Abstract
Transplant decisions for children with acute lymphoblastic leukemia (ALL) in second complete remission (CR2) are often based on the type of available donor. In many cases, allogeneic hematopoietic cell transplantation (HCT) is considered only if a human leukocyte antigen (HLA) matched sibling donor (MSD) is available. The role of unrelated donor (URD) HCT in this patient population is not well established. As advances in supportive care and donor selection have improved, the use of URD HCT in such patients should be reevaluated. We analyzed the outcomes of 87 consecutive children with ALL in CR2 who underwent allogeneic HCT at the University of Minnesota between 1990 and 2007. Donor sources included MSD bone marrow (n = 32), well and partially matched (M, n = 18) and mismatched (MM, n = 16) URD bone marrow and URD umbilical cord blood (UCB, n = 21). Although the incidence of neutrophil recovery was similar in all groups, the overall incidence of grades II–IV acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD) was 37% and 9%, respectively, with a higher incidence of aGVHD in recipients of URD grafts. Leukemia-free survival (LFS) at 5 years was lower in recipients of MM-URD grafts, but was comparable in all other groups. Although relapse at 5 years was highest in recipients of MSD (50%), results were not significantly different compared to recipients of M-URD (17%), MM-URD (6%), and UCB (33%) (P =.17). The development of grades II–IV aGVHD and a first remission >3 years were associated with a lower risk of relapse (relative risk [RR] 0.2, P =.03; RR 0.2. P =.01 respectively). Together, these results support the continued investigation of URD HCT for ALL in CR2, and suggest the timing of HCT in these children should be based primarily on the risk of relapse with conventional chemotherapy and not on the type of donor available.
Keywords: Acute lymphoblastic leukemia, Second complete remission, Unrelated donor hematopoietic cell transplantation, Umbilical cord blood
INTRODUCTION
Although the majority of children diagnosed with acute lymphoblastic leukemia (ALL) have a high probability of leukemia-free survival (LFS) at 5 years with standard chemotherapy, 20% to 25% will ultimately relapse [1]. Salvage therapy for recurrent ALL is often not curative [2]. Intensive reinduction chemotherapy will result in a second complete remission (CR2) in >70% of patients [3,4]; however, the optimal strategy for long-term survival remains unclear. Potential treatment options are maintenance chemotherapy or allogeneic hematopoietic cell transplantation (HCT) using human leukocyte antigen (HLA) matched sibling donors (MSD) or HLA compatible alternate (HLA mismatched related or HLA matched [M]/mismatched [MM] unrelated) donors.
Prospective and retrospective studies have suggested a survival advantage in MSD recipients compared to those treated with maintenance chemotherapy [3,5–10]. In 1999, Boulad et al. [3] reported a series of 75 children with ALL in CR2 treated with either HCT using an MSD bone marrow (n = 38) or chemotherapy (n = 37). LFS was significantly better at 5 years in the transplanted group (62% and 26%, respectively [P = .03]), principally because of a reduced risk of relapse after HCT (19% versus 67% [P = .01]). These results were independent of the length of first remission, characteristics at diagnosis, and intensity of prior treatment [3]. Similarly, in a collaborative study between the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research (CIBMTR), Eapen et al. [10] performed a retrospective analysis in 374 children with ALL in CR2 after a marrow relapse who received either an MSD HCT (n = 186) or maintenance chemotherapy (n = 188). Significantly better LFS was observed in transplant recipients who had been treated with total body irradiation (TBI) and had a history of early relapse (<36 months after diagnosis). Despite the limitations of retrospective studies, the general practice has been to offer early allogeneic HCT to those children with ALL in CR2 for whom an MSD is available [3,5–10].
With the limited availability of suitable related donors, we and others have explored the safety and efficacy of alternate sources of hematopoietic stem cells (HSC) for transplantation. Data are emerging that suggest alternate donor HCT may offer a long-term survival advantage, particularly in those children with ALL in CR2 with early or very early bone marrow (BM) relapse compared to those who received chemotherapy [11]. Although some centers are focused on the use of haploidentical related donors, the vast majority of alternate donor transplants in children are from adult volunteer unrelated donors who are HLA M (M-URD) or 1 antigen MM (MM-URD) and partially matched umbilical cord blood (UCB) (personal communication, CIBMTR). The incidence of treatment-related mortality (TRM) has historically been high with alternate donor transplants, but recent improvements in donor selection and supportive care warrant a reexamination of the outcomes. The aim of this single-center study was to compare the results of MSD and alternate donor HCT in children with ALL in CR2 and to assess risk factors that might potentially influence transplant outcomes.
MATERIALS AND METHODS
Study Design
Outcomes were retrospectively evaluated in 87 consecutive children (<18 years of age at the time of HCT) with ALL in CR2 who underwent allogeneic HCT at the University of Minnesota between 1990 and 2007. Complete remission was defined morphologically as ≤5% bone marrow blasts immediately prior to HCT. All treatment protocols were reviewed and approved by the institutional review board at the University of Minnesota, and all patients/guardians provided signed informed consent.
Stem cell sources included HLA MSD BM, URD BM, and unrelated UCB. For the UCB units, patients and donors were typed for HLA-A and -B at the antigen level and for -DRB1 at the allele level. Eleven were mismatched at 1 HLA locus and 8 were mismatched at 2 HLA loci. For the other unrelated donors, patients and donors had antigen level typing at HLA-A and -B and allele level typing at HLA-DRB1 until June 2005, when testing at the allele level for all loci was implemented. All matched sibling donors had antigen level typing at HLA-A, -B, and -DRB1 unless adequate family typing was not available to determine haplotypes, in which case allele level DNA typing was performed at all loci. Prospective allele level typing for HLA-C was incorporated for all donor sources beginning in June 2004. Based on the recent report by Weisdorf et al. [12], aimed at standardizing interpretations of retrospective analyses in URD transplantation, we divided our cohort into the following groups: HLA MSD (n = 32), M-URD (n = 18; 1 well-matched and 17 partially matched), MM-URD (n = 16), and UCB (n = 21).
Preparative Therapy and Supportive Care
All patients received a cyclophosphamide (Cy, 60–120 mg/kg) and TBI (1320–1375 cGy) containing regimen, but 20 MSD, 3 M-URD, and 4 MM-URD recipients also received etoposide (VP16, 30 mg/kg) and 7 UCB recipients also received fludarabine (Flu, 75 mg/kg). Prophylaxis for acute graft-versus-host disease (aGVHD) always included cyclosporin A (CsA) with some receiving methotrexate (MTX, n = 56), mycophenolate mofetil (MMF, n = 7), methylprednisone (MP, n = 14) or T cell depletion (n = 10). All patients who received T cell-depleted grafts (n = 10) were participants in local clinical trials.
Patients were hospitalized in single rooms ventilated with high-efficiency particulate air filtration systems. Patients at high risk for recurrence of herpes simplex virus (HSV) (titer ≥1:8) received prophylactic acyclovir. Patients at high risk of cytomegalovirus (CMV) reactivation (recipient or donor with a titer ≥1:8) received prophylactic high-dose acyclovir until day 100. Documented CMV reactivation or infection was treated with ganciclovir with or without .v. immunoglobulin. Broad-spectrum antibiotics were administered for fever during aplasia, and antifungals were added for persistent fever unresponsive to antibiotic therapy. All patients received fluconazole for prophylaxis of yeast infections for 100 days, trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis (carinii) jiroveci after engraftment for 12 months after transplantation, and antibiotic prophylaxis (penicillin, gatifloxacin, or levofloxacin) for Gram-positive organisms during the treatment of GVHD. Intravenous granulocyte-colony stimulating factor (G-CSF) (5 µg/kg/day) was administered daily until neutrophil engraftment.
Outcome Endpoints and Definitions
Study endpoints included neutrophil engraftment, aGVHD (grades II–IV and grades III–IV), chronic GVHD (cGVHD), LFS, relapse, and TRM. Neutrophil engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count (ANC) >0.5 × 109/L. Primary graft failure was defined as failure to achieve an ANC >0.5 × 109/L by day +42 or date of death (if after day +21). Secondary graft failure was defined as primary engraftment followed by a decrease in the ANC to <0.5 × 109/L without recovery until a new stem cell infusion is required or the patient died. aGVHD and cGVHD was evaluated and graded according to previously published criteria, with histopathologic confirmation when possible [13,14]. All patients with engraftment were considered evaluable for GVHD. LFS was defined as the time from transplant (day 0) until disease recurrence, death, or last patient contact, whichever came first. Survival was defined as the time from transplant day 0 until death or last contact. Relapse was defined as a recurrence of ALL after HCT.TRM was defined as death in the first 100 days after HCT for any reason other than relapse.
Statistical Analysis
Patient and transplant characteristics by donor type were analyzed using the chi-square test for categoric data and the Wilcoxon rank-sum test for continuous data. The cumulative incidence of neutrophil recovery was calculated by treating patients without an ANC >0.5 × 109/L at day 42 or with autologous marrow reconstitution as primary graft failures. The cumulative incidence of aGVHD and cGVHD, TRM, and relapse were calculated by treating deaths from other causes as competing risks [15]. LFS was estimated by the Kaplan-Meier method [16]. Event times were measured from the date of transplantation to the event or the date of last contact. Statistical comparisons of the time-to-event curves were completed by the log-rank test.
Proportional hazards regression modeling was used for multiple regression analysis with Cox regression and the Gray and Fine competing hazards method as appropriate [17,18]. Variables considered in the models included the main effect variable of donor type (MSD versus M-URD versus MM-URD versus UCB), along with conditioning regimen, GVHD prophylaxis, age at diagnosis and transplant, sex, CMV serostatus, aGVHD as a time-dependent variable, length of first remission, and type of ALL (T cell versus B cell).
RESULTS
Patient Characteristics
Table 1 shows demographic and treatment characteristics by stem cell source. Although there was no significant difference between the groups with respect to age at diagnosis, type of ALL (pre-B cell versus T cell), length of CR1, age at transplant, sex, CMV status or cell dose, the median length of follow-up among survivors was shorter in the UCB group, MM-URD transplants were more frequent prior to 1999, and UCB transplants were more likely to have occurred after 1999 (P < .01). Although all patients received Cy and TBI, recipients of MSD marrow were more likely to receive VP16 and recipients of UCB were more likely to receive Flu as part of their conditioning (P < .01). Most patients transplanted with marrow received CsA/MTX for GVHD immunoprophylaxis, and most transplanted with UCB received CsA/MMF. T cell depletion of URD marrow occurred in 3% of MSD, 28% of M-URD, and 25% of MM-URD transplants (P < .01).
Table 1.
Patient Demographics and Treatment Characteristics
| MSD | M-URD (“Well Matched” and “Partially Matched” URD)* |
MM-URD* | UCB | P-value | |
|---|---|---|---|---|---|
| Number of patients | 32 | 18 | 16 | 21 | |
| Age at diagnosis | .36 | ||||
| < 1 year | 1 (3%) | 1 (6%) | 3 (19%) | 2 (10%) | |
| ≥ 1 year | 31 (97%) | 17 (94%) | 13 (81%) | 19 (90%) | |
| Median | 4.6 years | 3.4 years | 5.4 years | 4.2 years | .13 |
| T cell ALL | 4 (13%) | 0 | 3 (19%) | 2 (10%) | .33 |
| Median Length of CR1 (range) | 2.1 years (0.4–7.6) | 2.0 years (0.6–3.9) | 1.1 years (0.1–3.5) | 2.2 years (0.1–5.6) | .17 |
| Median age at HCT (range) | 8.0 years (3.1–17.9) | 7.0 years (1.7–17.7) | 6.9 years (0.9–17.2) | 7.0 years (2.1–17.9) | .44 |
| Sex | .91 | ||||
| Male | 24 (75%) | 13 (72%) | 11 (69%) | 14 (67%) | |
| Female | 8 (25%) | 5 (28%) | 5 (31%) | 7 (33%) | |
| Year of HCT | <.01 | ||||
| 1990–1994 | 10 (31%) | 7 (39%) | 8 (50%) | 0 | |
| 1995–1999 | 15 (47%) | 9 (50%) | 8 (50%) | 6 (29%) | |
| 2000–2007 | 7 (22%) | 2 (11%) | 0 | 15 (71%) | |
| Conditioning regimen | <.01 | ||||
| Cy/TBI | 12 (38%) | 15 (83%) | 12 (75%) | 14 (67%) | |
| Cy/TBI/Etop | 20 (62%) | 3 (17%) | 4 (25%) | 0 | |
| Cy/TBI/Flu | 0 | 0 | 0 | 7 (33%) | |
| Total number of nucleated cells infused (×108/kg) |
|||||
| Overall median (range) | 2.0 (1.4–2.2) | 2.0 (2.0–2.2) | 2.0 (2.0–2.0) | 0.33 (0.15–1.32) | <.01 |
| Non-T cell deplete median (range) | n = 31 | n = 13 | n = 12 | n = 21 | |
| 2.0 (1.4–2.2) | 2.0 (2.0–2.2) | 2.0 (2.0–2.0) | 0.33 (0.15–1.32) | ||
| T cell deplete median (range) | n = 1 | n = 5 | n = 4 | n = 0 | |
| 1.88 | 0.6 (0.27–0.7) | 0.36 (0.25–0.73) | |||
| GVHD prophylaxis | <.01 | ||||
| MTX based | 31 (97%) | 13 (72%) | 12 (75%) | 0 | |
| MP/CsA | 0 | 0 | 0 | 14 (67%) | |
| MMF/CsA | 0 | 0 | 0 | 7 (22%) | |
| T cell depletion | 1 (3%) | 5 (28%) | 4 (25%) | 0 | |
| Median length of follow-up among survivors (range) |
6.6 years (2.9–11.0) | 9.3 years (3.4–14.5) | 7.8 years (7.0–13.5) | 4.1 years (1.0–7.8) | .03 |
CR1 indicates first complete remission; Cy, cyclophosphamide; TBI, total body irradiation; Etop, etoposide; Flu, fludarabine; MTX, methotrexate; MP, methylprednisone; CsA, cyclosporine A; MMF, mycophenolate mofetil; ALL, acute lymphoblastic leukemia; GVHD, graft-versus-host disease; UCB, umbilical cord blood; MSD, matched sibling donor; M, matched; MM, mismatched; HCT, hematopoietic cell transplantation.
Matching for URDs are according to Weisdorf et al. [12] matching algorithm.
Hematopoietic Recovery and Engraftment
Rates of neutrophil engraftment were similar among HSC sources. The incidence of neutrophil recovery by day +42 was 97% (95% confidence interval [CI] 91%–100%) in recipients of MSD, 100% in recipients of M-URD, 86% (95% CI 69%–100%) in recipients of MM-URD, and 95% (95% CI 86%–100%) in recipients of UCB (P = .28). Time to neutrophil recovery was also similar between the HSC sources, with a median of 25 days (range: 13–38 days), 24 days (range: 11–34 days), 25 days (range: 12–32 days), and 23 days (range: 9–35 days) in the HLA MSD, M-URD, MM-URD, and UCB groups, respectively (P = .76). Primary graft failure occurred in 4 patients (MSD [n = 1], MM-URD [n = 2], and UCB [n = 1]), and secondary graft failure occurred in 3 patients (M-URD [n = 2] and MM-URD [n = 1]). Therefore, the probability of neutrophil engraftment was 97% (95% CI 91%–100%) in the HLA MSD group, 87% (95% CI 70%–100%) in the M-URD group, 80% (95% CI 60%–100%) in the MM-URD group, and 95%(95% CI 86%–100%) in the UCB group (P=.18).
aGVHD
The cumulative incidence of grades II–IV aGVHD for the entire cohort was 37% (95% CI 27%–47%) at day 100. Grades II–IV aGVHD was higher in recipients of MM-URD transplants (75% [95% CI 49%–100%]) when compared to MSD recipients (22% [95% CI 8%–36%]), M-URD recipients (44% [95% CI 20%–68%]), and UCB recipients 24% (95% CI 0%–22%) (P < .01). Using the sibling donor group as the reference category in a multiple regression analysis (Table 2), the MM-URD group had a significantly higher risk of grades II–IV aGVHD (relative risk [RR] 5.8, 95% CI 2.3%–13.2%, P < .01). Despite the high degree of donor-recipient MM, the risk of GVHD after UCB transplant was not significantly different from that observed in recipients of MSD marrow (Table 2). The cumulative incidence of grades III–IV aGVHD for the entire cohort was 15% (95% CI 8%–22%) at day 100. In multiple regression analysis, there was a higher risk of grade III–IV aGVHD in recipients of MM-URD grafts (RR 5.6, 95% CI 1.1%–27.8%, P = .03). The risk in recipients of M-URD and UCB grafts were similar to that observed in recipients of MSD marrow (Table 2).
Table 2.
Multivariate Analysis of Acute and Chronic GVHD
| Donor type | Relative Risk of Grades II–IV Acute GVHD (95% CI) |
P-Value | Relative Risk of Grades III–IV Acute GVHD (95% CI) |
P-Value | Relative Risk of Chronic GHVD (95% CI) |
P-Value |
|---|---|---|---|---|---|---|
| MSD* | 1.0 | 1.0 | 1.0 | |||
| M-URD | 2.4 (0.9–6.5) | .08 | 3.9 (0.7–20.3) | .11 | 2.8 (0.5–15.8) | .25 |
| MM-URD | 5.8 (2.3–13.2) | <.01 | 5.6 (1.1–27.8) | .03 | 2.2 (0.3–15.2) | .44 |
| UCB | 1.2 (0.4–3.6) | .80 | 1.6 (0.2–11.7) | .63 | 0.8 (0.1–8.3) | .83 |
MSD indicates matched related sibling; M-URD, matched unrelated donor; MM-URD, mismatched unrelated donor, UCB, umbilical cord blood; CI, confidence interval, GVHD, graft-versus-host disease.
The above model is the result of multiple regression analysis on GVHD after testing the following independent variables: donor type, sex, pretransplant conditioning, GVHD prophylaxis, cytomegalovirus (CMV) serostatus, age, length of first remission, and type of ALL (T cell versus B cell).
Reference category.
cGVHD
Overall, only 8 patients developed cGVHD (3 limited, 5 extensive) by 1 year after transplant for an incidence of 9%(95%CI 3%–15%). There were 2 patients (6%) in the MSD group, 3 (17%) in the M-URD group, 2 (13%) in the MM-URD group, and 1 (5%) in the UCB group who developed cGVHD. In multiple regression analysis there was no difference in RR among the 4 groups (Table 2). Three of the 8 subjects diagnosed with cGVHD died during the follow-up period. The 5 remaining patients are alive, symptom free, and are no longer on immunosuppressive therapy.
LFS
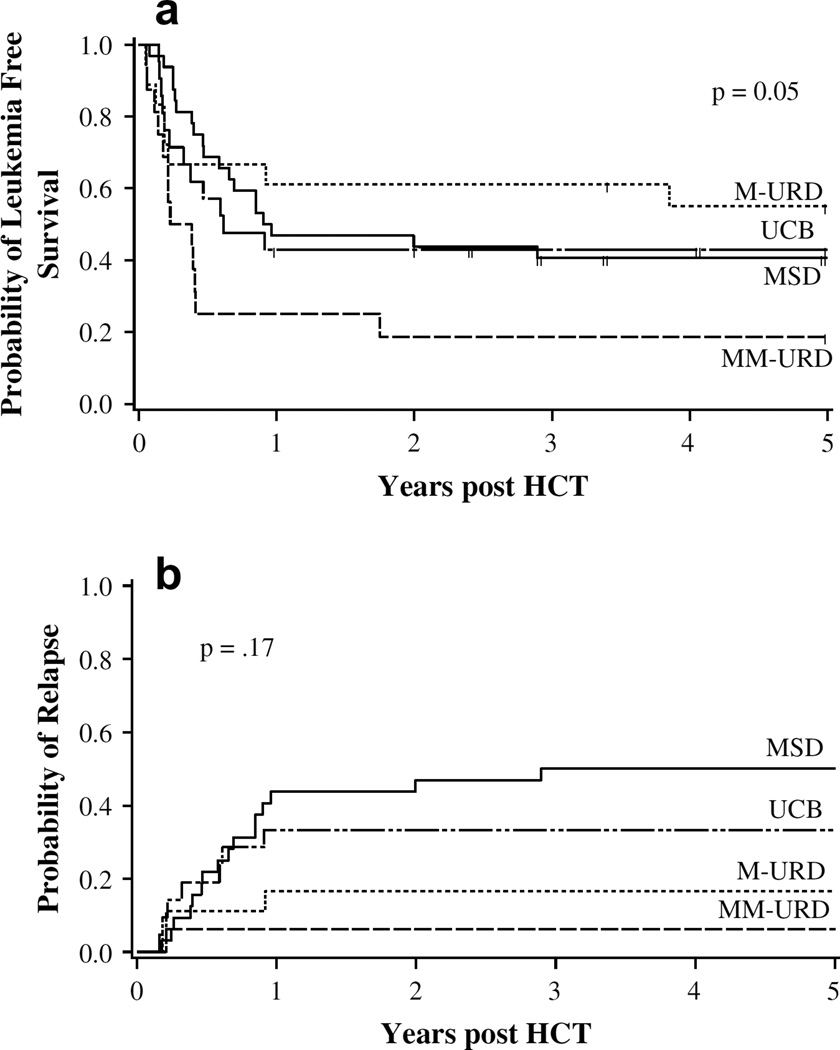
Probability of LFS for the entire cohort of patients was 45% (95% CI 35%–65%) and 40% (95% CI 30%–50%) at 1 and 5 years, respectively. By donor type, the 5-year LFS was 41% (95% CI 24%–68%) in recipients of a MSD, 57% (95% CI 35%–80%) in recipients of a M-URD, 19% (95% CI 5%–40%) in recipients of a MM-URD, and 43% (95% CI 23%–63%) in recipients of UCB (P=.05) (Figure 1a). For patients with a CR1 ≥3 years, 5-year LFS was significantly better at 56% (95% CI 35%–73%) compared to 33% (95% CI 21%–45%) in those patients with a CR1 <3 year (P = .02). Patients with T cell ALL (n = 9) had 0% LFS at 5 years compared to 46% in those with pre-B cell ALL (P = .04). In multivariate regression analysis MM-URD recipients had a 3.2 times increased relative risk of relapse or death (P = .01). In addition, patients with a CR1 >3 years or grades II–IV aGVHD had less than half the relative risk of relapse or death (P < .01 and.02, respectively) (Table 3a).
Figure 1.
Probability of (a) LFS and (b) relapse by HSC source. Legend: (a) probability of LFS by HSC source. The probability at 5 years was 0.41 in recipients of MSD, 0.57 in recipients of M-URD, 0.19 in recipients of MM-URD, and 0.43 in recipients of UCB. (b) Probability of relapse by HSC source. The probability at 5 years was 0.50 in recipients of MSD, 0.17 in recipients of M-URD, 0.06 in recipients of MM-URD, and 0.33 in recipients of UCB.
Table 3.
Multivariate Analysis of (a) Leukemia Free Survival and (b) Relapses
| a) | ||
|---|---|---|
| Relative Risk of Relapse or Mortality (95% CI) |
P-Value | |
| Donor type | ||
| MSD* | 1.0 | |
| M-URD | 1.1 (0.5–2.5) | .89 |
| MM-URD | 3.2 (1.3–7.7) | .01 |
| UCB | 1.2 (0.6–2.4) | .68 |
| Length of first remission | ||
| <1 Year* | 1.0 | |
| 1–3 Years | 0.6 (0.3–1.1) | .07 |
| >3 Years | 0.3 (0.2–0.8) | <.01 |
| Grade II–IV acute GVHD | ||
| No* | 1.0 | |
| Yes | 0.4 (0.2–0.9) | .02 |
| b) | ||
|---|---|---|
| Relative Risk of Relapse (95% CI) |
P-Value | |
| Donor type | ||
| MSD* | 1.0 | |
| M-URD | 0.5 (0.1–1.8) | .28 |
| MM-URD | 0.5 (0.1–5.2) | .60 |
| UCB | 0.8 (0.3–2.1) | .71 |
| Length of First Remission | ||
| < 1 Year* | 1.0 | |
| 1–3 Years | 0.7 (0.3–1.8) | .42 |
| >3 Years | 0.2 (0.1–0.7) | .01 |
| Grade II–IV Acute GVHD | ||
| No* | 1.0 | |
| Yes | 0.2 (0.1–0.8) | .03 |
MSD indicates matched related sibling; M-URD, matched unrelated donor; MM-URD, mismatched unrelated donor, UCB, umbilical cord blood; CI, confidence interval, GVHD, graft-versus-host disease.
Legend: the above model is the result of multiple regression analysis on GVHD after testing the following independent variables: donor type, sex, pretransplant conditioning, GVHD prophylaxis, CMV serostatus, age, length of first remission, and type of ALL (T cell versus B cell).
Reference category.
Relapse
A total of 27 patients had disease recurrence after HCT for an overall incidence of 31% (95% CI 21%–41%) at 5 years. In univariate analysis, the probability of relapse at 5 years was 50% (95% CI 31%–71%) in those with an MSD, 17% (95% CI 0%–33%) in those with an M-URD, 6% (95% CI 0%–16%) in those with an MM-URD, and 33% (95% CI 13%–53%) after UCB transplant (P = .17) (Figure 1b). For patients with a CR1 ≥3 years, the probability of relapse at 5 years was 16% (95% CI 2%–29%) compared to 38% (95%CI 25%–51%) in those with a CR1 <3 years (P < .01). In multivariate regression analysis, the only factors that had a significant impact on the risk of relapse were the development of grade II–IV aGVHD (RR 0.2, 95% CI 0.1%–0.8%, P = .03) and having a CR1 of >3 years (RR 0.2, 95% CI 0.1%–0.7%, P = .01). Donor type did not have a significant impact on the risk of relapse (Table 3b).
Mortality
Incidence of TRM at 1 year for the entire cohort was 26% (95% CI 17%–35%). TRM was higher in those with an MM-URD (69%, 95% CI 43%–95%) compared to those with MSD (9%, 95% CI 0%–19%), M-URD (22%, 95% CI 4%–40%), and UCB grafts (24%, 95% CI 6%–42%). When examining the contributing causes of death by donor source, disease recurrence was the most frequent cause in recipients of MSD marrow (72% [n = 14]) and UCB (58% [n = 7]) compared to that in recipients of M-URD (33% [n = 3]) and MM-URD marrow (8% [n = 1]) (P < .01). aGVHD was more common in recipients of MM-URD (69% [n = 9]) compared to those who received MSD (12% [n = 2]), M-URD (38% [n = 3]), or UCB (25% [n = 3]) grafts (P = .06).
DISCUSSION
We have summarized the transplant results at a single center comparing transplant outcomes by HSC source specifically for children with ALL in CR2. Although the number of patients studied is limited, our results suggest that transplant outcomes are remarkably similar in recipients of MSD, M-URD, and UCB grafts. Specifically, there were no obvious differences in LFS, risk of relapse, and most importantly, risk of early TRM.
Our results also show that patients receiving MM-URD grafts have a higher risk of developing grades II–IV aGVHD than the MSD group. Such findings are not surprising given the association of HLA matching and GVHD [19]. Although there was a trend toward a higher incidence in the M-URD group, this did not reach statistical significance. When compared to the MSD group, UCB recipients did not differ in the risk of aGVHD despite considerable HLA disparity. Although there is a higher risk of aGVHD in recipients of MM-URD BM and a trend toward a higher risk in recipients of M-URD marrow, increased mortality from the GVHD was only observed in the MM-URD recipients. In addition, those patients who developed grades II–IV aGVHD had a significantly lower risk of relapse than those with GVHD. The overall incidence of cGVHD at 1 year posttransplant (9%) in our cohort of patients is less than what would be expected based on the literature [14,20], and we did not observe a difference in cGVHD based on HSC source. Such results are encouraging because risks of TRM and debilitating complications of cGVHD have been the principal reasons for delayed referral for URD HCT, whether from a marrow or UCB donor.
The improved outcome after alternate donor transplant for ALL in CR2 that we and others [21–23] have observed is likely secondary to better supportive care measures and unrelated donor selection strategies. In recent years, the adverse effect of HLA-C mismatching [24] and the importance of high-resolution allele level typing in unrelated donor marrow transplantation has been firmly established [25]. The prospective addition of these HLA typing strategies may even further improve outcomes seen after alternative donor HCT.
Advantages of this study include the fact that it is single-center data with homogenous patient selection criteria and supportive care measures. Consequently, though, the sample size is relatively low, resulting in diminished power. In addition, because the study period spanned a number of years, the standards for HLA matching shifted to include allele level typing and typing at HLA-C, which makes analyses based on matching more difficult.
Another limitation of this study is the absence of data on health-related quality of life (HRQL). Although it could be argued that HRQL may be poorer in recipients of unrelated donor transplants compared to that after an MSD transplant, there are few studies to date in long-term survivors specifically addressing this hypothesis. There are many reports, though, that show that the major contributor to decreased HRQL after HCT is the presence of cGVHD [14,26,27]. Although our analysis clearly demonstrates a higher incidence of aGVHD in recipients of MM-URD marrow, nearly all survivors at 2 years were off immune suppressive therapy. Furthermore, the incidence of cGVHD was only 9%, with no difference between sources. This similarity in the cumulative incidence cGVHD and prevalence of immune suppressive therapy use between groups would suggest that long-term HRQL is also likely similar. Future trials, though, should include HRQL assessments to more accurately determine if true differences exist between the groups.
One remaining question is whether a particular HSC source is optimal. Recent reports indicate similar, if not improved, LFS with HLA matched or mismatched UCB for children with acute leukemia [28]. In addition, although the mechanism is unclear, preliminary data suggest that using more than 1 UCB unit may decrease the risk of relapse in patients with acute leukemia [29]. This strategy is currently being tested in a multicenter trial where patients are randomized to receive either a single or double UCB transplant. Although there are advantages and disadvantages to each alternate donor source, timing of availability, prior infectious disease screening, and the ability to readily find a suitable donor for patients of all ethnicities are important factors to consider in transplantation for malignant diseases like acute leukemia. Compared to BM UCB offers the advantages of immediate availability and a high probability of finding a suitable match regardless of the ethnicity of the patient [28,30].
In the absence of a prospective, randomized trial that would compare 5-year LFS and HRQL in patients with ALL in CR2 treated with allogeneic HCT (MSD versus M-URD versus UCB) and conventional chemotherapy, we will have to rely on retrospective analyses from single centers or registries. Although registry data benefits from greater patient numbers, single-center results benefit from less heterogeneity in patient selection criteria and supportive care measures between groups. Given the nature of the disease and preconceived notions regarding each treatment approach, randomization is even more difficult, as exemplified by the Children’s Cancer Group study in which patients without an MSD were to be randomized to maintenance chemotherapy, autologous HCT, or alternate donor HCT (M-URD or haploidentical) [31]. Although the study design was appropriate, many enrolled patients later refused the randomization because of patient and physician preferences, thereby limiting the interpretation of the results. This study and others [21–23] suggesting similar outcomes in patients with ALL in CR2 regardless of donor source, should reassure pediatric oncologists and support the development and implementation of such a randomized trial. In the meantime, additional retrospective analyses are needed in this patient population comparing alternate donor HCT to MSD HCT in larger numbers of patients.
With our current standards of HLA typing and continued advances in supportive care measures, this study supports further investigation of the use of URD HCT in children with ALL in CR2, and suggests that the timing of HCT in these children should be based primarily on the risk of relapse with conventional chemotherapy and not on the type of donor available.
Acknowledgments
Financial disclosure: This work was in part supported by grants from the National Cancer Institute (PO1-CA65493) and the Children’s Cancer Research Fund.
REFERENCES
- 1.Harned T, Gaynon P. Relapsed acute lymphoblastic leukemia: current status and future opportunities. Curr Oncol Rep. 2008;10:453–458. doi: 10.1007/s11912-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 2.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–587. doi: 10.1111/j.1365-2141.2005.05773.x. [DOI] [PubMed] [Google Scholar]
- 3.Boulad F, Steinherz P, Reyes B, et al. Allogeneic bone marrow transplantation versus chemotherapy for the treatment of childhood acute lymphoblastic leukemia in second remission: a single-institution study. J Clin Oncol. 1999;17:197–207. doi: 10.1200/JCO.1999.17.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Lawson SE, Harrison G, Richards S, et al. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000;108:531–543. doi: 10.1046/j.1365-2141.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AJ, Horowitz MM, Pollock BH, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med. 1994;331:1253–1258. doi: 10.1056/NEJM199411103311902. [DOI] [PubMed] [Google Scholar]
- 6.Bacigalupo A, Van Lint MT, Frassoni F, et al. Allogeneic bone marrow transplantation versus chemotherapy for childhood acute lymphoblastic leukaemia in second remission. Bone Marrow Transplant. 1986;1:75–80. [PubMed] [Google Scholar]
- 7.Schroeder H, Gustafsson G, Saarinen-Pihkala UM, et al. Allogeneic bone marrow transplantation in second remission of childhood acute lymphoblastic leukemia: a population-based case control study from the Nordic countries. Bone Marrow Transplant. 1999;23:555–560. doi: 10.1038/sj.bmt.1701617. [DOI] [PubMed] [Google Scholar]
- 8.Torres A, Alvarez MA, Sanchez J, et al. Allogeneic bone marrow transplantation vs chemotherapy for the treatment of childhood acute lymphoblastic leukaemia in second complete remission (revisited 10 years on) Bone Marrow Transplant. 1999;23:1257–1260. doi: 10.1038/sj.bmt.1701802. [DOI] [PubMed] [Google Scholar]
- 9.Uderzo C, Valsecchi MG, Bacigalupo A, et al. Treatment of childhood acute lymphoblastic leukemia in second remission with allogeneic bone marrow transplantation and chemotherapy: ten-year experience of the Italian Bone Marrow Transplantation Group and the Italian Pediatric Hematology Oncology Association. J Clin Oncol. 1995;13:352–358. doi: 10.1200/JCO.1995.13.2.352. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 12.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz ME, Sullivan KM. Chronic graft-versus-host disease. Blood Rev. 2006;20:15–27. doi: 10.1016/j.blre.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Fine JPGR. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Petersdorf EW. HLA matching in allogeneic stem cell transplantation. Curr Opin Hematol. 2004;11:386–391. doi: 10.1097/01.moh.0000143701.88042.d9. [DOI] [PubMed] [Google Scholar]
- 20.Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006;13:426–435. doi: 10.1097/01.moh.0000245689.47333.ff. [DOI] [PubMed] [Google Scholar]
- 21.Al-Kasim FA, Thornley I, Rolland M, et al. Single-centre experience with allogeneic bone marrow transplantation for acute lymphoblastic leukaemia in childhood: similar survival after matched-related and matched-unrelated donor transplants. Br J Haematol. 2002;116:483–490. [PubMed] [Google Scholar]
- 22.Munoz A, Diaz-Heredia C, Diaz MA, et al. Allogeneic hemopoietic stem cell transplantation for childhood acute lymphoblastic leukemia in second complete remission-similar outcomes after matched related and unrelated donor transplant: a study of the Spanish Working Party for Blood and Marrow Transplantation in Children (Getmon) Pediatr Hematol Oncol. 2008;25:245–259. doi: 10.1080/08880010802016557. [DOI] [PubMed] [Google Scholar]
- 23.Oakhill A, Pamphilon DH, Potter MN, et al. Unrelated donor bone marrow transplantation for children with relapsed acute lymphoblastic leukaemia in second complete remission. Br J Haematol. 1996;94:574–578. doi: 10.1046/j.1365-2141.1996.d01-1834.x. [DOI] [PubMed] [Google Scholar]
- 24.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 26.Matthes-Martin S, Lamche M, Ladenstein R, et al. Organ toxicity and quality of life after allogeneic bone marrow transplantation in pediatric patients: a single centre retrospective analysis. Bone Marrow Transplant. 1999;23:1049–1053. doi: 10.1038/sj.bmt.1701754. [DOI] [PubMed] [Google Scholar]
- 27.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 29.Verneris MR, DeFor TE, et al. Risk of relapse after umbilical cord blood transplantation in patients with acute leukemia: marked reduction in recipients of two units. ASH Abstr. 2005 [Google Scholar]
- 30.Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 31.Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children’s Oncology Group study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]