We show that electroconvulsive therapy (ECT)-like stimulation greatly enhances synaptic potentiation induced by dopamine at the excitatory synapse formed by the hippocampal mossy fiber in mice. The effect of ECT-like stimulation on the dopaminergic modulation was rapidly induced, maintained for more than 4 wk after repeated treatments, and most likely mediated by increased expression of the dopamine D1 receptor. These effects may be relevant to fast-acting strong antidepressant action of ECT.
Keywords: electroconvulsive therapy, dopamine, mossy fiber, hippocampus, antidepressant
Abstract
Electroconvulsive therapy (ECT) is an established effective treatment for medication-resistant depression with the rapid onset of action. However, its cellular mechanism of action has not been revealed. We have previously shown that chronic antidepressant drug treatments enhance dopamine D1-like receptor-dependent synaptic potentiation at the hippocampal mossy fiber (MF)-CA3 excitatory synapse. In this study we show that ECT-like treatments in mice also have marked effects on the dopaminergic synaptic modulation. Repeated electroconvulsive stimulation (ECS), an animal model of ECT, strongly enhanced the dopamine-induced synaptic potentiation at the MF synapse in hippocampal slices. Significant enhancement was detectable after the second ECS, and further repetition of ECS up to 11 times monotonously increased the magnitude of enhancement. After repeated ECS, the dopamine-induced synaptic potentiation remained enhanced for more than 4 wk. These synaptic effects of ECS were accompanied by increased expression of the dopamine D1 receptor gene. Our results demonstrate that robust neuronal activation by ECS induces rapid and long-lasting enhancement of dopamine-induced synaptic potentiation at the MF synapse, likely via increased expression of the D1 receptor, at least in part. This rapid enhancement of dopamine-induced potentiation at the excitatory synapse may be relevant to the fast-acting antidepressant effect of ECT.
NEW & NOTEWORTHY We show that electroconvulsive therapy (ECT)-like stimulation greatly enhances synaptic potentiation induced by dopamine at the excitatory synapse formed by the hippocampal mossy fiber in mice. The effect of ECT-like stimulation on the dopaminergic modulation was rapidly induced, maintained for more than 4 wk after repeated treatments, and most likely mediated by increased expression of the dopamine D1 receptor. These effects may be relevant to fast-acting strong antidepressant action of ECT.
electroconvulsive therapy (ECT) has been established as an effective treatment for medication-resistant depression. The therapeutic efficacy of ECT for depression is characterized by the fast onset of action and high response rates (Husain et al. 2004). Although ECT has been widely used to treat psychiatric disorders for more than 70 years, the cellular mechanism of action remains to be elucidated. Since most of currently available antidepressant drugs act on the central monoaminergic neurotransmission, it is conceivable that ECT can also modify functioning of the monoaminergic system. Among central monoaminergic systems, the serotonergic and noradrenergic systems have been the major targets of pharmacological treatments for depression. Given possible involvement of dopaminergic dysfunction in medication-resistant depression (Dunlop and Nemeroff 2007), the effect of ECT on the dopaminergic system would be worth investigating. Indeed, alterations of the dopaminergic system have been implicated in the mechanism of action of ECT (Baldinger et al. 2014). In experimental animals, repeated electroconvulsive stimulation (ECS) increases activity of dopaminergic neurons in the ventral tegmental area (West and Weiss 2011; but see Tsen et al. 2013) and increases the expression of dopamine receptors in the striatum and nucleus accumbens (Lammers et al. 2000; Smith et al. 1995; Strome et al. 2007). However, the change in the dopamine receptor expression is relatively small. In addition, it has not been characterized how neuromodulatory effects of dopamine are changed after ECS.
The hippocampal mossy fiber (MF)-CA3 excitatory synapse has been implicated in the mechanism of action of antidepressant drugs (Kobayashi 2009; Kobayashi et al. 2008, 2010; Wang et al. 2015). Morphological studies have suggested that the hippocampal MF system could be a potential target of ECT (Gombos et al. 1999; Lamont et al. 2001, 2005; Vaidya et al. 1999). We have previously shown that dopamine selectively potentiates hippocampal MF-CA3 synaptic transmission in the hippocampal CA3 neuronal circuit via activation of D1-like receptors (Kobayashi and Suzuki 2007) and that this dopamine-induced potentiation is enhanced by chronic treatments with serotonergic antidepressant drugs in adult mice (Kobayashi et al. 2012, 2013). Therefore, we hypothesized that ECT/ECS may also have a profound effect on the dopaminergic modulation at the MF synapse. In the present study, we tested this hypothesis using hippocampal slices from ECS-treated mice.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice at the age of 7–8 wk (Japan SLC or Charles River Japan) were singly housed in the institutional standard condition (14:10-h light-dark cycle; lights on at 6:00 AM through 8:00 PM) with ad libitum access to food and water. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Animal Care and Use Committee of Nippon Medical School, the Committee of Animal Research of Kyoto University, Faculty of Pharmaceutical Sciences, and the Animal Care and Use Committee of Tokyo University of Science.
Electroconvulsive stimulation.
Bilateral ECS (current, 25 mA; pulse width, 0.5 ms; frequency, 100 Hz; shock duration, 1 s) was administered with a pulse generator (ECT Unit; Ugo Basile) via ear-clip electrodes. To avoid sudden unexpected death associated with ECS-induced immediate seizures, mice were anesthetized with isoflurane (1.5–2%). In repeated treatments, ECS was started at the age of 9 wk and administered 4 times a week for up to 3 wk. Mice did not show spontaneous seizures in their home cages during the course of treatments, and population spikes recorded in hippocampal slices looked normal. The sham-treated animals were handled in an identical manner to the ECS-treated animals without shock administration.
Real-time PCR.
RNA extraction and real-time PCR were performed as described previously (Imoto et al. 2015). Primer sequences are 5′-acagcagcccctccgatag-3′ and 5′-gttagacctgggcagatgaag-3′ for Drd1, 5′-acctgtcctggtacgatgatg-3′ and 5′-gcatggcatagtagttgtagtgg-3′ for Drd2, 5′-ctcggcaacgtcctagtgtg-3′ and 5′-aatgccacgaagaggtctgag-3′ for Drd5, and 5′-gaggccctgtaattggaatgag-3′ and 5′-gcagcaactttaatatacgctattgg-3′ for 18S rRNA.
Electrophysiology.
Mice were decapitated under deep halothane anesthesia 24 h after the last ECS unless otherwise stated. Field excitatory postsynaptic potentials (EPSPs) arising from the MF synapses or associational/commissural synapses were recorded in the CA3 region of transverse hippocampal slices (380 μm) and analyzed as described previously (Kobayashi and Suzuki 2007). Single electrical stimulation was delivered at a frequency of 0.05 Hz unless otherwise stated. SCH23390 was purchased from Tocris Bioscience (Bristol, UK). Dopamine was obtained from Wako Pure Chemical Industries (Osaka, Japan).
Drug treatments.
Diazepam (Wako) was dissolved in dimethyl sulfoxide at 10 or 20 mg/ml and diluted in the drinking water. Cycloheximide (Wako) and 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP; Sigma-Aldrich) were dissolved in saline and intraperitoneally administered.
Statistics.
All data are mean ± SE. Experiments with two groups were compared with unpaired two-tailed Student's t-test unless otherwise specified, and experiments with more than two groups were subjected to one-way ANOVA, followed by Dunnett's test or Tukey's test. Statistical interaction was assessed with two-way ANOVA, followed by Bonferroni's test. Statistical significance was set at P < 0.05. The number of data n represents the number of mice used unless otherwise specified.
RESULTS
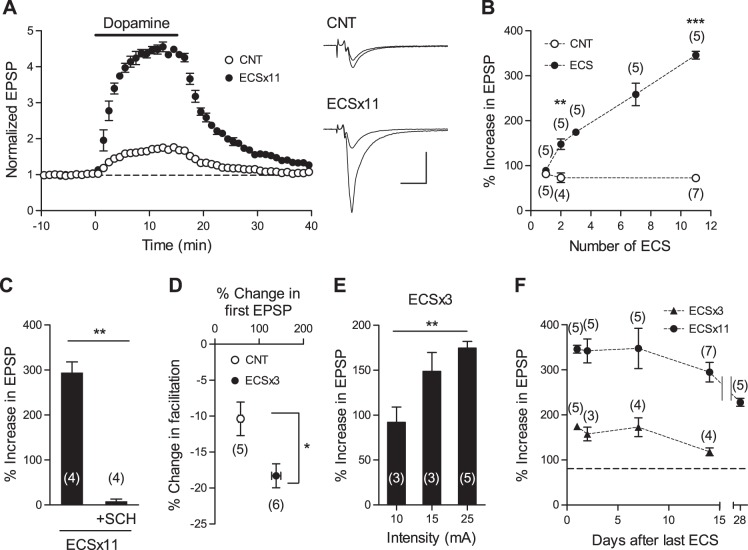
We first examined the effect of repeated ECS on the dopamine-induced synaptic potentiation at the MF-CA3 synapse. Mice were subjected to 11 times of ECS (ECS×11) or sham treatments, and the electrophysiological experiments were performed 24 h after the last treatment. In ECS-treated mice, synaptic potentiation induced by dopamine was dramatically enhanced (to 172.6 ± 4.2% of baseline in control, to 445.9 ± 8.7% of baseline in ECS; Fig. 1A). Whereas single ECS failed to induce a detectable effect, significant enhancement was observed after the second ECS (Fig. 1B). Further repetition of ECS up to 11 times monotonously increased the magnitude of the dopamine-induced potentiation. The dopamine-induced potentiation after ECS×11 was nearly completely suppressed by the D1-like receptor antagonist SCH23390 (Fig. 1C). There were no significant changes in basal EPSP-to-fiber volley ratios after ECS×3 (control: 1.45 ± 0.17, n = 5; ECS: 1.51 ± 0.14, n = 6) or input-output relationship after ECS×11 (Imoto Y, Segi-Nishida E, Suzuki H, and Kobayashi K, unpublished observation). Dopamine presynaptically potentiates MF synaptic transmission, as evidenced by a reduction of synaptic facilitation induced by repetitive stimulation (Kobayashi and Suzuki 2007). This reduction of facilitation was also enhanced after ECS×3 (Fig. 1D). The magnitude of the enhancement of the dopaminergic modulation increased with the intensity of electrical currents in mice treated with ECS×3 (Fig. 1E), suggesting a graded effect of electrical stimulation. Although the effects of ECS gradually decayed with time, the synaptic potentiation remained enhanced at 4 wk after ECS×11 (Fig. 1F). Dopamine had no significant effects on the associational/commissural-CA3 synapse after ECS×3 (103.4 ± 2.1% of baseline, n = 4 slices). These results indicate that ECS causes the rapid and long-lasting enhancement of the dopaminergic modulation at the MF synapse.
Fig. 1.
Enhancement of dopaminergic modulation at the MF synapse by ECS. A: repeated ECS enhances synaptic potentiation induced by dopamine (10 μM, 15 min), applied in the bath at the horizontal bar. Sample recordings show averages of 15 consecutive EPSPs before and during dopamine application in control (CNT) and ECS-treated mice. Scale bar: 10 ms, 0.5 mV. B: dependence of enhancement of the dopaminergic modulation on the number of ECS (**P = 0.0025; ***P < 0.0001). C: in slices pretreated with SCH23390 (50 nM), the effect of dopamine after 11 repetitions of ECS (ECS×11) was suppressed (**P = 0.0013). D: in parallel with enhancement of synaptic potentiation, ECS×3 augmented dopamine-induced reduction of triple-pulse facilitation at 200-ms intervals (P = 0.02). E: effects of ECS×3 delivered at different current intensities [Tukey's test: **P < 0.01; 1-way ANOVA: F(2,8) = 9.238, P = 0.0083]. F: effects of ECS×3 and ECS×11 assessed at different time intervals after the last ECS. The number of data (n) is shown on graphs in Figs. 1–4 and represents the number of slices in C and D of Fig. 1.
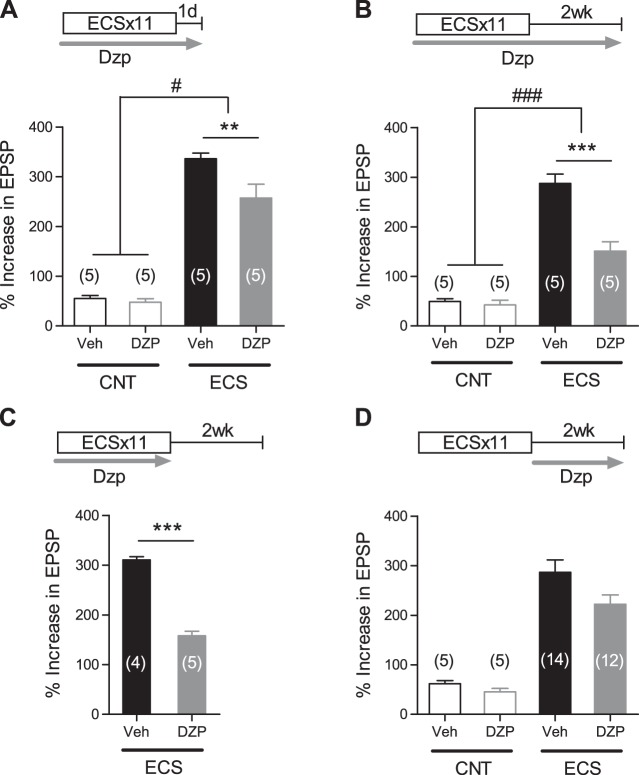
We next examined the role of neuronal activity in the maintenance of the enhanced dopaminergic modulation by using diazepam, a positive allosteric modulator of GABAA receptors. Chronic oral treatments with diazepam had no significant effects on the dopamine-induced potentiation in the control mice (Fig. 2). Diazepam applied during and after the period of ECS×11 significantly reduced the effects of ECS on the dopaminergic modulation assessed 1 day or 2 wk after the last ECS (Fig. 2, A and B), confirming the effectiveness of this oral treatment. Diazepam applied only during the period of ECS×11 similarly reduced the magnitude of the enhancement at 2 wk after ECS (Fig. 2C). On the other hand, diazepam applied only after ECS×11 did not significantly affect the magnitude of the enhancement at 2 wk after ECS (Fig. 2D). These results suggest that the lasting enhancement of the dopaminergic modulation is largely determined by neuronal activation during the period of ECS treatments and is not supported by neuronal activity during the maintenance phase.
Fig. 2.
Effects of augmented GABAergic inhibition on ECS-induced enhancement of dopaminergic modulation. A and B: diazepam (Dzp; 5 mg/kg per day) attenuates effects of ECS on the dopamine-induced synaptic potentiation assessed 24 h (A) and 2 wk (B) after the last ECS [Bonferroni's test: **P < 0.01, ***P < 0.001; 2-way ANOVA: interaction (drug treatment × ECS), F(1,16) = 5.16, #P = 0.0373 for 24 h and F(1,16) = 19.62, ###P = 0.0004 for 2 wk]. Veh, vehicle. C: effects of Dzp (5 mg/kg per day) applied during the period of ECS on lasting enhancement of dopaminergic modulation (***P < 0.0001). D: effects of Dzp (10 mg/kg per day) applied after ECS on lasting enhancement of dopaminergic modulation [interaction (drug treatment × ECS), F(1,32) = 0.8402, P = 0.3662].
Next, we examined mechanisms underlying the enhancement of the dopaminergic modulation by ECS. ECS was delivered twice at an interval of 48 h, and CPP, an antagonist of glutamate NMDA receptors, was injected 30 min before each ECS. In mice treated with CPP, ECS caused a significant enhancement of the dopaminergic modulation, and there was no significant difference in the effectiveness of ECS compared with the saline-treated mice (Fig. 3A). We then examined the effect of the protein synthesis inhibitor cycloheximide. Cycloheximide applied in the same way as CPP significantly attenuated the effect of ECS on the dopaminergic modulation (Fig. 3B). These results suggest that the protein synthesis, but not activation of NMDA receptors, is essential for the rapid enhancement of the dopaminergic modulation.
Fig. 3.
Protein synthesis-dependent enhancement of dopaminergic modulation by ECS. A: no significant effects of CPP (40 mg/kg ip) on rapid enhancement of dopaminergic modulation by ECS [Bonferroni's test: **P < 0.01, ***P < 0.001; 2-way ANOVA: interaction (drug treatment × ECS), F(1,20) = 0.8776, P = 0.36]. The number of data (n) represents the number of slices. B: effects of cycloheximide (Chx; 100 mg/kg ip) on rapid enhancement of dopaminergic modulation by ECS [Bonferroni's test: ***P < 0.001; 2-way ANOVA: interaction (drug treatment × ECS), F(1,16) = 8,831, ##P = 0.009].
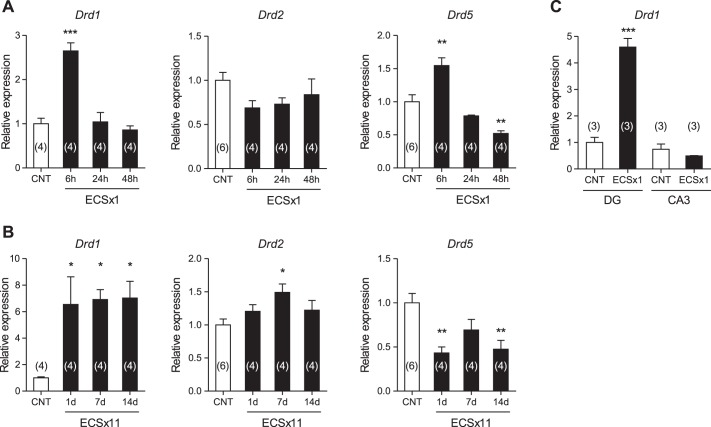
The dopamine-induced potentiation at the MF synapse is mediated by D1-like receptors, comprising D1 and D5 receptors, most likely expressed in the granule cells of the dentate gyrus (DG), which project their MF axons to CA3 (Kobayashi and Suzuki 2007). Hippocampal neurons including the granule cells express dopamine D1 and D5 receptors (Fremeau et al. 1991; Gangarossa et al. 2012; Mansour et al. 1991; Rocchetti et al. 2015; Sariñana et al. 2014). We next examined the effect of ECS on the expression of the dopamine receptor genes in DG. Single ECS transiently upregulated the expression of the dopamine D1 (Drd1) and D5 (Drd5) receptor genes, but not D2 (Drd2) gene (Fig. 4A). Repeated ECS caused a robust increase in the Drd1 expression level that lasted more than 2 wk after ECS (Fig. 4B), which is consistent with the lasting enhancement of the dopaminergic synaptic modulation after repeated ECS. Repeated ECS caused only a small increase in Drd2 expression and significantly reduced Drd5 expression (Fig. 4B). Single ECS had no significant effect on Drd1 expression in CA3 (Fig. 4C). These results suggest that the enhanced dopaminergic modulation in ECS-treated mice is expressed at least in part by upregulation of the D1 receptor expression in DG.
Fig. 4.
Upregulation of dopamine D1 receptor gene expression by ECS. A: single ECS caused transient increase in Drd1 and Drd5 expression in DG [Dunnett's test: **P < 0.001, ***P < 0.001 compared with CNT; 1-way ANOVA: F(3,12) = 27.77, P < 0.0001 for Drd1 and F(3,14) = 20.36, P < 0.0001 for Drd5]. Gene expression levels were assessed at different time intervals as indicated. B: a lasting increase in Drd1 expression after repeated ECS in DG [Dunnett's test: *P < 0.05, **P < 0.001; 1-way ANOVA: F(3,12) = 5.247, P = 0.0152 for Drd1, F(3,14) = 3.331, P = 0.0505 for Drd2, and F(3,14) = 7.056, P = 0.004 for Drd5]. C: single ECS increased Drd1 expression in DG, but not in CA3 (***P = 0.0007).
DISCUSSION
ECS has been shown to induce MF sprouting (Gombos et al. 1999; Lamont et al. 2001, 2005; Vaidya et al. 1999) and increase the immunoreactivity for neuropeptide Y in MFs (Ma et al. 2002). However, it has not been demonstrated how ECS modulates physiological properties of MF synaptic transmission. The present study has demonstrated that ECS causes rapid and lasting enhancement of the dopamine D1-like receptor-dependent synaptic potentiation at the hippocampal MF-CA3 excitatory synapse. The therapeutic effects of ECT for depression emerge after a few sessions of treatments (Husain et al. 2004). The dopaminergic modulation at the MF synapse was increased about twofold after the second ECS. Such a rapid and robust change in the synaptic modulation may be relevant to the fast-acting strong antidepressant action of ECT. We have previously shown that chronic antidepressant drug treatments also enhance the dopamine D1-like receptor-dependent potentiation at the MF synapse (Kobayashi et al. 2012). Our results suggest that the enhancement of the dopaminergic modulation at the MF synapse can be a candidate cellular mechanism shared by pharmacological and physical antidepressant treatments.
The effect of ECS on the dopaminergic modulation at the MF synapse required protein synthesis and was independent of NMDA receptor activation. The NMDA receptor-independent effect of ECS may depend on regulation of the gene expression by other glutamate receptors such as metabotropic glutamate receptors (Wang et al. 2007) or by activation of voltage-dependent Ca2+ channels (Greer and Greenberg, 2008). It is also possible that neurotransmitters other than glutamate are involved. Indeed, ECS can cause a large increase in the extracellular concentration of dopamine (Zis et al. 1991). The enhanced dopaminergic modulation is likely expressed by upregulation of the D1 receptor expression in DG, at least in part. Since D1 receptor is expressed in GABAergic interneurons as well as in glutamatergic neurons (Gangarossa et al. 2012; Karunakaran et al. 2016; Rosen et al. 2015), GABAergic interneurons may be involved in signaling pathways mediating the effect of dopamine and/or its enhancement by ECS. After repeated ECS, the D1 gene expression level in DG was increased about sixfold (Fig. 4), which is in contrast to a relatively small effect of ECS on the dopamine receptor gene expression or ligand binding demonstrated in the striatum, nucleus accumbens, and cortical regions (Lammers et al. 2000; Smith et al. 1995; Strome et al. 2007). We have previously shown that chronic antidepressant treatments increases D1-like receptor ligand binding about twofold in a manner specific to DG and the MF terminal region (Kobayashi et al. 2012). Therefore, the DG appears to have a higher sensitivity to neuronal stimuli than other brain regions with respect to the stimulus-induced upregulation of the D1 receptor expression. The D1-like receptors have been shown to regulate long-term potentiation in DG (Kusuki et al. 1997; Sariñana et al. 2014). Since the dopaminergic innervation to DG and CA3 is sparse (Broussard et al. 2016; McNamara et al. 2014; Rosen et al. 2015), dopaminergic modulation of the dentate-to-CA3 neuronal circuit by endogenous dopamine is supposed to be weak in normal conditions. ECS could preferentially boost the D1-dependent component of such weak neuromodulation in this circuit without strongly affecting the brain regions with dense dopaminergic innervation.
GRANTS
This work was supported by Japan Society for the Promotion of Science KAKENHI Grants 25460096 (to E. Segi-Nishida), 25116525 (to K. Kobayashi), and 15H01296 (to K. Kobayashi), Takeda Science Foundation (to K. Kobayashi), and Japan Science and Technology Agency, Core Research for Evolutional Science and Technology (to K. Kobayashi and H. Suzuki).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K., E.S.-N., and H.S. conception and design of research; K.K., Y.I., F.Y., M.K., M.U., and E.S.-N. performed experiments; K.K., Y.I., F.Y., M.K., M.U., and E.S.-N. analyzed data; K.K. interpreted results of experiments; K.K. prepared figures; K.K. drafted manuscript; K.K. and E.S.-N. edited and revised manuscript; K.K., Y.I., F.Y., M.K., M.U., E.S.-N., and H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yasunori Mikahara, Yoko Oda, Tomoko Nishisaka, and Kayo Murayama for technical assistance.
REFERENCES
- Baldinger P, Lotan A, Frey R, Kasper S, Lerer B, Lanzenberger R. Neurotransmitters and electroconvulsive therapy. J ECT 30: 116–121, 2014. [DOI] [PubMed] [Google Scholar]
- Broussard JI, Yang K, Levine AT, Tsetsenis T, Jenson D, Cao F, Garcia I, Arenkiel BR, Zhou FM, De Biasi M, Dani JA. Dopamine regulates aversive contextual learning and associated in vivo synaptic plasticity in the hippocampus. Cell Rep 14: 1930–1939, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64: 327–337, 2007. [DOI] [PubMed] [Google Scholar]
- Fremeau RT Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci USA 88: 3772–3776, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Longueville S, De Bundel D, Perroy J, Hervé D, Girault JA, Valjent E. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus 22: 2199–2207, 2012. [DOI] [PubMed] [Google Scholar]
- Gombos Z, Spiller A, Cottrell GA, Racine RJ, McIntyre Burnham W. Mossy fiber sprouting induced by repeated electroconvulsive shock seizures. Brain Res 844: 28–33, 1999. [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59: 846–860, 2008. [DOI] [PubMed] [Google Scholar]
- Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, Biggs MM, O'Connor K, Rasmussen K, Litle M, Zhao W, Bernstein HJ, Smith G, Mueller M, McClintock SM, Bailine SH, Kellner CH. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 65: 485–491, 2004. [DOI] [PubMed] [Google Scholar]
- Imoto Y, Kira T, Sukeno M, Nishitani N, Nagayasu K, Nakagawa T, Kaneko S, Kobayashi K, Segi-Nishida E. Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain 8: 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Chowdhury A, Donato F, Quairiaux C, Michel CM, Caroni P. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat Neurosci 19: 454–464, 2016. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol 39: 24–36, 2009. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Haneda E, Higuchi M, Suhara T, Suzuki H. Chronic fluoxetine selectively upregulates dopamine D1-like receptors in the hippocampus. Neuropsychopharmacology 37: 1500–1508, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Asada M, Inagaki H, Kawada T, Suzuki H. Corticosterone facilitates fluoxetine-induced neuronal plasticity in the hippocampus. PLoS One 8: e63662, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Haneda E, Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci 28: 6272–6280, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, Suzuki H. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci USA 107: 8434–8439, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Suzuki H. Dopamine selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. Neuropharmacology 52: 552–561, 2007. [DOI] [PubMed] [Google Scholar]
- Kusuki T, Imahori Y, Ueda S, Inokuchi K. Dopaminergic modulation of LTP induction in the dentate gyrus of intact brain. Neuroreport 8: 2037–2040, 1997. [DOI] [PubMed] [Google Scholar]
- Lammers CH, Diaz J, Schwartz JC, Sokoloff P. Selective increase of dopamine D3 receptor gene expression as a common effect of chronic antidepressant treatments. Mol Psychiatry 5: 378–388, 2000. [DOI] [PubMed] [Google Scholar]
- Lamont SR, Paulls A, Stewart CA. Repeated electroconvulsive stimulation, but not antidepressant drugs, induces mossy fibre sprouting in the rat hippocampus. Brain Res 893: 53–58, 2001. [DOI] [PubMed] [Google Scholar]
- Lamont SR, Stanwell BJ, Hill R, Reid IC, Stewart CA. Ketamine pre-treatment dissociates the effects of electroconvulsive stimulation on mossy fibre sprouting and cellular proliferation in the dentate gyrus. Brain Res 1053: 27–32, 2005. [DOI] [PubMed] [Google Scholar]
- Ma XM, Mains RE, Eipper BA. Plasticity in hippocampal peptidergic systems induced by repeated electroconvulsive shock. Neuropsychopharmacology 27: 55–71, 2002. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou QY, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience 45: 359–371, 1991. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci 17: 1658–1660, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetti J, Isingrini E, Dal Bo G, Sagheby S, Menegaux A, Tronche F, Levesque D, Moquin L, Gratton A, Wong TP, Rubinstein M, Giros B. Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol Psychiatry 77: 513–525, 2015. [DOI] [PubMed] [Google Scholar]
- Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci 18: 1763–1771, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariñana J, Kitamura T, Künzler P, Sultzman L, Tonegawa S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc Natl Acad Sci USA 111: 8245–8250, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Lindefors N, Hurd Y, Sharp T. Electroconvulsive shock increases dopamine D1 and D2 receptor mRNA in the nucleus accumbens of the rat. Psychopharmacology (Berl) 120: 333–340, 1995. [DOI] [PubMed] [Google Scholar]
- Strome EM, Zis AP, Doudet DJ. Electroconvulsive shock enhances striatal dopamine D1 and D3 receptor binding and improves motor performance in 6-OHDA-lesioned rats. J Psychiatry Neurosci 32: 193–202, 2007. [PMC free article] [PubMed] [Google Scholar]
- Tsen P, El Mansari M, Blier P. Effects of repeated electroconvulsive shocks on catecholamine systems: electrophysiological studies in the rat brain. Synapse 67: 716–727, 2013. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 89: 157–166, 1999. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem 100: 1–11, 2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang D, Lu XY. Dentate gyrus-CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant-like responses to leptin. Mol Psychiatry 20: 509–519, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on activity of dopaminergic neurons in the ventral tegmentum. Int J Neuropsychopharmacol 14: 201–210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis AP, Nomikos GG, Damsma G, Fibiger HC. In vivo neurochemical effects of electroconvulsive shock studied by microdialysis in the rat striatum. Psychopharmacology (Berl) 103: 343–350, 1991. [DOI] [PubMed] [Google Scholar]