Abstract
Diminished ovarian reserve (DOR) and premature ovarian failure are associated with elevated FMR1 CGG repeat alleles. We assessed pretest attitudes about potentially carrying the FMR1 premutation (FXP) (>55 CGG repeats) among reproductive age women compared with attitudes after learning their non-carrier status. Ninety-two women with DOR, regular menses and no family history of Fragile X Syndrome underwent FMR1 testing and completed attitudinal questionnaires before (T1) and 3 months after learning the test results (T2). The analysis utilized signed rank tests and α=0.05. Very few women thought they were likely to have a FXP (6.6%). More participants thought FMR1 premutations were “serious” at T2 (62.9%) than at T1 (46.1%, p<0.0003). When asked at T1 to “describe your feelings when you consider that you are potentially a carrier” of a FXP, 10% had negative feelings, 50% felt ambivalent, and 40% had positive feelings. At T2, feelings about not being a carrier were significantly more favorable (p<0.0001): negative (0%), ambivalent (6.5%), positive (93%). Corroborating prior reports, few women had a negative view of FXP, perhaps anticipating that carrying the FXP explains their infertility. Perception of the seriousness of FXP increased after learning they did not carry the FXP, which would be predicted by health belief models.
Keywords: attitudes, diminished ovarian reserve, female infertility, FMR1, fragile X, genetic counseling, genetic screening, genetic carrier
INTRODUCTION
Infertility affects approximately 9% of couples worldwide (Boivin, Bunting, Collins, & Nygren, 2007), including approximately 72 million women aged 20–44 (Boivin et al., 2007). A reduction in oocyte quantity and quality with advanced age is a normal physiologic occurrence termed diminished ovarian reserve (DOR)(Sharara, Scott, & Seifer, 1998). DOR is diagnosed in approximately 10% of women seeking fertility assistance (Levi et al., 2001; Scott & Hofmann, 1995). While the average age of female infertility due to normal ovarian aging is mid-forties, some women experience DOR much earlier and become prematurely infertile. Women with DOR have regular menstrual periods. Thus, their diagnosis is often a surprise as they believe they are fertile if they menstruate regularly (Friese, Becker, & Nachtigall, 2006).
Fragile X syndrome (FXS) is the most common heritable form of mental retardation in males. Individuals with fragile X syndrome have a CGG trinucleotide expansion in the 5′-untranslated region of the Fragile X Mental Retardation (FMR1) gene. While the normal number of CGG repeats in this gene is less than 45, individuals with FXS have an expansion of over 200 repeats, referred to as a full mutation. Repeats in the range of 55–199 are termed “premutation” and repeats in the range of 45–54 are termed “gray zone” (Kronquist, Sherman, & Spector, 2008; Maddalena et al., 2001). The likelihood of expansion of a maternal FMR1 premutation to the full mutation in offspring increases with the size of her premutation allele. If the mother has approximately 100 CGG repeats in the FMR1 gene, there is a nearly 100% chance that her child(ren) will have the full mutation (Nolin et al., 2003; Nolin et al., 2011), although this risk is moderated when the FMR1 CGG repeat is interspersed with one or more AGG’s (Nolin et al., 2013). Research since the 1990’s has provided evidence that women with a premutation allele (Karimov et al., 2011; S. L. Sherman, 2000), and potentially women with a high normal (Pastore et al., 2012; Streuli et al., 2009) or gray zone (Gleicher, Weghofer, & Barad, 2009; Karimov et al., 2011) level repeat (35–44 and 45–54 CGG repeats, respectively), have an increased risk of primary ovarian insufficiency (specifically, a diagnosis of premature ovarian failure and/or DOR). Thus, there is reproductive relevance to women from being tested for the FMR1 premutation. In fact, The American College of Medical Genetics (S. Sherman, Pletcher, & Driscoll, 2005) and the Genetics Committee of the American College of Obstetrics & Gynecology(ACOG_Committee_of_Genetics, 2010) have recommended FMR1 testing for reproductive-age women with elevated follicle stimulation hormone (FSH), which would encompass women with DOR.
A recent qualitative study of seven women with DOR who were undergoing FMR1 testing reported that most women had a sense of hope about not carrying the premutation (Pastore et al., 2013). Prior to learning their test results, many acknowledged that the results would potentially have an impact on their future reproductive decisions. For many women a positive FMR1 premutation test result would provide an explanation for their infertility and possibly a sense of closure. Some women felt that the decision for the FMR1 testing was easily made due to the benefit of a potential explanation for their fertility challenges.
There is limited research on the cognitive and emotional reactions to FMR1 premutation testing in women without a family history of FXS (Anido, Carlson, & Sherman, 2007; Anido, Carlson, Taft, & Sherman, 2005; Cizmeli, Lobel, Franasiak, & Pastore, 2013; Pastore et al., 2008). These reports demonstrated conflicting reactions amongst women (e.g., both relief and concerns) and various reproductive implications (e.g., concerns for subsequent generations). (For research related to the reaction to premutation screening among females with a positive family history, the reader is referred elsewhere (McConkie-Rosell, Spiridigliozzi, Iafolla, Tarleton, & Lachiewicz, 1997; McConkie-Rosell, Spiridigliozzi, Sullivan, Dawson, & Lachiewicz, 2000; McConkie-Rosell, Spiridigliozzi, Sullivan, Dawson, & Lachiewicz, 2001; K. Raspberry & D. Skinner, 2011; K. A. Raspberry & D. Skinner, 2011)).
Given the current recommendations that women with elevated FSH levels for their age be offered FMR1 testing, it is important to understand how women with fertility challenges perceive this genetic test. This study assessed pre-test and post-test attitudes towards FMR1 testing, including emotional reactions to potentially being a carrier of the FMR1 premutation, in a sample of women diagnosed with diminished ovarian reserve.
METHODS
Participants
This was a prospective multi-center cohort study of infertile women diagnosed with DOR who were enrolled between March 2005 – November 2012. This cohort was recruited from five sites: academic Reproductive Endocrinology and Infertility clinics in Virginia (17.4% of participants), California (35.9%), and North Carolina (14.1%), plus private fertility practices in Virginia (28.3%) and North Carolina (4.3%). This study was approved by the Institutional Review Board at all academic sites (11448 at University of Virginia, 11-1535 at University of North Carolina at Chapel Hill, 16182 at Stanford University).
Eligibility requirements included: diagnosis of DOR (cycle day 2–5 FSH > 10 mIU/mL, OR FSH > 12 mIU/mL after 5 days of 100 mg clomiphene citrate medication, OR fewer than 6 antral follicles sized 2–10 mm, OR low anti-mullerian hormone for her age as detailed below), age at DOR diagnosis ≤ 42 years, and regular menstrual cycles for the past 6 months. The antral follicle count (AFC) was used for enrollment at only one site. The anti-mullerian hormone (AMH) criteria were stratified by age as follows:
age <= 30 AMH <= 1.1 ng/mL
age 31 – 35 AMH <= 0.675 ng/mL
age 36 – 40 AMH <= 0.34 ng/mL
age 41 – 42 AMH <= 0.25 ng/mL, which was based on the nomogram of Nelson et al. (Nelson, Messow, Wallace, Fleming, & McConnachie, 2011).
The criteria for exclusion were: known cause of elevated FSH for one’s age unrelated to fragile X (e.g., surgical removal of either one or both ovaries, chemotherapy or radiation therapy, Turners Syndrome, autoimmune disease), or a family history of FXS or premutation. Knowing that FSH values can vary by assay (Scriver, Baker, Young, Behr, & Pastore, 2010; Taieb et al., 2002), de-identified samples were run at each satellite site and the primary site (University of Virginia), and adjusted accordingly to ensure consistency in the enrollment criteria across sites as described previously (Pastore et al., 2012; Scriver et al., 2010).
One hundred-three women enrolled between March 2005 – November 2012, of whom the following were excluded from this analysis: 1 because she carried the FMR1 premutation, 2 because they did not want to learn their FMR1 test results and therefore were not asked to complete the attitudinal questionnaire, and another 8 because they did not complete the 3-month follow-up questionnaire. The one carrier was excluded due to small numbers for analysis. Thus, there were 92 non-carrier women in the longitudinal dataset.
Study Procedures
After signing an informed consent, women provided a single blood sample for FMR1 trinucleotide assessment and received pretest genetic counseling. Genetic counseling sessions were conducted, in person or by telephone, by an experienced, board certified genetic counselor and followed a standardized procedure that involved a discussion about the association of FMR1 premutations and ovarian dysfunction and the mechanisms of inheritance of fragile X syndrome, including a discussion of full mutations, premutations and gray zone alleles. The clinical phenotype of fragile X syndrome was discussed in detail. A full family history was not elicited, however, specific questions were asked regarding family history of mental retardation and/or fragile X syndrome as related to the previously mentioned study exclusion criteria. Questionnaires completed at the study visit and/or medical record reviews were the source of all demographic, reproductive and family medical history variables. All materials were coded with an assigned ID to maintain anonymity.
Two questionnaires were self-administered at baseline/pretest to assess these topics: (1) infertility/reproductive history; (2) smoking history; (3) demographics; (4) attitudes regarding a fragile X premutations (McConkie-Rosell et al., 2001); and (5) the Health Orientation Scale (HOS) (Wooldridge & Murray, 1988), which measures the psychological implications of being identified as a carrier of a genetic mutation. Items 4 and 5 were only posed to the participant if she wanted to learn the genetic test results, and were repeated 3 months after communication of those results.
Statistical Analysis
All emotional reactions and opinions were measured using 9-point scales where 1 = “not at all” and 9 = “very much.” Wilcoxon signed rank nonparametric statistics (Wuensch, 2004) were used to compare any scale question where there were paired results. T-tests were used for the corresponding analyses of continuous variables (HOS). The paired comparisons were from time 1 (T1) at baseline before the test results were known and time 2 (T2), which was 3 months after learning they did not carry the FXP. The data compared included:
the HOS questionnaire results at T1 for potentially being a carrier of the FMR1 premutation (“Without knowing your test result, how would you describe your feelings at this moment when you consider that you are potentially a carrier of a fragile X premutation?”) vs. T2 reaction about not being a carrier of the FMR1 premutation using the HOS tool (“How would you describe your feelings at this moment when you think about the fact that you are NOT a carrier of a fragile X premutation? “),
attitudes regarding the seriousness of FMR1 premutations at T1 v T2 (“How serious do you think fragile X premutations are as a medical condition?”),
perception that “being a carrier of a genetic condition is considered bad by others” at T1 v T2,
perception that “others think positively about carriers of genetic conditions” at T1 v T2, and
perceived likelihood of carrying the FMR1 premutation at T1 (“How likely do you think it is that you are a carrier of a fragile X premutation?”).
Potential effect modification at T1 by race (white vs. Asian due to sample size limitations for other race/ethnicity groups) or gravidity (0 vs. 1+ prior pregnancies) or age were evaluated using Wilcoxon signed rank, Kruskal Wallis and t tests (T+, KW and t, respectively). Three categories of age at the time of study consent were created based on age below the 25th percentile (up to 35 years and 5 months), the 25th–75th percentile (between 35.5 and 41 years), and over the 75th percentile (at least 41 years and 1 month). All statistical analyses were based on two-sided tests with an alpha=0.05 using SAS software version 9.3.
RESULTS
Baseline Characteristics
Among the 92 subjects, the mean age at diagnosis of DOR was 37.0 years (range 25–42), which was, on average, 1 year younger than their age at the time of study participation (mean age 38.3 years and range of 27–49, Table I). A total of 26% of subjects were non-white and 5% were Hispanic. Eighty percent of participants were nulliparous and 41% were nulligravid.
Table I.
Demographics of study participants (N=92)
| Factor | n (%) |
|---|---|
| Age at diagnosis of DOR (years) | Mean 37.0 (sd 3.0) |
| Median 40.0 | |
| Range 25 – 42 | |
| Age at survey (years) | Mean 38.3 (sd 3.0) |
| Median 40.0 | |
| Range 27–49 | |
| Race | |
| White | 68 (74%) |
| Asian | 14 (15%) |
| Mixed race | 6 (7%) |
| Black | 4 (4%) |
| Ethnicity | |
| Non-Hispanic | 86 (95%) |
| Hispanic | 5 (5%) |
| Nulligravid | 38 (41%) |
| Nulliparous | 65 (80%)1 |
| BMI | Mean 24.8 (sd 5.1) |
| Median 22.6 | |
| Range 19.7 – 34.2 | |
DOR = Diminished Ovarian Reserve
n=81
Likelihood and Seriousness
As shown in Table II, at T1 the majority of participants were neutral (52%) or did not (42%) consider themselves likely to carry the FMR1 premutation. Stratification by gravidity indicated that women who had never been pregnant thought they were more likely to carry the FMR1 premutation than women who had been pregnant (T+=1332, p=0.04, data not shown). No differences by race/ethnicity (T+=662, p=0.23) or age category (KW χ2(2 df)=2.23, p=0.33, data not shown) were found.
Table II.
Responses to questions before (T1) and 3 months after learning the FMR1 test results (T2), N=92.
| Question | Time | “Not at all” or “disagree” | Neutral | “Very” or “agree” | p-value (T1 vs T2) |
|---|---|---|---|---|---|
| “How likely do you think it is that you are a carrier of a fragile X premutation?” | T1 | 42% | 52% | 7% | |
| “How serious do you think fragile X premutations are as a medical condition?” | T1 | 9% | 45% | 46% | <0.0003* |
| T2 | 3% | 34% | 63% | ||
| “Overall, being a carrier of a genetic condition is considered bad by others” | T1 | 34% | 30% | 36% | <0.03 |
| T2 | 21% | 33% | 46% | ||
| “In general, others think positively about carriers of genetic conditions” | T1 | 48% | 39% | 12% | >0.18 |
| T2 | 40% | 47% | 13% |
Responses to these questions were obtained by a 1–9 numerical scale, where responses 1–3 correspond to “not at all” or “disagree,” responses 4–6 correspond to “neutral,” and responses 7–9 correspond to “very” or “agree.”
At T1 46% regarded carrying the FMR1 premutation as a serious condition while 45% had a neutral position regarding the seriousness of carrying the premutation (Table II). More participants (63%) considered this a serious condition at T2 than T1 (Signed rank S=531, p<0.003). Stratification by gravidity indicated that women who had never been pregnant were more likely to consider FMR1 premutations to be a serious condition (T+=1915, p=0.005). No differences by race/ethnicity (T+=525, p=0.60) or age (KW χ2(2 df)=0.90, p=0.64, data not shown) were found.
Perception of Genetic Carriers
When asked about other people’s perceptions of genetic carriers pre- and post FMR1 genetic testing, answers did not vary greatly (Table II). Initially 36% of participants thought being a carrier of a genetic condition was considered bad by others. The same response was obtained by 46% of subjects at T2 (Signed rank S=331, p<0.03). More individuals answered “Not at all” to this question at T1 (34%) than T2 (21%). At baseline 48% of participants thought that others do not think positively of carriers for genetic conditions while 40% gave the same answer after receiving their test results (Signed rank S=161, p>0.18). The majority of survey responders at T2 (47%) were neutral regarding how positively others think about carriers of genetic mutations, which was higher than at T1 (39%). None of the T1 responses were significantly different when stratified by gravidity or race or age (data not shown, p>0.07).
Health Orientation Scale
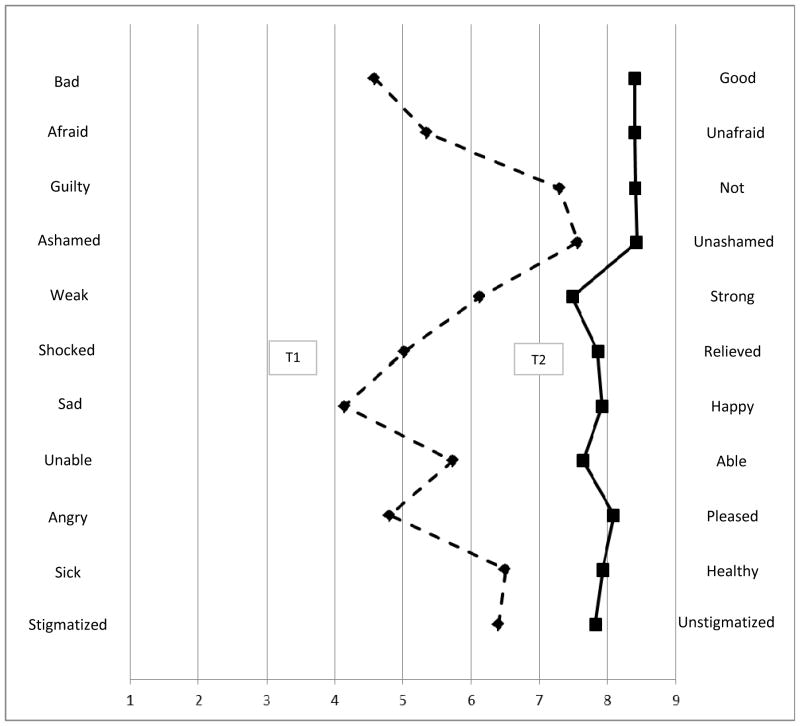
Participants had overall significantly different perspectives about carrying the FMR1 gene premutation at T1 and T2. Mean scores on each component of the Health Orientation Scale (Figure 1) were consistently higher at T2 when compared to responses at T1 (p≤0.0002 for each subscale). Mean scores at T1 ranged from a low of 4.1 on the sad/happy subscale up to 7.6 on the ashamed/unashamed subscale). In contrast, at T2 the mean scores ranged from a low of 7.5 on the weak/strong subscale up to 8.4 on four subscales: bad/good, afraid/unafraid, guilty/not guilty, and ashamed/unashamed. Using the terminology of the original scale authors (Wooldridge & Murray, 1988), the summary HOS measure at T1 indicated that 0% reacted very unfavorably, 9.8% unfavorably, 50.0% ambivalently, 33.7% favorably, and 6.5% very favorably at T1 to “describe their feelings when you consider that you are potentially a carrier of a fragile X premutation.” At T2, their reaction about not carrying the FMR1 premutation was ambivalent (6.5%), favorable (35.9%), or very favorable (57.6%), which was significantly more positive than at T1 (t=13.01, p<0.001). There were no differences in the total HOS score when stratified by gravidity (equal variances t(87 df)=−0.32, p=0.75) or race/ethnicity (unequal variances t(23 df)=0.85, p=0.40). At T1, older women had higher HOS scores (i.e., felt more positive) than younger women (KW χ2(2 df)=15.52, p=0.0004), although the mean scores were fairly close (3.1 for the youngest quarter of participants, 3.2 for the middle half, 3.9 for the oldest quarter).
Figure 1.
Comparison between responses to Health Orientation Scale prior to (dashed line, Time 1) and 3 months after (solid line, Time 2) genetic testing for the FMR1 gene (n=92 women).
DISCUSSION
Overall, the women in our study diagnosed with DOR initially perceived fragile X premutations as serious. In comparison to other populations, our participants viewed FXP as serious as women with a family history of fragile X syndrome (pretest mean= 6.2 on our 1–9 scale vs. pretest mean= 6.9 on a 0–10 visual analog scale, respectively)(McConkie-Rosell et al., 2001). This contrasts with a prior report of women with DOR (N=20) where the mean score for seriousness was 5.4 (Pastore et al., 2008). Among women with DOR, attitudes regarding the seriousness of carrying the FXP increased after learning they were not carriers, which would be predicted by health belief models (Champion, 1999) and may be related to their individual expectations of being a non-carrier. Significantly more participants considered FXP to be a serious condition at T2 than T1, which was also true among non-carriers from FXS families undergoing FXP testing (McConkie-Rosell et al., 2001). This potentially indicates that some women may perceive carrying the FXP as more grave once they know definitely that they are not at risk. Additionally, a woman may downplay her perception of the seriousness of FXP when she thinks she is still at risk for carrying the FXP. Qualitative interviews reported previously (Pastore et al., 2013) with a subset of this study population, also support a change in attitude over time towards fragile X, in that the relief expressed at not carrying the FXP was much greater than the concern expressed about carrying the FXP before their test results were known.
Only 6% of the participants thought it was likely that they carried the FXP. This percentage coincidentally reflects the estimated prevalence expected among women with premature ovarian failure without a family history of FXS (Gersak, Meden-Vrtovec, & Peterlin, 2003; Uzielli et al., 1999). Pre-enrollment educational material provided to all participants included a summary of the relevant science; therefore they were informed prior to testing that most participants would not carry the FXP.
Corroborating prior reports (Pastore et al., 2008), few women viewed potentially carrying the FXP as unfavorable, perhaps in anticipation that carrying the FXP would explain their DOR. Our qualitative research indicated that women view a positive genetic result as a plausible explanation for their fertility challenges (Pastore et al., 2013). The strongest pretest negative emotion was sadness at potentially carrying the FXP. All subscale means were lower than our earlier report with 20 participants (subset of the current cohort) (Pastore et al., 2008). The current study group was larger and from multiple locations, thus it is likely that this report more accurately captured the thoughts and feelings of women with DOR.
There is limited research on emotional reactions to and impact on reproductive decision making from carrier testing for x-linked disorders. Carrier testing for autosomal and x-linked-recessive disorders has been associated with relief from fear and is useful in reproductive planning (Fanos & Johnson, 1993; Pastore et al., 2013; Williams & Schutte, 1997). Relief from fear was reported whether they already had children (afraid they had put their children at risk to pass on the disease to grandchildren) or not (had feared having a baby with the disorder) (Williams & Schutte, 1997). A study of population screening for FMR1 premutations (Anido et al., 2005),(Anido et al., 2007) found that while women had active coping mechanisms, they also had concerns for the implications of their carrier status for their children or grandchildren, the results impacted reproductive decisions whether in hindsight or for their own future, and the women’s positive carrier status generally had minimal relevance unless it pertained to a current stage of life.
Study Strengths and Limitations and Research Recommendations
This research provides important information relevant to current medical society recommendations to screen women with elevated FSH levels for FXP. This publication adds to the scant literature on genetic testing among women with DOR. This sample consisted of non-carriers only, thus the T2 results should not be extrapolated to carriers. However, given that most women affected by the recommendations for FMR1 testing will have negative test results, it is important to understand how these women feel about receiving a potentially positive test result when they initially make the decision to undergo testing. Our observations are limited by the patient population of fertility clinics, who are generally in higher socioeconomic groups (Jain, 2006), and further limited to women diagnosed with DOR. No statistical correction was made for multiple comparisons, thus there is the possibility that some of our findings are due to chance. Lastly, our sample only included women who chose to be tested, thus they may have been more psychologically prepared to effectively cope with the results. Future research on the unique genetic counseling needs and pretest educational needs of women outside of FXS families undergoing FMR1 testing in fertility clinics would be very beneficial, especially as the use of genetic testing expands in clinical practice.
Practice Implications
The significance of the FMR1 premutation testing for individual women who are still seeking conception with their own ovum cannot be understated. While premutation carriers do not have FXS, they can pass the full mutation to their children. Provision of information regarding the chance of having a son with fragile X syndrome, or a daughter with the full mutation or a premutation, would likely have a significant impact on reproductive decisions made by women with diminished ovarian reserve.
We anticipated that women with fertility difficulties would react very differently to this genetic testing process than women with a relative with FXS, where the impact of the full mutation is readily apparent. Women seek fertility assistance because they want to become pregnant, and are unlikely to anticipate that their conception challenges are related to a genetic condition. Our findings could be interpreted to indicate that these women under endorse their concerns or fears prior to testing, and may endorse stronger emotions regarding FMR1 after discovering that they do not carry the premutation. Alternatively, the findings could reflect that these women were creating meaning around the test results and using their experience and knowledge acquired as they participated in the study over time in their perception of FMR1. With either interpretation, this study provides valuable insights to clinicians and genetic counselors regarding FMR1 premutation testing among fertility clinic patients.
The interpretation of current FMR1 testing recommendations varies in clinical practice. Clinics affiliated with our research study vary widely in their practices from testing only those with a family history suggestive of FXS, to routine screening of all women undergoing IVF regardless of the fertility diagnosis. As not all fertility clinics have genetic counseling services, it may be safe to assume that not all women being screened for fragile X premutations in this population receive counseling as part of the testing process. Recommendations from the National Society of Genetic Counselors call for pre- and post-test genetic counseling for centers engaged in population screening for FMR1 (Finucane et al., 2012). The European Society of Human Reproduction and Embryology (ESHRE) recommends genetic counseling if the results are positive, but does not support pre-test counseling with a trained genetic counselor (Harper et al., 2013). Counseling concerns, such as the scope of family history queries needed for this gene due to disparate phenotypes (Finucane et al., 2012), complexity of information to relay to the patient given disparate potential phenotypes(Finucane et al., 2012; Harper et al., 2013; McConkie-Rosell et al., 2007), uncertainty regarding the interpretation of intermediate alleles given inconsistent research findings (Finucane et al., 2012), and discussions with parents about communicating risk to their children (McConkie-Rosell, Spiridigliozzi, Sullivan, Dawson, & Lachiewicz, 2002) are reviewed elsewhere in these and other publications.
In summary, current recommendations now endorse screening women with elevated FSH levels for fragile X premutations, and most women affected by those recommendations will have negative test results. Our results suggest that clinicians providing this testing can expect mixed reactions to testing, both prior to and following negative test results. A significant portion of women who request FMR1 testing may perceive FMR1 premutations as serious before testing, however, many will feel ambivalent or have positive feelings about potentially being a carrier. In contrast, perception of the seriousness of FXP may increase after learning that they have a negative result. Furthermore, FMR1 screening in a population of women with infertility issues involves complex, and somewhat unique genetic counseling considerations. In women with DOR, a positive test result may provide closure or a “reason” for fertility challenges, thus these women may have different attitudes towards this test than the majority of women having routine screening for FMR1 premutations who can readily conceive. Our findings regarding the attitudes of women with diminished ovarian reserve towards testing for fragile x premutations can inform health care providers in fertility clinics about likely perceptions to testing in this unique population.
Acknowledgments
We thank the participants in this study, the study co-investigators (Dr. Steven Young, University of North Carolina at Chapel Hill; Dr. Christopher D. Williams, Reproductive Medicine and Surgery Center of Virginia; Dr. Valerie Baker, Stanford University; Dr. Lawrence Silverman and Dr. Ani Manichaikhul, University of Virginia; Dr. Joel Finkelstein, Massachusetts General Hospital), and Dr. Kelly Gurka for analytic assistance. We also thank the clinical research coordinators at all participating clinics: Parchayi Dalal, Hannah Spencer, Amy Brown, Amanda DeSmit, Angie Morey, Rebecca Briggs, and Janetta Phillips. This work was supported by the Eunice K. Shriver National Center for Child Health and Human Development at the National Institutes of Health (grants R03HD052768, R21HD057485 and R01HD068440 to LMP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Location of study: Charlottesville, VA; Chapel Hill, NC; Stanford, CA
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- ACOG_Committee_of_Genetics. Carrier Screening for Fragile X Syndrome. Obstetrics & Gynecology. 2010;116(4):1008–1010. doi: 10.1097/AOG.0b013e3181fae884. [DOI] [PubMed] [Google Scholar]
- Anido A, Carlson LM, Sherman SL. Attitudes toward fragile X mutation carrier testing from women identified in a general population survey. Journal of Genetic Counseling. 2007;16(1):97–104. doi: 10.1007/s10897-006-9049-0. [DOI] [PubMed] [Google Scholar]
- Anido A, Carlson LM, Taft L, Sherman S. Women’s attitudes toward testing for fragile X carrier status: a qualitative analysis. Journal of Genetic Counseling. 2005;14(4):295–306. doi: 10.1007/s10897-005-1159-6. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human Reproduction. 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Champion V. Revised Susceptibility, Benefits, and Barriers Scale for Mammography Screening. Research in Nursing & Health. 1999;22:341–348. doi: 10.1002/(sici)1098-240x(199908)22:4<341::aid-nur8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cizmeli C, Lobel M, Franasiak J, Pastore LM. Levels and associations among self-esteem, fertility distress, coping, and reaction to potentially being a genetic carrier in women with diminished ovarian reserve. Fertility and Sterility. 2013 doi: 10.1016/j.fertnstert.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanos JH, Johnson J. Barriers to carrier testing for CF siblings. American Journal of Human Genetics. 1993;53(3, Suppl):51. [Google Scholar]
- Finucane B, Abrams L, Cronister A, Archibald A, Bennett R, McConkie-Rosell A. Genetic counseling and testing for FMR1 gene mutations: Practice guidelines of the National Society of Genetic Counselors. Journal of Genetic Counseling. 2012 doi: 10.1007/s10897-012-9524-8. [DOI] [PubMed] [Google Scholar]
- Friese C, Becker G, Nachtigall RD. Rethinking the biological clock: eleventh-hour moms, miracle moms and meanings of age-related infertility. Social Science & Medicine. 2006;63(6):1550–1560. doi: 10.1016/j.socscimed.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Gersak K, Meden-Vrtovec H, Peterlin B. Fragile X premutation in women with sporadic premature ovarian failure in Slovenia. Human Reproduction. 2003;18(8):1637–1640. doi: 10.1093/humrep/deg327. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Müllerian hormone. Fertility and Sterility. 2009;91(5):1700–1706. doi: 10.1016/j.fertnstert.2008.01.098. [DOI] [PubMed] [Google Scholar]
- Harper JC, Geraedts J, Borry P, Cornel MC, Dondorp W, Gianaroli L, … Macek M., Jr Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. European Society of Human Genetics and European Society of Human Reproduction and Embryology. European Journal of Human Genetics. 2013;21(Suppl 2):S1–21. doi: 10.1038/ejhg.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain T. Socioeconomic and racial disparities among infertility patients seeking care. Fertility and Sterility. 2006;85(4):876–881. doi: 10.1016/j.fertnstert.2005.07.1338. doi: http://dx.doi.org/10.1016/j.fertnstert.2005.07.1338. [DOI] [PubMed] [Google Scholar]
- Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, … Welt CK. Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Human Reproduction. 2011;26(8):2077–2083. doi: 10.1093/humrep/der168. [DOI] [PubMed] [Google Scholar]
- Kronquist KE, Sherman SL, Spector EB. Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genetics in Medicine. 2008;10(11):845–847. doi: 10.1097/GIM.0b013e31818b0c8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT., Jr Reproductive outcome in patients with diminished ovarian reserve. Fertility & Sterility. 2001;76(4):666–669. doi: 10.1016/s0015-0282(01)02017-9. [DOI] [PubMed] [Google Scholar]
- Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, … Wolff DJ. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genetics in Medicine. 2001;3(3):200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkie-Rosell A, Abrams L, Finucane B, Cronister A, Gane LW, Coffey SM, … Hagerman RJ. Recommendations from Multi-disciplinary Focus Groups on Cascade Testing and Genetic Counseling for Fragile X-associated Disorders. Journal of Genetic Counseling. 2007;16(5):593–606. doi: 10.1007/s10897-007-9099-y. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Spiridigliozzi GA, Iafolla T, Tarleton J, Lachiewicz AM. Carrier testing in the fragile X syndrome: attitudes and opinions of obligate carriers. American Journal of Medical Genetics. 1997;68(1):62–69. doi: 10.1002/(sici)1096-8628(19970110)68:1<62::aid-ajmg12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Spiridigliozzi GA, Sullivan JA, Dawson DV, Lachiewicz AM. Carrier testing in fragile X syndrome: effect on self-concept. American Journal of Medical Genetics. 2000;92(5):336–342. doi: 10.1002/1096-8628(20000619)92:5<336::aid-ajmg8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Spiridigliozzi GA, Sullivan JA, Dawson DV, Lachiewicz AM. Longitudinal study of the carrier testing process for Fragile X Syndrome: perceptions and coping. American Journal of Medical Genetics. 2001;98:37–45. [PubMed] [Google Scholar]
- McConkie-Rosell A, Spiridigliozzi GA, Sullivan JA, Dawson DV, Lachiewicz AM. Carrier testing in fragile X syndrome: when to tell and test. American Journal of Medical Genetics. 2002;110(1):36–44. doi: 10.1002/ajmg.10396. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Messow MC, Wallace AM, Fleming R, McConnachie A. Nomogram for the decline in serum antimüllerian hormone: a population study of 9,601 infertility patients. Fertility and Sterility. 2011;95(2):736–741. e733. doi: 10.1016/j.fertnstert.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Nolin SL, Brown WT, Glicksman A, Houck GE, Jr, Gargano AD, Sullivan A, … Sherman SL. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. American Journal of Human Genetics. 2003;72(2):454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin SL, Glicksman A, Ding X, Ersalesi N, Brown WT, Sherman SL, Dobkin C. Fragile X analysis of 1112 prenatal samples from 1991 to 2010. Prenatal Diagnosis. 2011;31(10):925–931. doi: 10.1002/pd.2815. [DOI] [PubMed] [Google Scholar]
- Nolin SL, Sah S, Glicksman A, Sherman SL, Allen E, Berry-Kravis E, … Hadd AG. Fragile X AGG analysis provides new risk predictions for 45–69 repeat alleles. American Journal of Medical Genetics Part A. 2013;161(4):771–778. doi: 10.1002/ajmg.a.35833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore LM, Karns LB, Ventura K, Clark ML, Steeves RH, Callanan NP. Longitudinal Interviews of Couples Diagnosed with Diminished Ovarian Reserve Undergoing Fragile X Premutation Testing. Journal of Genetic Counseling. 2013 doi: 10.1007/s10897-013-9616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore LM, Morris WL, Karns LB, Pastore LM, Morris WL, Karns LB. Emotional reaction to fragile X premutation carrier tests among infertile women. Journal of Genetic Counseling. 2008;17(1):84–91. doi: 10.1007/s10897-007-9129-9. [DOI] [PubMed] [Google Scholar]
- Pastore LM, Young SL, Baker VM, Karns LB, Williams CD, Silverman LM. Elevated Prevalence of 35–44 FMR1 Trinucleotide Repeats in Women with Diminished Ovarian Reserve. Reproductive Sciences. 2012;19(11):1226–1231. doi: 10.1177/1933719112446074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspberry K, Skinner D. Enacting genetic responsibility: experiences of mothers who carry the fragile X gene. Sociology of Health & Illness. 2011;33(3):420–433. doi: 10.1111/j.1467-9566.2010.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspberry KA, Skinner D. Negotiating desires and options: how mothers who carry the fragile X gene experience reproductive decisions. Social Science & Medicine. 2011;72(6):992–998. doi: 10.1016/j.socscimed.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RT, Jr, Hofmann GE. Prognostic assessment of ovarian reserve. Fertility & Sterility. 1995;63(1):1–11. [PubMed] [Google Scholar]
- Scriver J, Baker V, Young S, Behr B, Pastore L. Inter-laboratory validation of the measurement of follicle stimulating hormone (FSH) after various lengths of frozen storage. Reproductive Biology and Endocrinology. 2010;8(1):145. doi: 10.1186/1477-7827-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharara FI, Scott JRT, Seifer DB. The detection of diminished ovarian reserve in infertile women. American Journal of Obstetrics and Gynecology. 1998;179(3):804–812. doi: 10.1016/s0002-9378(98)70087-0. [DOI] [PubMed] [Google Scholar]
- Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genetics in Medicine. 2005;7(8):584–587. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL. Premature ovarian failure in the fragile X syndrome. American Journal of Medical Genetics. 2000;97:189–194. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Streuli I, Fraisse T, Ibecheole V, Moix I, Morris MA, de Ziegler D. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertility and Sterility. 2009;92(2):464–470. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Taieb J, Olivennes F, Birr AS, Benattar C, Righini C, Frydman R, Lindenbaum A. Comparison of day 3 FSH serum values as determined by six different immunoassays. Human Reproduction. 2002;17(4):926–928. doi: 10.1093/humrep/17.4.926. [DOI] [PubMed] [Google Scholar]
- Uzielli ML, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, … Sereni A. Premature ovarian failure (POF) and fragile X premutation females: from POF to to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. American Journal of Medical Genetics. 1999;84(3):300–303. [PubMed] [Google Scholar]
- Williams JK, Schutte DL. Benefits and burdens of genetic carrier identification. Western Journal of Nursing Research. 1997;19(1):71–82. doi: 10.1177/019394599701900105. [DOI] [PubMed] [Google Scholar]
- Wooldridge EQ, Murray RFJ. The Health Orientation Scale: a measure of feelings about sickle cell trait. Social Biology. 1988;35(1–2):126–136. doi: 10.1080/19485565.1988.9988694. [DOI] [PubMed] [Google Scholar]
- Wuensch KL. Nonparametric Statistics. 2004 Retrieved 01-12-2005, from //core.ecu.edu/psyc/wuenschk/StatsLessons.htm.