of knowledge has been accumulated concerning the roles of Ca2+ as a second messenger in various types of eukaryotic cells (5, 11). In yeasts, Ca2+ plays an essential role in the mating process (9, 26), and the Ca2+ calmodulin-dependent protein phosphatase calcineurin plays crucial roles in a variety of cellular functions, including ion homeostasis, cytokinesis, and transcriptional regulation (14, 55, 63). In the budding yeast Saccharomyces cerevisiae, FKS1, which codes for a putative catalytic subunit of 1,3-β-d-glucan synthase, is predominantly expressed under optimal growth conditions in a cell cycle-dependent manner, while the transcription of FKS2, an alternative gene for the putative glucan synthase, is completely dependent on calcineurin in the absence of a functional Fks1p and in the presence of mating pheromone or a high extracellular [Ca2+] (42, 74). In the fission yeast Schizosaccharomyces pombe, 1,3-β-d- and 1,3-α-d-glucan synthases, both of which contribute to the mechanical strength of the cell wall (12, 23, 29, 32), are presumed to be the downstream targets of Pck2p, a protein kinase C homologue (4, 8, 32). The overexpression of pck2+ (OP-pck2+) has been shown to increase 1,3-β-d-glucan synthase activity to a significant extent, as well as to induce an extremely high intracellular [Ca2+] (4, 8). The effects of OP-pck2+ were reported to be abolished in the absence of Ehs1p, a homologue of the calcium channel component Mid1p of S. cerevisiae (9, 25). The ehs1-1 mutant displays several cell wall-related defective phenotypes, and these defects are suppressed by moderate OP-pck2+ levels, suggesting that Pck2p contributes, along with the Ehs1p calcium channel, to the integrity of the cell wall (9). More recently, another component, Cta4p, a cation P5-type ATPase that is also required for calcium homeostasis, has been identified (45). The null mutant displayed pleiotropic phenotypes, including defects in cytokinesis and microtubule dynamics, similar to the calcineurin null mutant phenotypes (73). These findings suggest that the regulation of intracellular [Ca2+] is critical to cell wall integrity, cytokinesis, and cytoskeletal organization, all of which are essential for fungal-cell morphogenesis. Transient and spatial changes in intracellular [Ca2+] are thus critical for generating signals that regulate cellular events or maintain ion homeostasis; the processes are mediated by Ca2+ channels, Ca2+ antiporters, and Ca2+-transporting ATPases, which are localized in the plasma or vesicle membranes to allow the transport of ions into or out of the cell or organelles via the membranes. However, at present, little is known concerning the molecular mechanisms of calcium signaling required for these cellular processes during the cell cycle. To address these issues, the molecular characterization of the mutants that show calcium-sensitive phenotypes might be useful.
Fourteen genes, named cps1 to cps14, were identified as mutant alleles that confer hypersensitivity to the mitotic poison isopropyl chlorophenyl carbamate (CIPC) (30). Among these, cps1+ was found to code for a putative β-1,3-d-glucan synthase subunit, and the cps1-12 mutation causes cells to lyse in the presence of cyclosporin A (CsA), a potent inhibitor of calcineurin (29). cps8-188 encodes a mutated actin molecule (G273D), and the mutant shows a depolarized and multiseptated morphology with a disorganized cell wall structure (28, 31, 35). These findings suggest that in fission yeast, both calcium signaling and the actin organization are crucial to the integrity of the cell wall. We have recently found that the cps5-138 mutant is incapable of growing in a medium depleted of calcium or at high temperature, under which conditions the cells show an abnormally rounded shape. In the present study, we demonstrate that cps5 is a mutant allele of pmr1+, a gene encoding a putative Ca2+/Mn2+-ATPase (SPBC31E1.02c) that plays a crucial role in the maintenance of cell wall integrity and cytokinesis. In addition, we show that the S. pombe Pmr1p is required for intracellular Ca2+ homeostasis, cooperating with a vacuolar Ca2+-ATPase homologue, Pmc1p (SPAPB2B4.04c), to prevent the lethal activation of calcineurin for cell growth.
MATERIALS AND METHODS
Strains and culture conditions.
The strains used in this study are listed in Table 1. Routine yeast extract (YE) medium and selected Edinburgh minimal medium (EMM) supplemented with the appropriate amino acids and sporulation medium have been described elsewhere (2, 20). Ca2+-free EMM is EMM from which CaCl2 was omitted and in which calcium panthothenate was replaced by sodium pantothenate. In some experiments, 0.1 and 10 mM EGTA was added to Ca2+-free liquid and solid EMM, respectively. The yeast cells were cultured at 28°C, unless otherwise specified, on a plate or in liquid medium with continuous shaking. The Escherichia coli strain DH5α was used for the routine propagation of plasmids, as described elsewhere (56).
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| 972 | h− wild type | NCYCa |
| 903 | h−leu1-32 | This study |
| 218 | h+cps1-12 leu1-32 | J. Ishiguro et al. (29) |
| CP13-8 | h−cps5-138 | J. Ishiguro et al. (30) |
| CP13-8-4D | h+cps5-138 leu1-32 | This study |
| A3-2D-2A | h+ura4-Δ18 leu1-32 cps5::ura4+ | This study |
| B2B4#6 | h−ura4-Δ18 leu1-32 pmc1::ura4+ | This study |
| 733 | h+cps1-12 cps5-138 leu1-32 | This study |
| #16 | h−ehs1-1 leu1-32 | Carnero et al. (9) |
| JY741 | h−ade6-M216 ura4-Δ18 leu1-32 | C. Shimoda |
| JY746 | h+ade6-M210 ura4-Δ18 leu1-32 | C. Shimoda |
National Collection of Yeast Cultures, AFRC Institute of Food Research, Norwich, United Kingdom.
Plasmids and DNA techniques.
DNA manipulations were carried out according to the standard methods described in Sambrook et al. (56). The plasmids pAL-KS and pREP1 are described elsewhere (29, 41, 65). The plasmids pDB248X-pck1+, pDB248X-pck2+, pREP3X-pck1+, and pREP3X-pck2+ were obtained from P. Pérez (4).
Gene cloning and mutation site determination.
The mutant strain CP13-8-4D was transformed with an S. pombe genomic library constructed in the pAL-KS vector (pTN-L1; prepared by T. Nakamura). Four transformants capable of growing on both EMM and YE plates containing 280 μM isopropyl N-3-chlorophenyl carbamate (CIPC; Sigma) were isolated, and the plasmids were recovered. Nucleotide sequence determination revealed that all four plasmids contained only one complete open reading frame (ORF), SPBC31E1.02c. The plasmid called pCP5-15, which contained an 8.8-kb genomic DNA insert, was used for further analysis. PCR was carried out to detect the mutation site, using the cps5-138 mutant genome as a template. The primers used were 5′-GGACTAGTCCTAAGAAACCAGCGGAAA GC-3′ (forward, with an SpeI site at the 5′ end) and 5′-CCCCCGGGGGTCGTTTTTGTTTTTGTATGA-3′ (reverse, with an SmaI site at the 5′ end). The amplified fragments were digested with SpeI/SacI, EcoRI/SacI, and EcoRI/SmaI, respectively, and the nucleotide sequence of each generated fragment (1.1, 1.1, and 0.7 kb, respectively) was determined by means of a Hitachi SQ5500E sequencer using the RPN2444 premixed cycle-sequencing kit (Amersham Pharmacia Biotech UK).
Gene disruption and overexpression.
The one-step gene disruption method (53) was employed to construct the cps5Δ and pmc1Δ (SPAPB2B4.04cΔ) strains. The cps5+ gene, amplified from the wild-type genome by using the same primers described above, was digested with SpeI/SmaI and ligated into pBluescript II KS(+) plasmids (Stratagene). The ura4+ gene was inserted into the BamHI site of the cps5+ ORF, and the SpeI/SmaI disruption fragment from the propagated plasmid was used for diploid (JY741XJY746) transformation. After diploid sporulation, tetrad analysis and PCR were performed on the haploid cells to check the uracil requirement phenotype and the correct ura4+ insertion, respectively. For pmc1+ disruption, a 1.5-kb fragment, which was amplified by PCR using the primers 5′-CGGGATCCCGTCGGTGTTAATTCATTTAAC-3′ (forward, with a BamHI site at the 5′ end) and 5′-CCCAAGCAATTGCTTTCACA-3′ (reverse), was digested with BamHI/SalI (0.6 kb) and ligated into pBluscript II KS(+) plasmids. The ura4+ gene was inserted into the HindIII site of the amplified fragment, and the BamHI/SalI disruption fragment (2.4 kb) from the propagated plasmid was used for diploid transformation. Gene disruption in the haploid cells was checked by tetrad analysis and PCR, as described above. For cps5+ overexpression, the SPBC31E1.02c ORF (also called pgak2) was amplified by PCR from pCP5-15 using the primers 5′-TCCCCCGGGGGAATTTAGGAATCCTTTACA-3′ (forward, with an SmaI site at the 5′ end) and 5′-TCCCCCGGGGGACATTGGAATTTTGTATTC-3′ (reverse, with an SmaI site at the 5′ end) and inserted into the pREP1 plasmid at the SmaI site. The plasmids bearing the cps5+ gene in the right orientation were selected and used to transform the strains CP13-8-4D, B2B4#6, and 903.
GFP tagging.
Two methods were employed to express a cps5+-GFP fused gene in cps5Δ cells; one was a plasmid-borne expression, and the other was a physiological level of expression using an integrated copy of the fused gene in the chromosome. The cps5+ gene bearing its own promoter region was amplified by PCR using the primers 5′-TCCCCCGGGGGATAACCTTTCCCAACTTGT-3′ (forward, with an SmaI site at the 5′ end) and 5′-GAAGATCTTCCCTACATTCCTTAGCAGATA-3′ (reverse, with a BglII site at the 5′ end). The amplified fragment (3.3 kb), which lacked the stop codon, was digested with SmaI/BglII and ligated in frame to the BglII site just before the ATG codon of the GFP ORF that was generated by PCR from the pEGFP vector (BD Biosciences and Clontech). The resulting cps5+-GFP fragment with a SacI site at the 3′ end of the GFP ORF was inserted between the SmaI and SacI sites of the pAL-KS plasmid and used to transform the cps5Δ strain, CP13-8-4D. To integrate a copy of cps5+-GFP into the chromosome, the cps5+-GFP fragment was inserted between the SmaI and SacI sites of pJK148 (12), and the plasmid was cut with XbaI at position −245 of the cps5+ ORF. The linearized plasmid was used to isolate leucine-nonrequiring transformants from the A3-2D-2A strain. For green fluorescent protein (GFP) tagging of pmc1+, essentially the same procedure was used. The primers used for the gene amplification were 5′-TCCCCCGGGGGAGACTTTTTGTCTTTTAAA-3′ (forward, with an SmaI site at the 5′ end) and 5′-CGGGATCCCGATGAACATTTGAGCTTTTTT-3′ (reverse, with a BamHI site at the 5′ end). The amplified fragment (4.4 kb) was digested with SmaI/BamHI and ligated in frame to the BamHI site just before the ATG codon of the GFP ORF. The resulting GFP-tagged fragment was inserted between the SmaI and SacI sites of the pAL-KS plasmid and used to transform the pmc1Δ strain, B2B4#6. For GFP tagging of cps11+/alg3+, the same procedure described in the case of cps5+-GFP integrated in pAL-KS was used. The primers used for gene amplification were 5′-TCCCCCGGGGGACCAGGCGTAGTAATCTAG-3′ (forward, with a SmaI site at the 5′ end) and 5′-GAAGATCTTCCCGGGTTTTCTGTAGTCGGA-3′ (reverse, with a BglII site at the 5′ end).
Fluorescence microscopy.
For septum visualization, cells were washed with phosphate-buffered saline (PBS) and stained with 100 μg of calcofluor (fluorescent brightener 28; Sigma)/ml for 30 min and then washed again with the same buffer. For F-actin visualization, cells were fixed with 4% formaldehyde (electron microscopy grade; Polysciences) and stained with rhodamine-conjugated phalloidin (R-415; Molecular Probes) as described elsewhere (2). F-actin and GFP-fused Cps5p were observed with a laser scanning confocal microscope system (Radiance 2100; Bio-Rad).
Analysis of acid phosphatase.
Acid phosphatase was analyzed according to the method described by Huang and Snider (24) with minor modifications (72). Logarithmic-phase cells, grown in YE medium, were transferred to YES-P (yeast extract sugar minus phosphate) medium, which contains a reduced amount of inorganic phosphate, and further cultivated for 8 h. Samples prepared from the cell lysate were subjected to electrophoresis on a 6% nondenaturing acrylamide gel, and then activity staining of acid phosphatase was carried out as described by Schweingruber et al. (58).
Cell wall analysis and 1,3-β-d-glucan synthase assay.
For the monitoring of enzymatic cell lysis, log-phase cells, grown at 28°C in YE, were washed with 50 mM citrate-phosphate buffer (pH 5.6), suspended in the same buffer containing 30 μg of Novozym234 (Novo Industries)/ml, followed by incubation at 30°C with shaking. The residual absorbance at 600 nm was monitored at hourly intervals, assuming an absorbance of 100% at time zero. Cell extracts for preparation of the enzyme and the 1,3-β-d-glucan synthase assay were carried out as described elsewhere (12). [14C]glucose labeling and the fractionation of cell wall polysaccharides were carried out as described previously (29).
RESULTS
cps5 mutants show defects in cell growth, morphology, and cytokinesis under low-extracellular-[Ca2+] or high-temperature conditions.
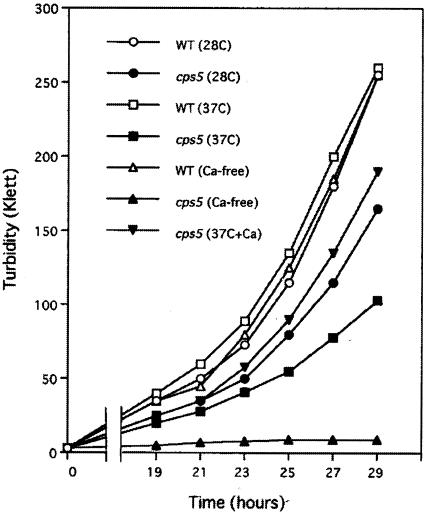
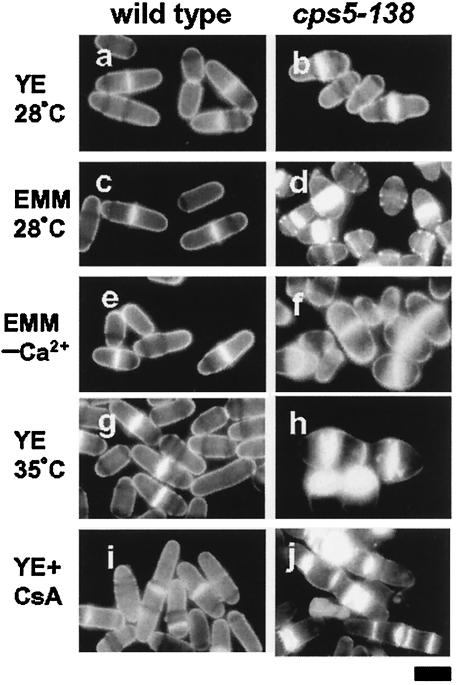
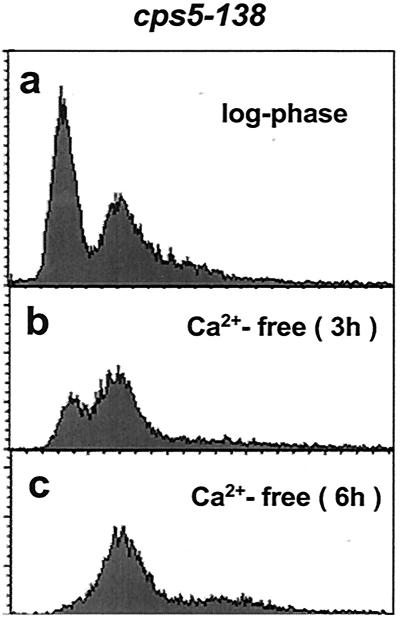
cps5-138 was originally identified as a mutant allele that confers hypersensitivity to the mitotic poison CIPC (30). cps5-138 mutant cell growth was also found to be slower than wild-type cell growth under normal conditions and defective under low-[Ca2+] or high-temperature conditions (Fig. 1). The mutant was found to be incapable of growing in the absence of Ca2+ (Ca2+-free EMM), in which the wild-type strain is capable of growing normally. The growth defect in EMM (0.1 mM CaCl2) at 37°C was significantly recovered by the addition of CaCl2 (50 mM) to the medium. These results indicate that the cps5-138 mutant requires a higher [Ca2+] in the medium for normal growth than the wild type. The mutant grown in EMM or in YE at high temperature (37°C) became lemon-like in shape, showing abnormal staining patterns with the dye calcofluor, which has a specific affinity for septum glucans (Fig. 2a to h). The mutant septum was stained much more intensely and broadly than the wild type, suggestive of the excessive deposition of septum materials. A prolonged exposure to high temperature caused cell wall materials to disperse from the medial plane to the cell surface. The rounded shape and abnormal septum staining observed in EMM reverted to the wild-type morphology when the [Ca2+] in the medium was increased to 10 mM (data not shown). The growth and morphology defects at 37°C can be restored to the wild-type phenotype in the presence of an osmotic stabilizer (1.2 M sorbitol), which suggests that the mutant has a cell wall defect (data not shown). Fluorescence-activated cell sorter (FACS) analysis showed that, when the mutant strain is exposed to Ca2+-free EMM, 4C cells with an unseparated septum accumulate gradually (Fig. 3). These results indicate that cell separation becomes defective in the cps5-138 mutant under restrictive conditions, because in S. pombe, DNA synthesis occurs before cell division is complete. As in the cases of other septation mutants, the cps5-138 mutant was found to be more sensitive to CsA, a specific inhibitor of calcineurin, than the wild type, giving rise to elongated cells with abnormally formed multisepta (Fig. 2i and j).
FIG. 1.
Defective cell growth of the cps5-138 mutant under restrictive culture conditions. Cell growth was monitored by measuring the turbidity at 640 to 700 nm (red filter) with a Klett-Summerson colorimeter. Wild-type (WT) (○) and cps5-138 (•) cells in EMM at 28°C, wild-type (□) and cps5-138 (▪) cells in EMM at 37°C, wild-type (▵) and cps5-138 (▴) cells in Ca2+-free EMM at 28°C, and cps5-138 cells in EMM plus 50 mM CaCl2 at 37°C (▾).
FIG. 2.
Abnormal cell morphology and septum formation of the cps5-138 mutant under restrictive culture conditions. Wild-type and cps5-138 mutant cells were stained with calcofluor. Log-phase cells of the wild type (a) and cps5-138 mutant (b) cultured in YE medium at 28°C and wild-type (c) and cps5-138 mutant (d) cells in EMM at 28°C. Log-phase cells of the wild type (e) and cps5-138 mutant (f) were transferred to Ca2+-free EMM and cultured for 3 h at 28°C. Wild-type (g) and cps5-138 mutant (h) cells cultured in YE medium at 35°C for 15 h. Wild-type (i) and cps5-138 mutant (j) cells cultured in YE medium plus CsA (10 μg/ml) at 28°C for 15 h. Bar, 5 μm.
FIG. 3.
Defective cytokinesis of the cps5-138 mutant under restrictive culture conditions. The cps5-138 mutant was cultured in YE medium at 28°C to the log phase (a), and the collected cells were transferred to Ca2+-free EMM and cultured for 3 (b) and 6 (c) h. Each cell culture was stained with propidium iodide (57) and analyzed with an LSR flow cytometer (BD Biosciences). The horizontal and vertical axes show relative DNA content and cell number, respectively.
cps5 mutants show a depolarized distribution of actin patches when exposed to low [Ca2+] or high temperature.
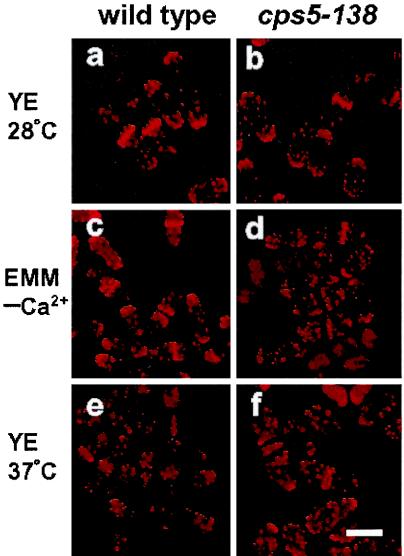
The F-actin cytoskeleton is known to be important for cell wall integrity and cytokinesis. To determine whether F-actin is affected by cps5 mutations, the cytoskeleton was visualized with rhodamine-conjugated phalloidin and observed during the cell cycle by fluorescence microscopy. The wild-type cells showed a polarized F-actin organization during the cell cycle; in interphase, actin patches were specifically localized to the growing end(s), and during late anaphase, they moved to either side of the actin ring, associating with the sites of septum wall formation (Fig. 4a). In the mutant cells, a polarized distribution was also observed under permissive conditions, although actin patches at the cell poles appeared to be somewhat dispersed compared with those in the wild type (Fig. 4b). When the mutant cells were exposed to low-[Ca2+] or high-temperature (37°C) conditions, the actin patches became randomized throughout the cell (Fig. 4c, d, e, and f). Therefore, the altered morphology of the cps5-135 mutant can be attributed to an altered actin distribution.
FIG. 4.
Depolarized actin distribution of the cps5-138 mutant under restrictive culture conditions. Wild-type and cps5-138 mutant cells were stained with rhodamine-conjugated phalloidin. Log-phase cells of the wild type (a) and cps5-138 mutant (b) cultured in YE medium at 28°C. Log-phase cells of the wild type (c) and cps5-138 mutant (d) were transferred to Ca2+-free EMM and cultured at 28°C for 3 h. Log-phase cells of the wild type (e) and cps5-138 mutant (f) were transferred to YE medium and cultured at 37°C for 6 h. Bar, 5 μm.
cps5+ encodes a homologue of the S. cerevisiae Pmr1 ATPase.
The cps5+ gene was cloned from a fission yeast genomic library by complementation of the CIPC-hypersensitive phenotype of the cps5-138 mutant strain. Four clones, which were capable of growing in EMM as well as in YE medium containing 280 μM CIPC, were isolated by the screening of 7 × 104 transformants. A nucleotide sequence analysis of the DNA fragments from the clones revealed that only one complete ORF, SPBC31E1.02c (The S. pombe Genome Project, The Wellcome Trust Sanger Institute; http://www.sanger.ac.uk/Projects/S_pombe/), is common to all the obtained DNA clones. Plasmids bearing this single ORF complemented not only the hypersensitivity but the abnormal cell morphology and defective cell growth of the cps5-138 mutant as well under restrictive conditions (data not shown). To determine whether the cloned gene was cps5+ itself, the ORF was amplified by high-fidelity PCR from the cps5-138 genome as a template, and the nucleotide sequence was determined. Analysis of three clones independently obtained by PCR showed that the guanine at nucleotide 1200 of the coding sequence had been replaced by adenine, resulting in the substitution of a nonsense codon (UGA) for the Trp codon. These results indicate that SPBC31E1.02c is cps5+ and not an extragenic multicopy suppressor. The ORF was next disrupted in a diploid strain (JY741 × JY746) by replacing cps5+ with a copy containing the ura4+ gene inserted at the BamHI site that is situated near the middle of the ORF. The transformed diploid was sporulated, and the resulting tetrads were examined for viability. The ura4+ disruptants grew normally on a YE plate (data not shown), indicating that cps5+ is not essential for cell growth under optimal growth conditions. No phenotypic differences between cps5-138 and cps5Δ were noted with respect to cell shape, actin distribution, and cell growth under restrictive conditions (data not shown). These results are consistent with the finding that the cps5-138 allele is a UGA opal mutation, predicted to encode only 399 amino acids instead of the 899 residues of SPBC31E1.02c. The effects of cps5+ overexpression in the wild-type background were examined using the pREP1 plasmid in the absence of thiamine (derepressed conditions), but no remarkable phenotype was observed (data not shown).
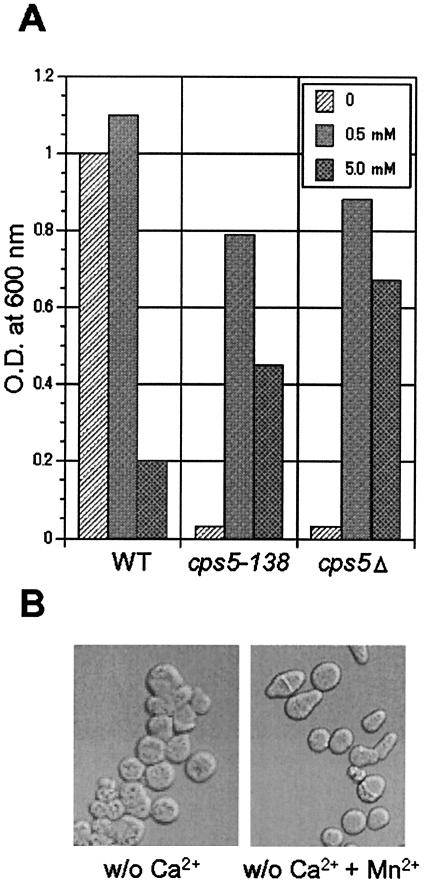
The predicted amino acid sequence of the SPBC31E1.02c protein reveals significant homology to proteins of the Pmr1 ATPase family (overall identity with S. cerevisiae Pmr1p, 53%) (46). Because the SPBC31E1.02c protein was identified very recently as S. pombe Pmr1p (37), we use the name Pmr1p in the following description instead of Cps5p. The S. pombe Pmr1p protein was shown to play an important role in cell morphology, cooperating with an Nramp-related metal transporter via the control of Mn2+ homeostasis (37). In this study, the effects of Mn2+ on the defective cell growth and morphology of the cps5-138 and cps5Δ cells under Ca2+-free conditions were also examined. Supplying 0.5 mM MnCl2 to the medium rescued the defective cell growth to a significant extent in both mutants (Fig. 5A) but did not suppress the aberrant cell morphology caused by the Ca2+-free medium (Fig. 5B).
FIG. 5.
Effects of MnCl2 on cell growth and morphology of the wild-type (WT), cps5-138, and cps5Δ strains. A: Each strain was cultured in Ca2+-free EMM and Ca2+-free EMM containing 0.5 or 5.0 mM MnCl2. After 48 h of cultivation, the optical density (O.D.) at 600 nm was measured to quantify the cell growth. B: The cps5-138 cells were grown in Ca2+-free EMM or Ca2+-free EMM containing 0.5 mM MnCl2 to the log phase and observed with a Nomarski interference microscope. w/o, without.
Pmr1p shows a localization pattern typical of endoplasmic reticulum (ER) proteins.
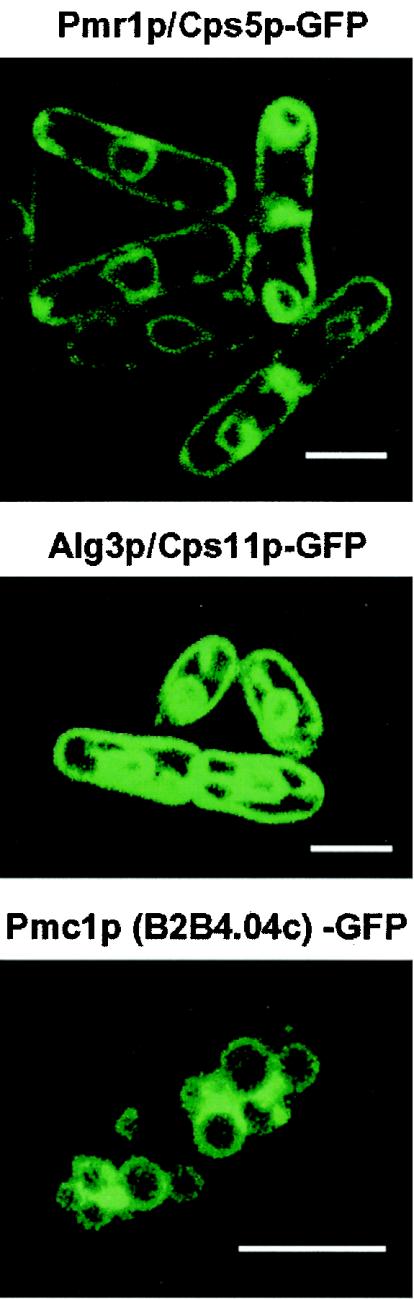
The intracellular localization of S. pombe Pmr1p was examined by fluorescence microscopy using GFP-tagged Pmr1p. The proteins were observed under two different conditions, plasmid-borne and chromosomal (an integrated single copy with its own promoter) expression of the fused gene in a cps5Δ strain. Both transformants are capable of growing in the absence of calcium with an almost normal morphology (data not shown), suggesting that the GFP-tagged Pmr1p is functional with respect to its pump activity. The typical localization pattern is shown in Fig. 6 (top panel). Most of the Pmr1p-GFP was observed in the nuclear and plasma membranes throughout the cell cycle. This localization pattern is typical of the ER proteins, since in S. pombe, the ER is associated with the nuclear membrane and extends longitudinally to the cell ends, reaching the entire plasma membrane (6, 49, 50). To confirm this, the ER was also stained with a GFP-tagged Alg3 mannosyltransferase homologue encoded by cps11+ (SPAC7D4.06c), because the protein is well established as localizing in the ER membrane. The Alg3p-GFP localization pattern was essentially the same as the case of Pmr1p-GFP (Fig. 6, middle panel). These results suggest that the fission yeast Pmr1p resides predominantly in the ER.
FIG. 6.
Intracellular localizations of GFP-tagged Pmr1p/Cps5p, Alg3p/Cps11p, and Pmc1p. Pmr1p was observed in cps5Δ cells capable of growing in Ca2+-free EMM with multicopy plasmids bearing the fused gene or the gene integrated in the chromosome. Almost the same fluorescence pattern was obtained in each case. The localizations of Alg3p and Pmc1p were examined in each disruptant with multicopy plasmids. Pmr1p-GFP (top panel) and Alg3p-GFP (middle panel) display a localization pattern typical of S. pombe ER proteins, and Pmc1p-GFP (bottom panel) shows the vacuole membrane localization. Bar, 5 μm.
cps5 mutants show decreased 1,3-β-d-glucan synthase activity with weakened cell walls.
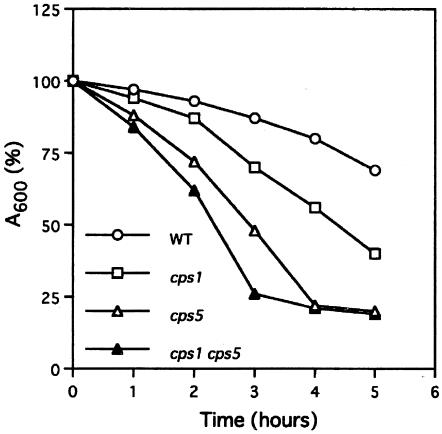
In yeast, the cell wall plays an essential role in determining cell shape, and therefore, a large number of morphological mutants have defects in cell wall integrity and cytokinesis (27). In order to examine whether this is the case for the cps5 mutant, physiological and biochemical analyses were carried out. Sensitivities to Nomozym234, a cell wall-digesting enzyme complex, in the mutant and wild-type strains were initially compared. The results are shown in Fig. 7. The cps5-138 mutant grown in YE medium at 28°C was much more sensitive to Novozym234 than the wild-type strain, suggesting that the mutant cell wall is altered, even under permissive conditions. The cps5Δ mutant showed the same sensitivity as cps5-138 (data not shown). The cps1-12 cps5-138 double mutant was found to be more sensitive to Novozym234 than any of the single mutants, suggesting that Pmr1p and Cps1p/Bgs1p regulate cell wall integrity by different mechanisms.
FIG. 7.
The cps5-138 mutant is hypersensitive to the cell wall-digesting enzyme complex Novozym234. Log-phase cells cultured in YE medium were incubated at 30°C with 30 μg of Novozym234/ml, and the residual absorbance was monitored at 600 nm. Average values obtained from triplicate experiments were plotted. Wild type (WT) (○), cps1-12 mutant (□), cps5-138 mutant (▵), and cps1 cps5 double mutant (▴).
Next, 1,3-β-d-glucan synthase activities were determined for the wild-type, cps5-138 mutant, and cps5Δ mutant strains grown under permissive and restrictive conditions (37°C in YE medium). As shown in Table 2, the specific activities of the mutant strains grown under permissive conditions in YE medium at 28°C were dramatically reduced by almost half for cps5-138 (56%) or even more for cps5Δ (45%) compared to the wild-type strain. Similar results were obtained when the mutant strains were grown in YE medium plus 1.2 M sorbitol at 28°C. Both cps5-138 and cps5Δ present a thermosensitive phenotype at 37°C that can be prevented by osmotic stabilization (1.2 M sorbitol), suggesting that it is due to a cell wall defect. The activity of 1,3-β-d-glucan synthase was assayed in both mutants grown at 37°C, either in the presence or in the absence of sorbitol. In the presence of sorbitol, the cells were normal in shape and growth, and the activity rose to almost 90% of that of the wild type. However, in the absence of sorbitol, in which the cells have a strong oval or rounded phenotype and grow slowly but are still viable (for 24 h cultivation), the activity increased considerably to >120% (cps5-138) and 140% (cps5Δ) that of the wild type (Table 2). Therefore, the thermosensitive morphological phenotype of the cps5 mutants is not due to a defect in 1,3-β-d-glucan synthase activity but rather to other cps5-related defects.
TABLE 2.
Characterization of (1,3)-β-d-glucan synthase activities from S. pombe wild-type, cps5-138, and cps5Δ strains grown at 28 and 37°C in the absence or presence of 1.2 M sorbitol
| Growth temp (°C) | Strain | Sp act (mean ± SD)a |
|---|---|---|
| 28 | Wild type | 10.1 ± 0.1 (100) |
| cps5-138 | 5.7 ± 0.2 (56) | |
| cps5Δ | 4.5 ± 0.2 (45) | |
| 28 + sorbitol | Wild type | 5.8 ± 0.2 (100) |
| cps5-138 | 3.3 ± 0.4 (57) | |
| cps5Δ | 2.3 ± 0.2 (40) | |
| 37b | Wild type | 4.6 ± 0.1 (100) |
| cps5-138 | 5.7 ± 0.0 (124) | |
| cps5Δ | 6.8 ± 0.7 (148) | |
| 37 + sorbitol | Wild type | 6.1 ± 0.1 (100) |
| cps5-138 | 5.5 ± 0.1 (90) | |
| cps5Δ | 5.2 ± 0.1 (85) |
Specific activity is expressed as milliunits per milligram of protein. The reaction mixture contained 150 μM GTP. The values were obtained from three independent experiments. Values in parentheses are percentages of specific activity compared to that of the wild type under the same conditions.
The cells were grown for 24 h at 37°C. Under these conditions, the thermosensitive morphological phenotype is completely expressed in the absence of sorbitol, and the cells are still viable and able to grow.
The cps5 mutants are unable to grow at 28°C in the absence of Ca2+ and grow normally but show an altered morphology in YE medium and EMM (0.1 mM Ca2+). The rounded shape is suppressed when both media contain 10 mM Ca2+. The cps1-12 mutant also displays hypersensitivity to high temperature, to cell wall-digesting enzymatic complexes, to CsA (29), and to Ca2+-free EMM. In addition, the thermosensitive phenotype is partially suppressed in the presence of 10 mM Ca2+. To confirm the relationship between cell wall weakness and extracellular [Ca2+] in these mutants, the 1,3-β-d-glucan synthase activity was analyzed under low (0.1 mM)- and high (10 mM)-[Ca2+] conditions (Table 3). The activity of the putative 1,3-β-d-glucan synthase mutant cps1-12 grown in the presence of 10 or 0.1 mM Ca2+ at 28°C was higher than that of the wild type (118 and 127%). A similar increase in activity was described for this mutant, based on an analysis of the thermosensitive phenotype (29). The increased activity may be the result of a compensatory mechanism taking place under conditions of cell wall stress (51). On the other hand, the absence of Ca2+ caused a decrease in the 1,3-β-d-glucan synthase activity of cps1-12 cells to 82% of that of the wild type. The activities of single cps5 and double cps1 cps5 mutants were similar in a medium containing 10 and 0.1 mM Ca2+. The cps5Δ mutant was more sensitive to [Ca2+], as the activity in cells grown in 0.1 mM Ca2+ decreased to 63%. Nonetheless, in the absence of Ca2+, both cps5-138 and cps5Δ showed a dramatic decrease to 50% of the activity of the wild type. Under these conditions, the cps1-12 mutant showed an epistatic phenotype, as the enzymatic activities were about the same in both single cps1-12 and double cps1-12 cps5-138 mutants (82 and 84%). These results led us to conclude that the Ca2+-hypersensitivity of cps5 mutants correlates with a decrease in 1,3-β-d-glucan synthase activity.
TABLE 3.
Characterization of (1,3)-β-d-glucan synthase activities from S. pombe wild-type; cps1-12, cps5-138, and cps5Δ mutant; and cps1-12 cps5-138 double mutant strains grown at 28°C in EMM in the presence or absence of calcium
| CaCl2, concn (mM) | Strain | Sp act (mean ± SD)a |
|---|---|---|
| 10 | Wild type | 7.4 ± 0.4 (100) |
| cps1-12 | 8.7 ± 0.7 (118) | |
| cps5-138 | 6.6 ± 0.6 (89) | |
| cps5Δ | 6.1 ± 0.4 (82) | |
| cps1-12 cps5-138 | 6.2 ± 0.5 (84) | |
| 0.1 | Wild type | 9.8 ± 0.1 (100) |
| cps1-12 | 12.4 ± 1.2 (127) | |
| cps5-138 | 8.7 ± 1.2 (89) | |
| cps5Δ | 6.2 ± 0.8 (63) | |
| cps1-12 cps5-138 | 8.0 ± 0.5 (82) | |
| 0b | Wild type | 15.0 ± 0.4 (100) |
| cps1-12 | 12.3 ± 0.9 (82) | |
| cps5-138 | 8.0 ± 0.7 (53) | |
| cps5Δ | 7.6 ± 1.3 (51) | |
| cps1-12 cps5-138 | 12.6 ± 1.1 (84) |
Values were obtained from three independent experiments as for Table 2. Values in parenthesis are percentages of specific activity compared to that of the wild type under the same conditions.
The cells were grown for 4 h in Ca2+-free EMM plus 0.1 mM EGTA. A longer incubation time produced the appearance of some cell lysis in the mutant cultures.
cps5 mutant cells grown under Ca2+-free conditions have no detectable cell wall galactomannan.
The results of both in vivo (sensitivity to Novozym234) and in vitro (1,3-β-d-glucan synthase activity) cell wall analyses of cps5 mutants suggest that the mutant cell wall has an altered composition. To confirm this possibility, a quantitative analysis of cell wall polysaccharides from cps5-138 and cps5Δ cells grown in the presence of 10 mM Ca2+ or in the absence of Ca2+ was carried out (Table 4). Even in the presence of a high [Ca2+], the mutant cell walls showed an almost 30% decrease in galactomannan content compared with the wild-type cell wall. Under Ca2+-free conditions, no detectable galactomannan appeared to be incorporated into the mutant cell walls. These results indicate that Pmr1p is involved in a general mechanism of wall protein galactosylation, and the process requires proper Ca2+ homeostasis brought about by Pmr1p. This is consistent with the finding that osmotic stabilization is not able to rescue the growth defect of the mutants under Ca2+-free conditions (data not shown). A significant increase in cell wall α-glucan (30%) in the mutant cells grown under Ca2+-free conditions could be explained by cell wall stress response. Indeed, it has been reported that when the level of Bgs3p, a putative 1,3-β-d-glucan synthase, is decreased using the 81X version of the repressible nmt1+ promoter, the amount of α-glucan in the cell wall increases significantly (39).
TABLE 4.
Incorporation of radioactivity from [14C]glucose into cell wall polysaccharides of S. pombe wild-type, cps5-138, and cps5Δ strains grown at 28°C in EMM in the presence or absence of calcium
| CaCl2 Concn (mM) | Strain | % Incorporation of [14C]glucose (mean ± SD)a
|
|||
|---|---|---|---|---|---|
| Cell wall | Galactomannan | α-Glucan | β-Glucan | ||
| 10 | Wild type | 34.3 ± 0.7 | 4.8 ± 0.2 (14.0) | 9.0 ± 0.4 (26.2) | 20.5 ± 0.1 (59.8) |
| cps5-138 | 34.0 ± 1.2 | 3.4 ± 0.1 (10.0) | 8.0 ± 0.5 (23.5) | 22.6 ± 0.6 (66.5) | |
| cps5Δ | 34.1 ± 0.7 | 3.4 ± 0.2 (10.0) | 8.2 ± 0.0 (24.0) | 22.5 ± 0.9 (66.0) | |
| 0b | Wild type | 34.3 ± 0.8 | 3.7 ± 0.0 (10.8) | 9.1 ± 0.3 (26.5) | 21.5 ± 0.9 (62.7) |
| cps5-138 | 37.4 ± 1.2 | <0.01 (0) | 12.8 ± 0.6 (34.2) | 24.6 ± 0.6 (65.8) | |
| cps5Δ | 40.8 ± 0.8 | <0.01 (0) | 13.7 ± 0.4 (33.6) | 27.1 ± 0.4 (66.4) | |
Percent incorporation of [14C]glucose is the counts per minute incorporated per fraction × 100/total counts per minute incorporated. The values were obtained from three independent experiments. Values in parentheses are percentages of the corresponding polysaccharide in the cell wall composition.
The cells were grown for 4 h in Ca2+-EMM plus 0.1 mM EGTA.
Pmr1p is involved in protein glycosylation.
S. cerevisiae Pmr1p has been shown to play a role in Golgi functions, including protein glycosylation and secretion (3, 19, 54). The analysis of cell wall galactomannan contents in cps5 mutants also suggests the involvement of S. pombe Pmr1p in protein glycosylation. To confirm this, acid phosphatase was analyzed by means of nondenaturing acrylamide gel electrophoresis. Since acid phosphatase is highly glycosylated, activity staining of the enzyme shows a smeared broad band in the upper part of the gel, while the enzyme moves faster when it is incompletely glycosylated (58). The acid phosphatase from the cps5 mutants exhibited increased electrophoretic mobility compared with that from the wild type (Fig. 8), indicating that the mutants synthesized an incompletely glycosylated acid phosphatase, even under optimal growth conditions. The electrophoretic patterns are similar to those from gmh3Δ and gms1Δ strains, the genes of which encode a galactosyltransferase and UDP-galactose transporter, respectively (66, 72), consistent with the finding that cps5 mutants contain no detectable galactomannan in the cell walls.
FIG. 8.
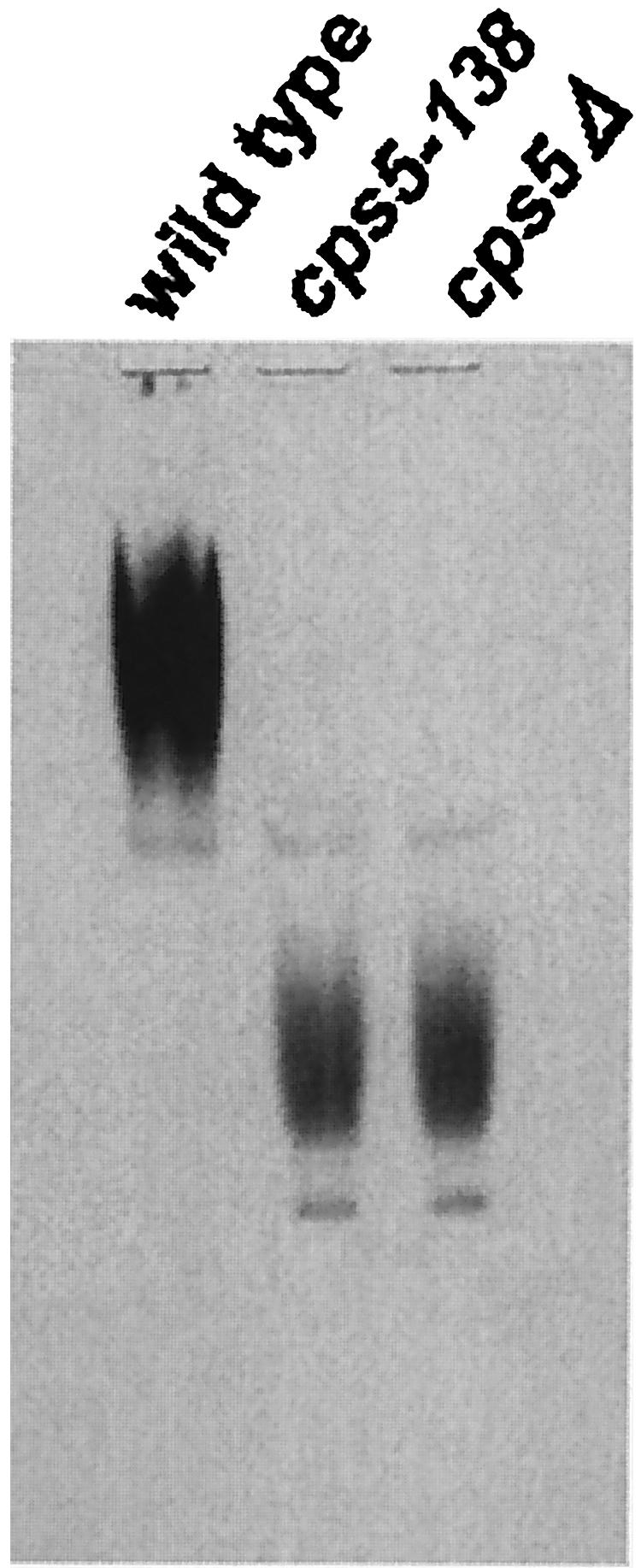
Activity staining of acid phosphatase on a nondenaturing polyacrylamide gel. Lysates from wild-type, cps5-138, and cps5Δ cells induced for acid phosphatase expression were subjected to electrophoresis on a 6% gel and stained for acid phosphatase activity.
Pmr1p shares its function with the Pmc1p homologue to maintain intracellular Ca2+ homeostasis.
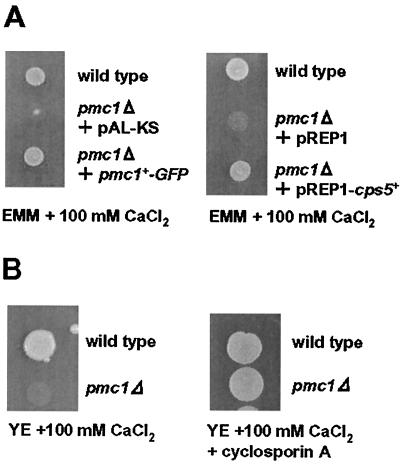
In S. cerevisiae, it was shown that Pmr1p acts together with Pmc1p to deplete cytosolic Ca2+, by which a lethal activation of calcineurin is prevented (15). To examine whether this is also true in S. pombe, the PMC1 homologue, SPAPB2B4B.04c, was disrupted, and the phenotypes were examined. The pmc1Δ strain B2B4#6 was found to grow almost normally in YE medium but was incapable of growing in the presence of high [Ca2+] in medium (>100 mM), conditions under which wild-type cells are able to grow normally (Fig. 9B). This result suggests that Pmc1p is not essential under normal growth conditions but is required for high-Ca2+ conditions. The high-Ca2+-sensitive phenotypes of pmc1Δ were significantly suppressed either in the presence of CsA, a potent inhibitor of calcineurin, in the medium or by overexpression of the cps5+ gene in pmc1Δ cells (Fig. 9A and B). These results suggest that the growth defect is due to an inappropriate activation of calcineurin, which is caused by an elevated intracellular [Ca2+], and that Pmr1p also acts to deplete cytosolic Ca2+, cooperating with the Pmc1p homologue. In S. cerevisiae, the double mutation of PMR1 and PMC1 is synthetic lethal (14). To examine whether this is also the case in S. pombe, a tetrad analysis of the heterozygous diploids (CP13-8-4D × B2B4#6) was carried out. Five sets of four meiotic progeny (PD type), 9 sets of two progeny (NPD), and 23 sets of three progeny (T) were obtained. None of the double mutants grew on YE plates. These results strongly suggest that the two Ca2+-ATPase homologues, Pmr1p and Pmc1p, in S. pombe share an essential role in depleting cytosolic Ca2+ to maintain a proper level of calcineurin activity for cell growth, even under standard culture conditions.
FIG. 9.
Complementation of the high-Ca2+-sensitive cell growth of the pmc1Δ strain. A: The cell suspension from the pmc1Δ strain transformed with pAL-KS, pREP1, pAL-KS bearing pmc1+-GFP, or pREP1 bearing cps5+ was spotted on an EMM plate containing 100 mM CaCl2. B: The pmc1Δ cell suspension was spotted on a YE plate containing 100 mM CaCl2 or 100 mM CaCl2 plus CsA (10 μg/ml). The spotted plates were incubated at 28°C for 4 to 5 days.
In S. cerevisiae, Pmc1p, which is 40% identical to the plasma membrane Ca2+-ATPase of mammalian cells, was shown to be localized to the vacuole membrane (15). To identify the intracellular localization of the Pmc1p homologue in S. pombe, GFP-tagged Pmc1p was observed by fluorescence microscopy. The Pmc1p-GFP fusion protein was capable of complementing high-Ca2+-sensitive phenotypes (Fig. 9A), suggesting that the fusion protein is functional with respect to its pump activity. As shown in Fig. 6 (bottom panel), Pmc1p-GFP was essentially localized to the vacuole membrane, which is similar to the case of budding yeast.
DISCUSSION
In the fission yeast S. pombe, 14 putative P-type ATPase genes were identified by a homology search of ORFs deduced from the S. pombe genome project (46, 71). At least three putative calcium ATPases have been assigned, namely, Cta4p (45), Pmc1p (SPAPB2B4.04c), and Pmr1p/Cps5p (SPBC31E1.02c) (37). At present, however, knowledge concerning the roles of the pump activity in cellular functions and how the activities are coordinately regulated in response to changes in intra- and extracellular [Ca2+] is very limited. The SPBC31E1.02c protein shows significant sequence similarity to the Pmr1 family of ATPases, which are present in a wide range of fungi and animal tissues (the fission yeast Pmr1p protein is most closely related to that of Yarrowia lipolytica [48], with a CLUSTALW score of 52.8). It has been well established in S. cerevisiae that Pmr1p is required for a variety of cellular functions, including N-linked and O-linked protein glycosylation, protein sorting in secretory pathways, and ER-associated protein degradation via its Ca2+ Mn2+ transporting activity, and that the pump is localized primarily in the medial Golgi (3, 19, 36, 44, 54, 60). In S. pombe, however, GFP-tagged Pmr1p appears to be localized predominantly in the ER rather than in the Golgi apparatus. It has been shown that the budding yeast Pmr1p is required for ER functions, such as Ca2+ homeostasis in the ER lumen, oligosaccharide trimming, and ER quality control, despite its Golgi localization (19, 62, 70) and that Cod1p/Spf1p, a putative P-type ATPase, is involved in processing the outer chain of carbohydrates in the Golgi, despite its localization in the ER (13, 64). We have recently found that a mutation in the cps11+ gene, which codes for the Alg3p homologue of S. cerevisiae, a Dol-P-Man-dependent mannosyltransferase residing in the ER (1, 59), causes defects of cell wall integrity and cell growth under the same restrictive conditions as in the case of cps5 mutations (unpublished result). As expected, the GFP-tagged Cps11p/Alg3p showed essentially the same localization pattern as Pmr1p. These findings support the view that the fission yeast Pmr1p resides predominantly in the ER membrane. This notion does not exclude the possibility that the Pmr1p pump activity is required for the Golgi functions, because it is quite possible to supply Ca2+ and Mn2+ ions to the Golgi apparatus despite the ER localization or by a residual pump activity from a small number of Golgi-resident Pmr1 molecules (19). In S. cerevisiae, GFP-tagged Pmr1p was reported to show a typical Golgi localization pattern, even when expressed from multicopy plasmids (38). However, it has also been observed that hemagglutinin-tagged Pmr1p is localized in the Golgi but the GFP-tagged version is localized in the ER under certain experimental conditions. Therefore, the possibility should be noted that the fission yeast Pmr1p was mislocalized or accumulated in the ER owing to GFP tagging, although the fusion protein is able to rescue the cps5 mutant phenotypes.
A phenotypic relationship between loss of Pmr1p function and an abnormal cell wall morphology was first reported in Kluyveromyces lactis (67). Klpmr1Δ cells revealed several defective phenotypes, including incomplete protein glycosylation, aberrant chitin deposition, and a thickened cell wall with an unbalanced ratio of insoluble to soluble glucans. These results suggest that the cell wall and wall-related glycoproteins, including the 1,3-β-d-glucan synthases, are affected by pmr1 mutations, resulting in changes in their enzymatic activities and/or in their subcellular distribution. Consistent with this hypothesis, we found that the in vitro 1,3-β-d-glucan synthase activity decreased by nearly 50% in cps5 mutant cells, even those grown in a standard YE medium. In the wild type, specific activity levels were higher under calcium-free conditions than in the presence of 0.1 or 10 mM calcium, and the level of α- and β-glucans increased to some extent under Ca2+-free conditions, particularly in the mutant cell walls. The reason for this is unclear, but it could be the result of a cell wall stress response, as described in Results. The 1,3-β-d-glucan synthase of the cell surface could be hyperactivated during the early stages of starvation as a survival mechanism and could be gradually inactivated during longer periods of starvation. Alternatively, the 1,3-β-d-glucan synthase activity measured in vitro may be different from the activity responsible for the synthesis of 1,3-β-d-glucan in vivo, as is the case in S. cerevisiae for differences between in vivo chitin synthesis and in vitro chitin synthase activities measured for Chs1p, Chs2p, and Chs3p (69). In S. pombe, four putative 1,3-β-d-glucan synthases have been identified, and at least three of them (Bgs1p/Cps1p, Bgs3p, and Bgs4p) are responsible for cell wall synthesis during the vegetative cell cycle (12, 18, 39; J. C. Ribas, unpublished results). We do not know yet which enzyme or enzymes are responsible for the activity detected in vitro and how they are regulated in response to changes in environmental conditions. However, it is clear that the specific activity levels of 1,3-β-d-glucan synthase were always much lower in the mutants than in the wild type under the same experimental conditions. An important finding is that there was almost no detectable galactomannan in the mutant cell walls, with an unbalanced ratio of α- and β-glucans, when the mutant cells were grown in the absence of calcium; the former could be caused by a defect in protein glycosylation, and the latter could be induced by changes in 1,3-β-d-glucan synthase activity. Cell wall weakness thus can be attributed to the dramatic changes in the composition of the mutant cell wall. It should be noted that deletion of S. cerevisiae PMR1 does not result in a detectable change in cell wall composition, in spite of the glycosylation defects (67).
Very recently, the S. pombe Pmr1p was reported to play an important role in Mn2+ homeostasis, cooperating with Pdt1p (an Nramp-related metal transporter) to regulate cell morphogenesis (37). The pmr1Δ pdt1Δ double mutants displayed more severe morphological defects than each single mutant when they were grown in EMM. Supplying Mn2+ to the medium suppressed defects of the double mutant in both cell morphology and the glycosylation of acid phosphatase to a great extent (37), consistent with the fact that Mn2+ is an important cofactor for protein glycosylation. In this study, we also confirmed that Mn2+ has a rescuing effect on the defective growth of cps5-138 and cps5Δ cells in Ca2+-depleted medium, but it was incapable of restoring their cell morphology in the absence of calcium. As already mentioned, the growth defect in the absence of calcium was not rescued even in the presence of sorbitol. These findings suggest that, in addition to protein glycosylation, which requires Mn2+ homeostasis in the secretory pathway, Pmr1p plays an important role in cell growth and cell-wall morphogenesis via control of cytosolic and/or organelle Ca2+ concentrations.
In S. cerevisiae, it has been shown that cell wall synthesis requires the coordination of a variety of cellular events, including the recruiting of cell wall materials and the glucan synthases themselves to the plasma membrane, enzyme activation by the GTP-binding protein Rho1p, and glycosylation of the glycosyl phosphatidylinositol anchor and stress sensor proteins (7, 10, 17, 33, 47). Another important component is the actin cytoskeleton (27, 52). F-actin is required for maintenance of cell wall structure and development (21, 34), as well as the polarized localization of Fks1p (16). Actin patch motility is also required for Fks1p movement, by which the structure and function of the cell wall are maintained (68). In S. pombe, the actin cytoskeleton was suggested to be involved in the secretion of cell wall materials, such as 1,6-β-glucan, α-glucan, and α-galactomannan (35). Although the molecular mechanisms for such interdependence of the actin cytoskeleton and cell wall synthesis remain unclear, the actin cytoskeleton is presumed to act as transporting machinery in the secretory pathway. Since Ca2+ is an important regulatory factor in the organization of F-actin, as well as in vesicle transport in mammalian cells, similar mechanisms could also function in fungal systems (43).
In most cases, cell wall mutants display a simultaneous defect in the formation of the septum (27). In cps5 mutants, the early steps in septum formation may not be impaired, because an F-actin ring appears to form normally. The late steps requires enzymes responsible for the synthesis of glucans, as well as septum materials, to be properly transferred to the site where the septum is formed. It is easy to imagine that the unbalanced synthesis of polysaccharides observed in the mutant cell walls and/or the incomplete or missing glycosylation of wall proteins also impairs septum synthesis, resulting in a defect in cytokinesis followed by cell separation. Although an excessive deposition of septum materials was observed, the defective mechanism remains unclear. It may be worth noting that septated cells that accumulated in Ca2+-free medium for 3 h were reduced in number by almost one-third when shifted back to permissive conditions within 3 h. This implies that ill-formed septa are reactivated to divide under permissive conditions when the cells are exposed for a short period to restrictive conditions.
In S. cerevisiae, it has been shown that Ca2+ signals generated by mating pheromones or a high-Ca2+ environment induce the expression of certain genes, including PMR1 and PMC1, which encode the Golgi and vacuolar Ca2+ ATPases, respectively, and FKS2, which encodes a putative 1,3-β-d-glucan synthase catalytic subunit, via a calcineurin-dependent transcription factor, Tcn1p/Crz1p (40, 42, 61, 74). It was also reported that in S. pombe the expression of pmr1+ and pmc1+ is controlled through a calcineurin-dependent transcription factor, Prz1p (22, 37). In budding yeast, Pmr1p and Pmc1p cooperatively regulate cytosolic [Ca2+] to a level appropriate for the activation of calcineurin, implying the existence of a feedback mechanism for the calcineurin pathway via modulation of cytosolic [Ca2+] (15). In this study, we also observed the same functional interdependence between S. pombe Pmr1p and Pmc1p in preventing lethal activation of calcineurin. The reason why pmr1Δ causes cells to be low-calcium sensitive and pmc1Δ causes cells to be high-calcium sensitive is that Pmr1p is essential for the supply of Ca2+ and Mn2+ to the organelles but is not necessarily required for depleting excess cytosolic [Ca2+], because Pmc1p is fully active in the latter process, although simultaneous loss of Pmr1p and Pmc1p functions causes fatal damage to the cell. We have observed that pmc1Δ cells become somewhat resistant to the cell wall-digesting reagent Novozym234 (unpublished results), suggesting that cytosolic [Ca2+] may also affect cell wall integrity. Indeed, Ehs1p, a homologue of the Ca2+ channel component Mid1p of S. cerevisiae, is reported to be involved in the cell wall integrity pathway, functioning with Pck2p, a protein kinase C homologue (9). To determine whether Pmr1p pump activity is involved in the Pck2 pathway, pck2+ was overexpressed in the cps5 mutants. As a result, neither the pck2+ overexpression-related phenotype nor the cps5 mutant phenotypes were suppressed, consistent with the finding that cps5+ failed to rescue the ehs1-1 mutant phenotypes and vice versa (unpublished result). These results suggest that Pmr1p is not involved in the Ehs1p-Pck2p pathway.
cps1-12 mutants display hypersensitivity to CsA, a potent inhibitor of calcineurin, as well as to Ca2+-free EMM, and an increase of exogenous [Ca2+] (to 10 mM) is able to partially suppress the thermosensitive phenotype (reference 29 and unpublished results). CsA appears to aggravate cps5-138 mutant phenotypes, and the cps5-138 cps1-12 double mutant is more hypersensitive to Novozym234 than either of the single mutants. From these findings, it is tempting to speculate that the gene expression of a 1,3-β-d-glucan synthase homologue(s) other than Bgs1p/Cps1p could be controlled by a calcineurin-dependent mechanism and that Pmr1p is involved in the regulation of this pathway via the control of cytosolic and/or organelle [Ca2+].
Acknowledgments
We thank Chikashi Shimoda, Taro Nakamura, Issei Mabuchi, Takashi Toda, and Pilar Pérez for the genomic library and plasmids and Yolanda Sánchez for the ehs1-1 strain.
J.C.G.C. acknowledges support from a fellowship granted by Consejo Superior de Investigaciones Científicas (CSIC) (Spain). Part of this work was supported by grant BIO2000-1448 from the Comisión Interministerial de Ciencia y Tecnología (Spain).
REFERENCES
- 1.Aebi, M., J. Gassenhuber, H. Domdey, and S. te Heesen. 1996. Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae. Glycobiology 6:439-444. [DOI] [PubMed] [Google Scholar]
- 2.Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 3.Antebi, A., and G. R. Fink. 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localized in a novel Golgi-like distribution. Mol. Biol. Cell 3:633-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arellano, M., M. H. Valdivieso, T. M. Calonge, P. M. Coll, A. Duran, and P. Perez. 1999. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 112:3569-3578. [DOI] [PubMed] [Google Scholar]
- 5.Berridge, M. J. 1997. Elementary and global aspects of calcium signalling. J. Physiol. 499:291-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazer, S. C., H. P. Williams, T. G. Chappell, and W. Z. Cande. 2000. A fission yeast kinesin affects Golgi membrane recycling. Yeast 16:149-166. [DOI] [PubMed] [Google Scholar]
- 7.Cabib, E., D.-H. Roh, M. Schmidt, L. B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679-19682. [DOI] [PubMed] [Google Scholar]
- 8.Calonge, T. M., K. Nakano, M. Arellano, R. Arai, S. Katayama, T. Toda, I. Mabuchi, and P. Perez. 2000. Schizosaccharomyces pombe Rho2p GTPase regulates cell wall α-glucan biosynthesis through the protein kinase Pck2p. Mol. Biol. Cell 11:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnero, E., J. C. Ribas, B. García, A. Durán, and Y. Sánchez. 2000. Schizosaccharomyces pombe ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet. 264:173-183. [DOI] [PubMed] [Google Scholar]
- 10.Cid, V. J., A. Durán, F. del Rey, M. P. Snyder, C. Nombela, and M. Sánchez. 1995. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59:345-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapham, D. E. 1995. Calcium signaling. Cell 80:259-268. [DOI] [PubMed] [Google Scholar]
- 12.Cortés, J. C. G., J. Ishiguro, A. Durán, and J. C. Ribas. 2002. Localization of the (1,3)β-d-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115:4081-4096. [DOI] [PubMed] [Google Scholar]
- 13.Cronin, S. R., R. Rao, and R. Y. Hampton. 2002. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J. Cell Biol. 157:1017-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham, K. W., and G. R. Fink. 1994. Ca2+ transport in Saccharomyces cerevisiae. J. Exp. Biol. 196:157-166. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham, K. W., and G. R. Fink. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delley, P.-A., and M. N. Hall. 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas, C. M. 2001. Fungal β(1,3)-d-glucan synthesis. Med. Mycol. 39:55-66. [DOI] [PubMed] [Google Scholar]
- 18.Durán, A. and P. Pérez. 2004. Cell wall synthesis, p. 269-279. In R. Egel (ed.), Molecular biology of Schizosaccharomyces pombe. Genetics, genomics and beyond. Springer-Verlag, Berlin, Germany.
- 19.Dürr, G., J. Strayle, R. Plemper, S. Elbs, S. K. Klee, P. Catty, D. H. Wolf, and H. K. Rudolph. 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9:1149-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egel, R. 1984. Two tightly linked silent cassettes in the mating-type region of Schizosaccharomyces pombe. Curr. Genet. 8:199-203. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel, M., and M. Kopecká. 1995. Disruption of the actin cytoskeleton in budding yeast results in formation of an aberrant cell wall. Microbiology 141:891-899. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama, S., R. Sugiura, Y. Lu, T. Maeda, K. Kawagishi, M. Yokoyama, H. Tohda, Y. Giga-Hama, H. Shuntoh, and T. Kuno. 2003. Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. J. Biol. Chem. 278:18078-18084. [DOI] [PubMed] [Google Scholar]
- 23.Hochstenbach, F., F. M. Klis, H. van den Ende, E. van Donselaar, P. J. Peters, and R. D. Klausner. 1998. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA 95:9161-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, K. M., and M. D. Snider. 1995. Isolation of protein glycosylation mutants in the fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 6:485-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iida, H., H. Nakamura, T. Ono, M. S. Okumura, and Y. Anraku. 1994. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14:8259-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida, H., Y. Yagawa, and Y. Anraku. 1990. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J. Biol. Chem. 265:13391-13399. [PubMed] [Google Scholar]
- 27.Ishiguro, J. 1998. Genetic control of fission yeast cell wall synthesis: the genes involved in wall biogenesis and their interactions in Schizosaccharomyces pombe. Genes Genet. Syst. 73:181-191. [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro, J., and W. Kobayashi. 1996. An actin point-mutation neighboring the ′hydrophobic plug' causes defects in the maintenance of cell polarity and septum organization in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 392:237-241. [DOI] [PubMed] [Google Scholar]
- 29.Ishiguro, J., A. Saitou, A. Durán, and J. C. Ribas. 1997. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J. Bacteriol. 179:7653-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro, J., and Y. Uhara. 1992. Isolation and characterization of mutants supersensitive to the spindle poison, isopropyl N-3-chlorophenyl carbamate (CIPC) in the fission yeast Schizosaccharomyces pombe. Jpn. J. Genet. 67:97-109. [DOI] [PubMed] [Google Scholar]
- 31.Ishijima, S. A., M. Konomi, T. Takagi, M. Sato, J. Ishiguro, and M. Osumi. 1999. Ultrastructure of cell wall of the cps8 actin mutant cell in Schizosaccharomyces pombe. FEMS Microbiol. Lett. 180:31-37. [DOI] [PubMed] [Google Scholar]
- 32.Katayama, S., D. Hirata, M. Arellano, P. Pérez, and T. Toda. 1999. Fission yeast α-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J. Cell Biol. 144:1173-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klis, F. M. 1994. Cell wall assembly in yeast. Yeast 10:851-869. [DOI] [PubMed] [Google Scholar]
- 34.Kobori, H., N. Yamada, A. Taki, and M. Osumi. 1989. Actin is associated with the formation of the cell wall in reverting protoplasts of the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 94:635-646. [DOI] [PubMed] [Google Scholar]
- 35.Konomi, M., J. Ishiguro, and M. Osumi. 2000. Abnormal formation of the glucan network from regenerating protoplasts in Schizosaccharomyces pombe cps8 actin point mutant. J. Electron Microsc. 49:569-578. [DOI] [PubMed] [Google Scholar]
- 36.Lapinskas, P. J., K. W. Cunningham, X. F. Liu, G. R. Fink, and V. C. Culotta. 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15:1382-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda, T., R. Sugiura, A. Kita, M. Saito, L. Deng, Y. He, Y. Lu, Y. Fujita, K. Takegawa, H. Shuntoh, and T. Kuno. 2004. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: the importance of Mn2+ homeostasis. Genes Cells 9:71-82. [DOI] [PubMed] [Google Scholar]
- 38.Mandal, D., S. J. Rulli, and R. Rao. 2003. Packing interactions between transmembrane helices alter ion selectivity of the yeast golgi Ca2+/Mn2+-ATPase PMR1. J. Biol. Chem. 278:35292-35298. [DOI] [PubMed] [Google Scholar]
- 39.Martín, V., B. García, E. Carnero, A. Durán, and Y. Sánchez. 2003. Bgs3p, a putative 1,3-β-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot. Cell 2:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127-130. [DOI] [PubMed] [Google Scholar]
- 42.Mazur, P., N. Morin, W. Baginsky, M. El-Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondésert, G., D. J. Clarke, and S. I. Reed. 1997. Identification of genes controlling growth polarity in the budding yeast Saccharomyces cerevisiae: a possible role of N-glycosylation and involvement of the exocyst complex. Genetics 147:421-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okorokov, L. A., and L. Lehle. 1998. Ca2+-ATPases of Saccharomyces cerevisiae: diversity and possible role in protein sorting. FEMS Microbiol. Lett. 162:83-91. [DOI] [PubMed] [Google Scholar]
- 45.Okorokova Façanha, A. L., H. Appelgren, M. Tabish, L. Okorokov, and K. Ekwall. 2002. The endoplasmic reticulum cation P-type ATPase Cta4p is required for control of cell shape and microtubule dynamics. J. Cell Biol. 157:1029-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okorokova-Façanha, A. L., L. A. Okorokov, and K. Ekwall. 2003. An inventory of the P-type ATPases in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 43:273-280. [DOI] [PubMed] [Google Scholar]
- 47.Orlean, P. 1977. Biogenesis of yeast wall and surface components, p. 229-362. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Park, C. S., J. Y. Kim, C. Crispino, C. C. Chang, and D. D. Ryu. 1998. Molecular cloning of YlPMR1, a S. cerevisiae PMR1 homologue encoding a novel P-type secretory pathway Ca2+-ATPase, in the yeast Yarrowia lipolytica. Gene 206:107-116. [DOI] [PubMed] [Google Scholar]
- 49.Pidoux, A. L., and J. Armstrong. 1992. Analysis of the BiP gene and identification of an ER retention signal in Schizosaccharomyces pombe. EMBO J. 11:1583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pidoux, A. L., and J. Armstrong. 1993. The BiP protein and the endoplasmic reticulum of Schizosaccharomyces pombe: fate of the nuclear envelope during cell division. J. Cell Sci. 105:1115-1120. [DOI] [PubMed] [Google Scholar]
- 51.Popolo, L., T. Gualtieri, and E. Ragni. 2001. The yeast cell-wall salvage pathway. Med. Mycol. 39:111-121. [PubMed] [Google Scholar]
- 52.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. II. The role of the cortical actin cytoskeleton. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 53.Rothstein, R. J. 1983. One-step gene disruption in yeast. Methods Enzymol. 101:202-211. [DOI] [PubMed] [Google Scholar]
- 54.Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman, J. LeVitre, L. S. Davidow, J. Mao, and D. T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133-145. [DOI] [PubMed] [Google Scholar]
- 55.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 80:1483-1521. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Sazer, S., and S. W. Sherwood. 1990. Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J. Cell Sci. 97:509-516. [DOI] [PubMed] [Google Scholar]
- 58.Schweingruber, A.-M., F. Schoenholzer, L. Keller, R. Schwaninger, H. Trachsel, and M. E. Schweingruber. 1986. Glycosylation and secretion of acid phosphatase in Schizosaccharomyces pombe. Eur. J. Biochem. 158:133-140. [DOI] [PubMed] [Google Scholar]
- 59.Sharma, C. B., R. Knauer, and L. Lehle. 2001. Biosynthesis of lipid-linked oligosaccharides in yeast: the ALG3 gene encodes the Dol-P-Man:Man5GlcNAc2-PP-Dol mannosyltransferase. Biol. Chem. 382:321-328. [DOI] [PubMed] [Google Scholar]
- 60.Sorin, A., G. Rosas, and R. Rao. 1997. PMR1, a Ca2+-ATPase in yeast golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 272:9895-9901. [DOI] [PubMed] [Google Scholar]
- 61.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strayle, J., T. Pozzan, and H. K. Rudolph. 1999. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 18:4733-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugiura, R., S. O. Sio, H. Shuntoh, and T. Kuno. 2002. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7:619-627. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki, C., and Y. Shimma. 1999. P-type ATPase spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 32:813-823. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka, K., T. Yonekawa, Y. Kawasaki, M. Kai, K. Furuya, M. Iwasaki, H. Murakami, M. Yanagida, and H. Okayama. 2000. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol. Cell. Biol. 20:3459-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka, N., M. Konomi, M. Osumi, and K. Takegawa. 2001. Characterization of a Schizosaccharomyces pombe mutant deficient in UDP-galactose transport activity. Yeast 18:903-914. [DOI] [PubMed] [Google Scholar]
- 67.Uccelletti, D., P. Mancini, F. Farina, S. Morrone, and C. Palleschi. 1999. Inactivation of the KlPMR1 gene of Kluyveromyces lactis results in defective cell-wall morphogenesis. Microbiology 145:1079-1087. [DOI] [PubMed] [Google Scholar]
- 68.Utsugi, T., M. Minemura, A. Hirata, M. Abe, D. Watanabe, and Y. Ohya. 2002. Movement of yeast 1,3-β-glucan synthase is essential for uniform cell wall synthesis. Genes Cells 7:1-9. [DOI] [PubMed] [Google Scholar]
- 69.Valdivieso, M. H., A. Durán, and C. Roncero. 1999. Chitin synthases in yeast and fungi, p. 55-69. In P. Jollès and R.A.A. Muzzarelli (ed.), Chitin and chitinases. Birkhäuser Verlag, Basel, Switzerland. [DOI] [PubMed]
- 70.Vashist, S., C. G. Frank, C. A. Jakob, and D. T. W. Ng. 2002. Two distinctly localized P-type ATPases collaborate to maintain organelle homeostasis required for glycoprotein processing and quality control. Mol. Biol. Cell 13:3955-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, R. Lyne, A. Stewart, et al. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]
- 72.Yoko-o, T., S. K. Roy, and Y. Jigami. 1998. Differences in in vivo acceptor specificity of two galactosyltransferases, the gmh3+ and gma12+ gene products from Schizosaccharomyces pombe. Eur. J. Biochem. 257:630-637. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida, T., T. Toda, and M. Yanagida. 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 107:1725-1735. [DOI] [PubMed] [Google Scholar]
- 74.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]