Abstract
The aim of this study was to make direct measurements of the possible radiation-induced EPR signals in the teeth of volunteers who were residents in Fukushima within 80 km distance from the Fukushima Nuclear Power plant at the time of the disaster, and continued to live there for at least 3 month after the disaster. Thirty four volunteers were enrolled in this study. These measurements were made using a portable L-band EPR spectrometer, which was originally developed in the EPR Center at Dartmouth. All measurements were performed using surface loop resonators that have been specifically designed for the upper incisor teeth. Potentially these signals include not only radiation-induced signals induced by the incident but also background signals including those from prior radiation exposure from the environment and medical exposure. We demonstrated that it is feasible to transport the dosimeter to the measurement site and make valid measurements. The intensity of the signals that were obtained was not significantly above those seen in volunteers who had not had potential radiation exposures at Fukushima.
INTRODUCTION
Following the earthquake and tsunami on 11 March 2011 in Japan, the Fukushima Daiichi nuclear disaster occurred, which was a series of equipment failures, nuclear meltdowns, and release of radioactive materials at the Fukushima Daiichi Nuclear Power Plant. It was the largest nuclear disaster since the Chernobyl disaster of 1986. The Japanese government estimates the total amount of radioactivity released into the atmosphere was approximately one-tenth as much as that released during the Chernobyl disaster. Under such a situation, populations who live near the nuclear power plant potentially could have been exposed to significant doses of ionizing radiation released from the damaged reactor that could cause direct clinical effects. Fortunately, cases of high dose exposures in local residents, except for workers of Tokyo Electric Power Company (TEPCO) have not been found.
Ionizing radiation generates unpaired electron species in irradiated materials, including biologic tissues. The radicals generated in tooth enamel are very stable, persisting indefinitely at levels that are directly proportional to dose(1). Electron Paramagnetic Resonance (EPR) spectroscopy has been applied to perform retrospective radiation biodosimetry using extracted tooth enamel, following radiation accidents and exposures(2–5) using conventional EPR dosimetry. This approach involves the isolation of tooth enamel for measurements made at X-band ( 9.0–10 GHz). While this technique is well suited for use in limited populations with potential significant exposures, its use as a tool to perform screening after an event where a large number of people have potentially been exposed to clinically-relevant doses is severely limited by the need to extract the tooth and then to do remote processing. In vivo EPR tooth dosimetry using L-band (1.0–1.2 GHz) has several desirable characteristics for screening and providing guidance, for triage following a mass-exposure incident(6–9). The EPR technique is applicable to individual subjects and can provide an estimate of the absorbed dose within the oral cavity. We have developed a portable in vivo EPR spectrometer which can be used to directly measure the upper central incisors of human subjects. The aim of this study was to assess the feasibility of making on-site measurement of EPR signals in the teeth of residents who had the potential of having been exposed from the accident in Fukushima.
MATERIALS AND METHODS
Eligibility of volunteer
All volunteers were 18 years old or older and were able to understand the contents of this protocol and were able to give us their written consent. Thirty four volunteers (male 13, female 21, average age 50.2 years old) were enrolled in this study. All volunteers had at least one completely intact healthy upper incisor. All of them have lived in Fukushima prefecture since the disaster occurred. All residences where the volunteers lived are located at the distance of 30–80 km from Fukushima Nuclear power plant.
It should be noted that this protocol has been approved by the IRBs of NIPH (National Institute of Public Health, Japan), NIPH-IBRA #12092 and Kagawa University, Heisei #24-4.
EPR instrumentation and measurements
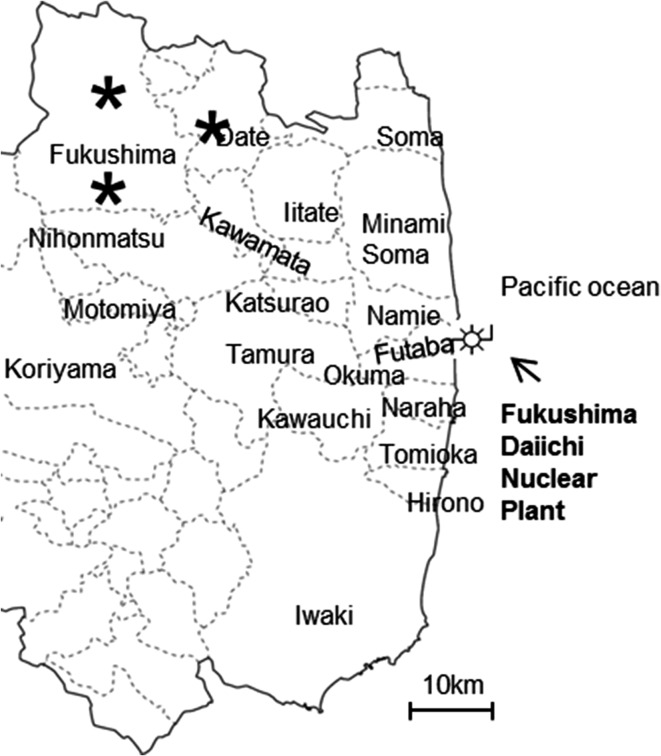
These measurements and developments were made using a deployable in vivo EPR tooth dosimeter designed by the EPR Center for the Study of Viable Systems, Geisel School of Medicine at Dartmouth(10, 11). This spectrometer operates in continuous wave (CW) mode with homodyne detection at an excitation frequency near 1.15 GHz (L-band) using a 41 mT dipole magnet weight 30 kg with 17 cm pole separation. Integrated field sweep and modulation coils provide 4 mT sweep range and 0.4 mT modulation at 20 kHz. All measurements are performed using surface loop resonators that have been specifically designed for upper incisor which were originally developed in the EPR Center at Dartmouth(12, 13). The version used in the present experiment had a detection loop with an inner diameter of 6.0 mm. The equipment, including the console and magnet, were designed to be completely transportable. Total weight of this system is around 100 kg (console and RF bridge, PC including rack with casters: 70 kg dipole magnet: 30 kg). (A version using compact electronics which do not require the heavy rack has recently been developed at Dartmouth with a total weight of less than 40 kg.) The EPR dosimeter was transported from NIPH to the sites of measurement by a small truck equipped with an air-suspension to protect the spectrometer. Assembly into operation status was easy and took about 10 min. The on-site measurements were made at three locations (Figure 1).
Figure 1.
The schema of Fukushima Prefecture is shown. On-site measurements were made at the locations marked by *. They were located at a distance of 70–80 km north-west from the Fukushima Nuclear power plant.
EPR data collection and analysis
The experimental procedures for in vivo measurements including subject immobilization, resonator positioning, and instrumental settings as described in our publications(8, 10, 14, 15). Spectra are acquired with a nominal modulation amplitude of 0.4 mT and an incident microwave power of 50 mW. Irradiated incisors (incisors were obtained from the volunteers at the Department of Oral and Maxillofacial Surgery, Kagawa University Hospital, Japan) were measured with our EPR dosimeter to confirm a relationship of the Radiation Induced Signals (RIS) and the radiation dose. The isolated tooth samples were irradiated by an X-ray generator (HITACHI MBR 1505R2 250 kV Al0.5 mm + Cu0.1 mm filter) with 0, 1.0, 5.0,10, 20 Gy, respectively.
Three sets of data were collected in vivo using the following instrumental settings: scan time 3 s, 30 sweeps, scan range 2.5 mT, time constant 0.03 s, modulation amplitude 0.4 mT. This required the volunteer to keep his/her mouth open for 90 s for acquisition of one set of data (Figures 2 and 3). After each set of the measurements, re-adjustment of the location of the loop resonator and tuning was performed. From three sets of individual scans collected in this period, mean or median values at each field-point were calculated and analyzed to estimate the amplitude of the RIS component (Figure 4). This amplitude, which is a voltage, is expressed here in arbitrary units (AU) to acknowledge variable spectrometer gains and instrumental conditions during the course of developments. A standard sample was measured simultaneously with spectral features which avoid significant overlap with the signals in the teeth of the volunteer. The standard material was 15N-substituted perdeuterated, 2,6,6-tetramethyl-4-oxopiperidine-1-oxyl (15N-PDT) which has two off-center EPR peaks separated by approximately 2.25 mT. The 15NPDT is sealed in a glass vial at a concentration of 8 mM in D2O and fixed to the transmission line of the resonator, such that its distal tip enters the sensitive volume of the resonator and the amplitude of the standard signal is comparable to that of an irradiated tooth (20 Gy). In this analysis, the linewidths and center separation of the RIS and PDT signals are fixed, and the modulation amplitude and peak-to-peak amplitudes are allowed to vary. Data analysis can be performed immediately online within the data acquisition software and also via post-processing of the recorded spectral data.
Figure 2.

Volunteers sat on the chair during the measurement. The time for each measurement was around 10 min including the tuning process.
Figure 3.

All measurements were performed using surface loop resonators that have been specifically designed for EPR measurements of the upper incisor teeth. The teeth were positioned by use of a bite plate system which gently held the upper lip and kept the resonator loop on the surface of the tooth. Bite plates are sterilizable and changed for each measurement.
Figure 4.
Typical EPR signal from volunteer (bracket shown). Arrowhead shows the signal from 15N-PDT as standard sample: sealed in a glass vial at a concentration of 8 mM in D2O attached on the loop of the resonator.
RESULTS
Figure 5 shows the relationship of the RIS (expressed as the ratio to the PDT standard to correct for changes in Q, etc.) vs. radiation dose (low energy X-rays with yield 4X that of Cs-137) of isolated incisors using our EPR dosimeter without correction factors for size, etc. EPR signal intensity shows a clear correlation with the dose of irradiation. Figure 6 shows the results of the signals from volunteers. The average of the RIS/PDT ratio was around 0.087. We did not estimate the apparent dose for the volunteers based on the results of the measured signal intensity, because for these measurements, valid in vivo calibration data were not available. However, the magnitude of the signals from the volunteers at Fukushima did not differ from that seen in volunteers who did not have possible exposures from nuclear fallout. This is consistent with the estimates of dose for these populations that have been developed by official agencies(16).
Figure 5.
RIS ratio vs. radiation dose (air kerma of X-rays whose radical yield is four times that of Cs-137) of isolated incisors. Each dot indicates the intensity of the EPR signal from one tooth. There were no corrections applied for variations in tooth size, etc.
Figure 6.
RIS ratio of measured EPR signals from the volunteers in Fukushima. Average 0.087 ± 0.051.
DISCUSSION
The fundamental basis for EPR tooth dosimetry is that radiation generates stable carbonate radicals in the calcium hydroxyapatite of the tooth enamel, and the relative density of these radicals can be measured using EPR and related to absorbed dose(1). Our system in vivo EPR tooth dosimetry has a number of valuable as well as some unique characteristics and capabilities that are particularly well suited for triage of a very large number of people with high throughput in a nuclear power plant disaster, e.g. Fukushima Daiichi. At the first stage of triage, the most important function of biodosimetry will be to help to quickly identify individuals likely to have received doses >2–3 Gy for whom acute medical care could affect survival(17). Also, a low false-negative rate is very desirable.
In this experiment, we did not obtain calibration data directly based on the population in Fukushima. Therefore, we did not attempt to estimate the dose from the EPR signal in the tooth enamel. However, our device is a clone of the tooth dosimeter developed by the Dartmouth group, with the same components (except for the resonator) and software. An indication of the feasibility and accuracy of in vivo measurements with this spectrometer is provided in a report by Bahar et al. where they have reported(18) an excellent correlation between measured signal amplitude and delivered dose in human teeth irradiated in vivo using the same tooth EPR dosimeter made by Dartmouth. The availability of such a calibration will be required when the method is actually used for triage, but was not essential for the purposes of this paper.
The tooth dosimetry system has been developed for use in field settings because of the intended use of the system. It needs to be able to be placed in a location nearby to the radiation event, to meet the need for rapid and high throughput in order to deal with large numbers of people(7, 14, 19). The purpose of this study was to demonstrate the ability to use the tooth dosimeter in the field through the actual measurements of volunteers in this study. Our results confirmed that the stability of the measurement with our portable EPR system is adequate, and that the set up of the system could be done very quickly even outside of the laboratory. Electric power supply (100 V 50/60 Hz) was easily available from commercial base electric services and if that was not available, could have been obtained from a small electric generator because electric power demand is relatively small. Through this experiment, we also have shown that our instrument is deployable and it is feasible to make a measurement of EPR signals from human incisors with a high throughput rate.
This field testing also has provided insights into improvements that are desirable for operation in the field. We found an improper positioning of the loop of the resonator on the surface of the tooth and motion during the measurement produced significant noise and reduced the S/N ratio of the signals. In order to get more accuracy in vivo settings, especially because of the need to have the system usable by non-expert operators, further improvements are needed to immobilize the resonator positioning system and the adjustment of the loop location in an automated way. Another problem is the background signal that is present in all teeth. However, we know the characteristics of the background signal from extensive measurements of unirradiated teeth, and our measurement indicated that the background signal is relatively consistent with variations that are small compared to the magnitude of the RIS and therefore, the background signal can be removed by use of an empirical calibration curve based on the measurements in vivo of teeth whose radiation dose is well characterized.
Because the carbonate radicals in the matrix of tooth enamel induced by ionizing radiation are very stable for long periods much greater than life-span, these measurements also could be useful for making measurements at any time after the event. This makes feasible, the potential application of in vivo tooth EPR dosimetry for estimating long-term risks/consequences of the residents long after a radiation event such as the Fukushima Daiichi Disaster.
Dose assessment including the accumulated exposure also could be important in improving the mental health care for many potentially affected people in such a scenario, even if doses were less than the level that causes acute effects, by providing them with objective information on their personal dose.
It also would be possible to lower the detectable dose by knowledge of the background levels of EPR signals in a population obtained prior to the possible exposure (e.g. in first responders or nuclear power plant operators), so that dose assessments could be more accurate by being able to remove the contribution s from background signals and prior exposures from medical and environmental sources.
Future studies will focus on:
More detailed analysis of the background signals and in vivo calibration by increasing the number of volunteers who are measured.
Determining the feasibility of longer measurements to lower the threshold level for detection of radiation-induced signals.
Determining the psychological benefits for volunteers who have been measured.
CONCLUSION
We were able to successfully carry out in vivo EPR on-site measurements from human upper incisors in volunteers from Fukushima. There were no indications of radiation-induced signals above the background in normal volunteers. This study also demonstrated that the EPR tooth dosimeter is capable of being transported to a distant site and successfully make estimates of radiation exposure.
ACKNOWLEDGMENTS
We would like to sincerely thank each of the volunteers in Fukushima subjects for their participation in this study.
FUNDING
This work was supported by Japan Society for the Promotion of Science KAKEN (grant number 26462841) and Japan Industrial Disease Clinical Research Grants (#150803-02) and partially supported by the Pilot Project Program of the Dartmouth Physically Based Center for Medical Countermeasures Against Radiation, with NIH funding from the National Institute of Allergy and Infectious Diseases (U19-AI091173).
REFERENCES
- 1.Brady J. M., Aarestad N. O. and Swartz H. M.. In vivo dosimetry by electron spin resonance spectroscopy. Health. Phys. 15(1), 43–47 (1968). [DOI] [PubMed] [Google Scholar]
- 2.Ikeya M. and Ishii H.. Atomic bomb and accident dosimetry with ESR: natural rocks and human tooth in-vivo spectrometer. Int. J. Rad. Appl. Instrum. A. 40(10–12), 1021–1027 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Tolstykh E. I., Degteva M. O., Kozheurov V. P., Shishkina E. A., Romanyukha A. A., Wieser A. and Jacob P.. Strontium metabolism in teeth and enamel dose assessment: analysis of the Techa river data. Radiat. Environ. Biophys. 39(3), 161–171 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Romanyukha A. A., Ivanov D., Schauer D. A., Thomas J. A. and Swartz H. M.. Spectrum file size optimization for EPR tooth dosimetry. Appl. Radiat. Isot. 62(2), 197–200 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Chumak V. V. The Chernobyl experience in the area of retrospective dosimetry. J. Radiol. Prot. 32(1), N59–N63 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Miyake M., Liu K. J., Walczak T. M. and Swartz H. M.. In vivo EPR dosimetry of accidental exposures to radiation: experimental results indicating the feasibility of practical use in human subjects. Appl. Radiat. Isot. 52(5), 1031–1038 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Swartz H. M., Flood A. B., Gougelet R. M., Rea M. E., Nicolalde R. J. and Williams B. B.. A critical assessment of biodosimetry methods for large-scale incidents. Health. Phys. 98(2), 95–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B. B., Dong R., Kmiec M., Burke G., Demidenko E., Gladstone D., Nicolalde R. J., Sucheta A., Lesniewski P. and Swartz H. M.. Development of in vivo tooth EPR for individual radiation dose estimation and screening. Health. Phys. 98(2), 327–338 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swartz H. M., Flood A. B., Williams B. B., Dong R., Swarts S. G., He X., Grinberg O., Sidabras J., Demidenko E. and Gui J. et al. Electron paramagnetic resonance dosimetry for a large-scale radiation incident. Health. Phys. 103(3), 255–267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams B. B., Flood A. B., Salikhov I., Kobayashi K., Dong R., Rychert K., Du G., Schreiber W. and Swartz H. M.. In vivo EPR tooth dosimetry for triage after a radiation event involving large populations. Radiat. Environ. Biophys. 53(2), 335–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams B. B., Dong R., Nicolalde R. J., Matthews T. P., Gladstone D. J., Demidenko E., Zaki B. I., Salikhov I. K., Lesniewski P. N. and Swartz H. M.. Physically-based biodosimetry using in vivo EPR of teeth in patients undergoing total body irradiation. Int. J. Radiat. Biol. 87(8), 766–775 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swartz H. M., Iwasaki A., Walczak T., Demidenko E., Salikov I., Lesniewski P., Starewicz P., Schauer D. and Romanyukha A.. Measurements of clinically significant doses of ionizing radiation using non-invasive in vivo EPR spectroscopy of teeth in situ. Appl. Radiat. Isot. 62(2), 293–299 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Salikhov I., Hirata H., Walczak T. and Swartz H. M.. An improved external loop resonator for in vivo L-band EPR spectroscopy. J. Magn. Reson. 164(1), 54–59 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Swartz H. M., Flood A. B., Williams B. B., Meineke V. and Dorr H.. Comparison of the needs for biodosimetry for large-scale radiation events for military versus civilian populations. Health. Phys. 106(6), 755–763 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Williams B. B., Dong R., Flood A. B., Grinberg O., Kmiec M., Lesniewski P. N., Matthews T. P., Nicolalde R. J., Raynolds T. and Salikhov I. K. et al. A deployable in vivo EPR tooth dosimeter for triage after a radiation event involving large populations. Radiat. Meas. 46(9), 772–777 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UNSCEAR 2013. Report to the General Assembly with Scientific Annexes Levels and effects of radiation exposure to the nuclear accident after the 2011 great east-Japan earthquake and tsunami United Nations Scientific Committee on the Effects of Atomic Radiation. Report Scientific Annex A Vol.1 (2013). [Google Scholar]
- 17.Grace M. B., Moyer B. R., Prasher J., Cliffer K. D., Ramakrishnan N., Kaminski J., Coleman C. N., Manning R. G., Maidment B. W. and Hatchett R.. Rapid radiation dose assessment for radiological public health emergencies: roles of NIAID and BARDA. Health. Phys. 98(2), 172–178 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Bahar N., Roberts K., Stabile F., Mongillo N., Decker R., Wilson L., Husain Z., Contessa J., Williams B. and Flood A. et al. SU-C-BRD-05: non-invasive in vivo biodosimetry in radiotherapy patients using electron paramagnetic resonance (EPR) spectroscopy. Med. Phys. 42(6), 3192–3193 (2015). [Google Scholar]
- 19.Flood A. B., Boyle H. K., Du G., Demidenko E., Nicolalde R. J., Williams B. B. and Swartz H. M.. Advances in a framework to compare bio-dosimetry methods for triage in large-scale radiation events. Radiat. Prot. Dosimetry. 159(1–4), 77–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]