Abstract
Purpose
To develop and evaluate an antibiotic-eluting suture for ophthalmic surgery.
Methods
Wet electrospinning was used to manufacture sutures composed of poly(L-lactide), polyethylene glycol (PEG), and levofloxacin. Size, morphology, and mechanical strength were evaluated via scanning electron microscopy and tensile strength, respectively. In vitro drug release was quantified using high performance liquid chromatography. In vitro suture activity against Staphylococcus epidermidis was investigated through bacterial inhibition studies. Biocompatibility was determined via histological analysis of tissue sections surrounding sutures implanted into Sprague-Dawley rat corneas.
Results
Sutures manufactured via wet electrospinning were 45.1 ± 7.7 μm in diameter and 0.099 ± 0.007 newtons (N) in breaking strength. The antibiotic release profile demonstrated a burst followed by sustained release for greater than 60 days. Increasing PEG in the polymer formulation, from 1% to 4% by weight, improved drug release without negatively affecting tensile strength. Sutures maintained a bacterial zone of inhibition for at least 1 week in vitro and elicited an in vivo tissue reaction comparable to a nylon suture.
Conclusions
There is a need for local, postoperative delivery of antibiotics following ophthalmic procedures. Wet electrospinning provides a suitable platform for the development of sutures that meet size requirements for ophthalmic surgery and are capable of sustained drug release; however, tensile strength must be improved prior to clinical use.
Translational Relevance
No antibiotic-eluting suture exists for ophthalmic surgery. A biocompatible, high strength suture capable of sustained antibiotic release could prevent ocular infection and preclude compliance issues with topical eye drops.
Keywords: ocular, drug delivery, medical device, biodegradable, levofloxacin
Introduction
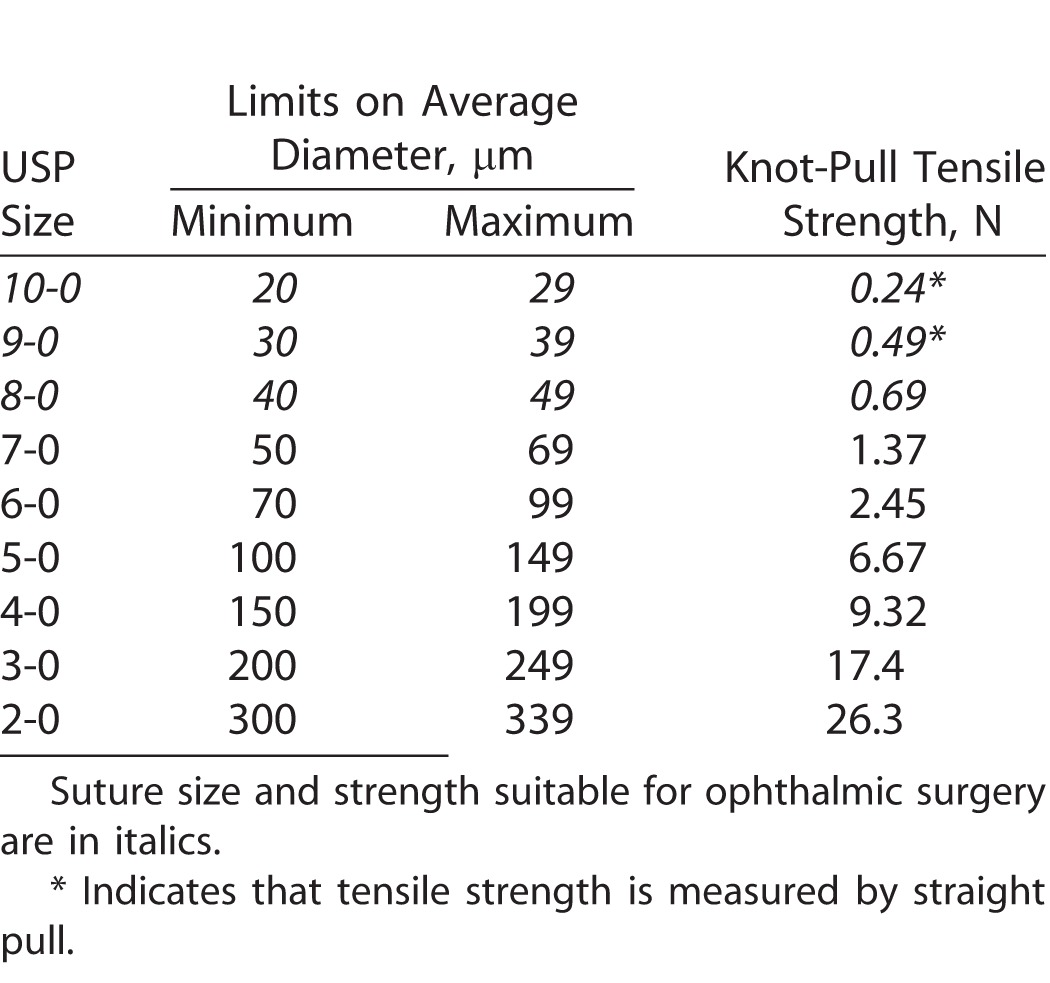
Interest in the development of drug-eluting sutures for a variety of clinical applications has grown over the past decade.1–3 Drug-eluting sutures may prevent complications and/or serve in a therapeutic role while simultaneously closing wounds and holding tissue together.4 Sutures are already a part of the surgical workflow, and this next generation of sutures could provide for additional functionality through local and sustained drug release.1 However, attempts to develop drug-eluting sutures have been limited by lack of sufficient tensile strength, sustained drug release, or scale needed for commercial viability.1,3,5–15 Furthermore, drug-eluting sutures thin enough to be used in ophthalmic surgery have not been described in the literature (Table), where, globally, more than 12 million procedures per year use nylon sutures to close ocular wounds and incisions.16–20 In 2002, Ethicon received approval to market a series of sutures coated with an antibiotic agent; however, none are indicated for ophthalmic use and, to date, there are no market offerings for antibiotic-eluting sutures for ocular surgery.21–24
Table.
United State Pharmacopeia (USP) Specifications for Absorbable Sutures.
Although infections of the eye, such as bacterial keratitis and endophthalmitis, are rare, they can lead to significant negative consequences, including corneal ulceration, edema, inflammation, and blindness.25 It has long been reported that the placement of foreign material into the body reduces the inoculum size of bacteria required for infection, and that the conventional nylon sutures used in ocular procedures can harbor bacteria and potentially facilitate infection.26,27 This phenomenon is further exacerbated when sutures become loose or break in situ. Heaven and coworkers28 reported that almost 40% of loose or broken nylon corneal sutures were contaminated with bacteria, and Staphylococcus epidermidis was isolated in more than 80% of cases. For this reason, it has become routine to prescribe expensive antibiotic drops off-label for prophylactic use after ophthalmic surgery; however, topical eye drops are associated with low patient compliance.29 Additionally, properly instilling eye drops is particularly difficult for pediatric patients and for those who are elderly and/or in cognitive decline.30,31 A potential alternative to frequent topical application would be to supply antibiotics directly from the surgical suture itself. For this purpose, the suture must: (1) be of suitable size, (2) be of high-strength to resist breakage and bacterial colonization, and (3) provide sustained antibiotic delivery. Such a suture might prevent ocular infections while providing convenience for both the patient and the surgeon.
Here, we describe the development of an absorbable suture loaded with levofloxacin, a third-generation fluoroquinolone and broad spectrum antibiotic used to treat ocular infection.32,33 The suture was manufactured via electrospinning, a simple technique first introduced in the early 1900s that employs electric forces to elongate and simultaneously decrease the diameter of a viscoelastic polymer stream, allowing for the formation of solid fibers ranging from nanometers to microns in diameter.34,35 Electrospinning provides a scalable and versatile platform, allowing for the incorporation of almost any polymer, such as the poly(L-lactide) (PLLA) and polyethylene glycol (PEG) used in this work. Both of these polymers are generally regarded as safe and have been used in medical devices approved by the United States Food and Drug Administration.36 We investigate the size, strength, drug release, bacterial inhibition, and biocompatibility of this new platform.
Methods
Microfiber Suture Fabrication
Levofloxacin microfiber sutures were manufactured using the wet electrospinning setup depicted in Figure 1A and described by Zhang and coworkers.37 Briefly, PLLA (221 kDa; Corbion, Amsterdam, Netherlands) at 86% to 89% (wt/wt) was mixed with levofloxacin (Sigma Aldrich, St. Louis, MO) at 10 wt% and either PEG (35 kDa; Sigma Aldrich) or Pluronic F127 (BASF, Florham Park, NJ) between 1 and 4 wt% and dissolved in chloroform (Sigma Aldrich) at room temperature for 24 hours. Levofloxacin concentration was held constant and PLLA concentration in chloroform was maintained at 15 wt% in all formulations. Sutures were produced by wet electrospinning the polymer/drug solution in a setup consisting of a high voltage power supply (Gamma High Voltage Research, Ormond Beach, FL), syringe pump (Fisher Scientific, Waltham, MA), and rotating metal collector with hexane (Sigma Aldrich) as the lending solvent. The polymer solution was ejected through a blunted 18-G needle (Fisher Scientific) at 13 mL/h with 4.7 kV of applied voltage 5 cm away from the collector rotating at 40 rpm. Fibers were then collected and desiccated for 2 days prior to storage at −20°C.
Figure 1.
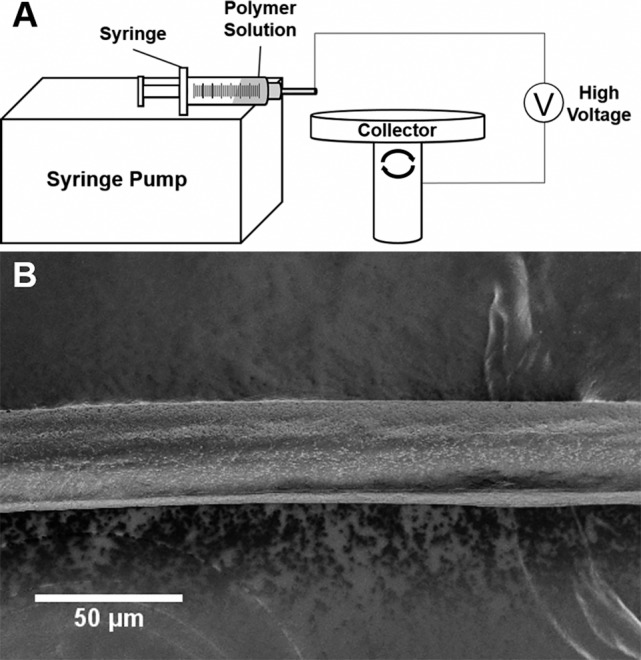
Electrospinning setup and microfiber suture. (A) Schematic of wet electrospinning setup. Voltage is applied to the polymer or polymer/drug solution that is driven into a rotating, grounded collector containing hexane. This provides for the manufacture of (B) long, uniform, defect-free, and drug-eluting monofilament sutures as shown via SEM.
Suture Characterization
Suture Size and Morphology
Sutures were serially dehydrated in ethanol (Sigma Aldrich) and dried prior to sputter coating with 10 nm of Au/Pd. Samples were then imaged via scanning electron microscopy (SEM) at 1 to 2 kV using a LEO Field Emission SEM (Zeiss, Oberkochen, Germany) and suture diameter measured using ImageJ software (n = 14 for each condition; http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD).
Tensile Strength Measurement
Mechanical properties of the sutures were evaluated using a DMA 6800 (TA Instruments, Timonium, MD). Three-centimeter long samples (n = 7 for each condition) were clamped vertically and force from a 5-newton (N) load cell was applied at 0.05 N/min to stretch the sample until breaking.
In Vitro Drug Release
Ten milligrams of suture (n = 3) was placed into 10 mL of 1× Dulbecco's phosphate buffered saline (PBS; ATCC, Manassas, VA) rotating at 37°C. At each time-point, 2-mL aliquots were withdrawn and replaced with fresh PBS. Aliquots were frozen, lyophilized, and resuspended in ultrapure water prior to high performance liquid chromatography (HPLC; Waters Corporation, Milford, MA) analysis. One hundred-microliter samples were injected into a Waters Symmetry 300 C18 5-μm column with a mobile phase of 0.1% vol/vol trifluoroacetic acid (Sigma Aldrich) in water:acetonitrile (75:25 vol/vol, Fisher Scientific) at a flow rate 1 mL/min. Elution was monitored by a 2998 photodiode array detector to detect levofloxacin with excitation at 290 nm and emission at 502 nm. Drug loading was determined by dissolving a 5-mg sample of suture into a mixture of tetrahydrofuran (Sigma Aldrich):acetonitrile (20:80) and injecting into the column under the same conditions as the release samples.
Assessment of Bacterial Inhibition
One-centimeter of suture was placed in 1 mL of PBS and incubated at 37°C for 1, 3, and 6 hours and 1, 2, 3, 4, 5, 6, and 7 days (n = 6 for each time-point). S. epidermidis (ATCC) was cultured overnight at 37°C on agar plates produced using nutrient agar (BD, Franklin Lakes, NJ). At each time-point, sutures were retrieved and placed on plated cultures in order to investigate bacterial inhibition. Bacterial inhibition zones around the sutures were measured and imaged 24 hours after suture placement.
Assessment of In Vivo Biocompatibility
Animals were cared for and experiments conducted in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University. Protocols are also in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. One millimeter of 8-0 Ethilon (nylon), Vicryl (poly(lactic-co-glycolic acid); PLGA) (Ethicon, Somerville, NJ) and 4% PEG/PLLA/levofloxacin sutures (n = 3) were implanted into the corneas of 6- to 8-week old, male Sprague-Dawley rats (Harlan Laboratories, Frederick, MD). Prior to implantation, rats were intraperitoneally anesthetized with a solution of Ketamine:Xylazine (75:5 mg/kg, Sigma Aldrich), and a drop of 0.5% proparacaine hydrochloride ophthalmic solution (Bausch & Lomb Inc., Tampa, FL) was applied to the cornea. Following implantation, the rats were evaluated for signs of infection every day for 7 days. The rats were then euthanized and eyes enucleated, fixed in formalin (Sigma Aldrich) for 24 hours, embedded in paraffin, cross-sectioned, and stained with hematoxylin and eosin for histological evaluation.
Statistical Analysis
Suture size, strength, and in vitro drug release are presented as mean ± standard error. Statistical significance was determined via one-way analysis of the variance (ANOVA) followed by Tukey test.
Results
Suture Fabrication and Characterization
Several different parameters, including needle gauge, flow rate, applied voltage, distance to collector, lending solvent, and collector rotation speed were optimized in order to manufacture sutures from a wet electrospinning setup described previously (Fig. 1A).37 Electrospinning of a 10 wt% polymer solution with application of 4.7 kV into a collector containing hexane and rotating at 40 rpm allowed for manufacture of a single, uniform, defect-free, cylindrical filament without beading, necking, or pores (Fig. 1B), which might adversely affect tensile strength and reproducibility. Microfibers manufactured with a collector speed of 40 rpm were thinner than those manufactured at lower speeds, and were more uniform in diameter than those manufactured at higher speeds where there was also significant fiber loss at the edge of the collector. PLLA and levofloxacin served as the core suture components, and the concentration of drug was held constant in the various formulations tested; however, the addition of F127 or different concentrations of PEG modified several suture properties. Four percent PEG along with the use of blunted 18-G needles and a flow rate of 13 mL/h provided for sutures 45.1 ± 7.7 μm in diameter (Fig. 2A). This qualifies as an 8-0 suture suitable for use in ophthalmic surgery, and would be stronger than 9-0 or 10-0 sized sutures manufactured from this setup. Under these conditions, it was possible to produce meters of suture material at a time.
Figure 2.
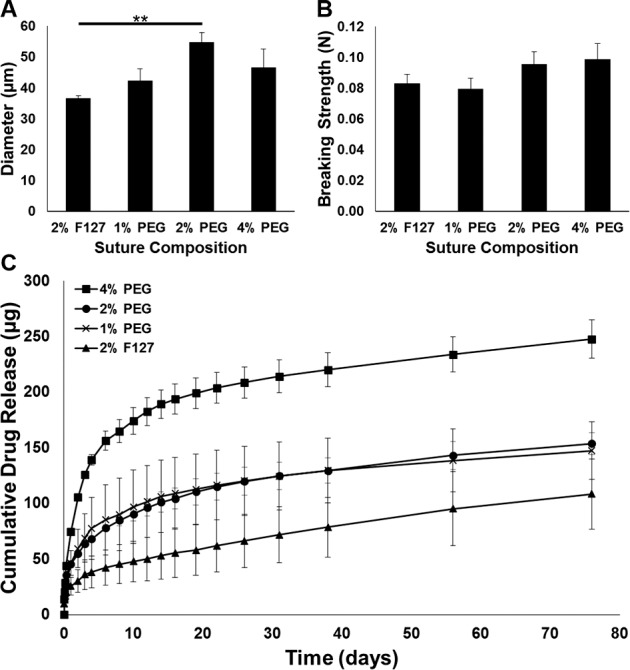
Suture diameter, tensile strength, and in vitro drug release. Monofilament suture (A) diameter, (B) breaking strength, and (C) in vitro drug release with the addition of different polymers to the core PLLA/levofloxacin formulation. Two percent F127, 1% PEG, and 4% PEG suture diameters are suitable for ophthalmic use. **P < 0.01. All formulations provide for sutures of similar strength. All formulations demonstrate sustained release of levofloxacin, while the 4% PEG formulation provides for a more rapid initial and greater overall release.
Following suture manufacture, fibers were desiccated and stored at −20°C preceding use in additional experiments. Prior to tensile testing, sutures were allowed to fully thaw and were cut into 3-cm segments. Tensile strength evaluation determined that the 4% PEG/PLLA/levofloxacin formulation also provided the highest breaking strength of all formulations tested at 0.099 ± 0.007 N. As the concentration of PEG increased from 1% to 4%, by weight, so did the average strength of the sutures (Fig. 2B), although it was not statistically significant.
In Vitro Levofloxacin Release
Preliminary studies indicated minimal drug release from PLLA/levofloxacin sutures manufactured via electrospinning. However, the addition of small percentages of F127 and PEG polymers to the formulation resulted in significant and sustained release of levofloxacin in vitro (Fig. 2C). Regardless of the addition to the core polymer formulation, all modified suture formulations demonstrated initial burst release in the first 48 hours followed by a slow, sustained, and linear release prior to ultimately reaching a plateau. The 4% PEG/PLLA/levofloxacin suture demonstrated the most significant burst release and also the highest cumulative release of all formulations tested. This suture formulation was found to have 4% drug loading and levofloxacin was detected in release media after more than 2 months with approximately 65% cumulative release.
Inhibition of S. epidermidis
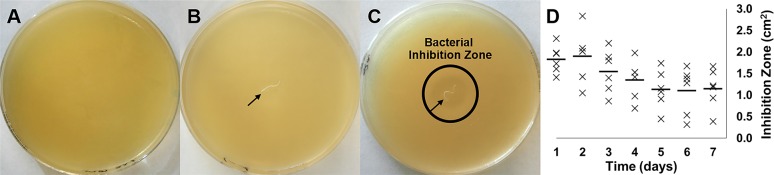
Bacterial inhibition zone experiments were conducted with S. epidermidis to determine whether levofloxacin released from sutures was capable of eliminating bacteria in an in vitro setting, and how long this effect might last in vivo. Four percent PEG/PLLA/levofloxacin sutures were cut to 1 cm in length and incubated in 37°C PBS from 1 hour up to 7 days. After each time-point, the suture was removed from solution and placed in the center of an agar plate that had been cultured with S. epidermidis for 24 hours. PBS, neat drug, and 4% PEG/PLLA sutures were used as controls (Figs. 3A–C). As depicted, PBS, and 4% PEG/PLLA did not inhibit bacterial growth, while the 4% PEG/PLLA/levofloxacin suture created a 2-cm inhibition zone after 24 hours of drug release in PBS. Further, after 7 days in release media (Fig. 3D), drug-loaded sutures still provided bacterial inhibition, confirming that biologically active antibiotic was being released from the suture in an amount sufficient to eliminate surrounding bacteria.
Figure 3.
In vitro bacterial inhibition. S. epidermidis cultured on agar plates in the presence of (A) PBS, (B) 4% PEG/PLLA suture, and (C) 4% PEG/PLLA/levofloxacin suture after 24 hours. Black arrow indicates suture location. (D) Inhibition zones were measured for sutures bled for up to 1 week. A bacterial inhibition zone is clearly present surrounding the 4% PEG/PLLA/levofloxacin suture after bleeding for 24 hours, and the sutures continue to show activity for at least 1 week.
In Vivo Performance and Biocompatibility
In order to evaluate the potential clinical value of an absorbable, antibiotic-eluting suture, electrospun sutures were implanted into the corneal stroma of male Sprague-Dawley rats. 8-0 Ethilon, 8-0 Vicryl, and 8-0 4% PEG/PLLA/levofloxacin sutures of approximately 1 mm in length were compared with each other and untreated controls after 7 days. Notably, 4% PEG/PLLA/levofloxacin sutures remained in the cornea and maintained integrity through the 7-day period, similar to the Ethilon and Vicryl sutures. Rats were monitored daily, and there were no gross signs of infection or inflammation among any of the animals for all sutures tested. Histological analysis (Figs. 4A–D) showed that the tissue reaction to the electrospun 4% PEG/PLLA/levofloxacin suture was indistinguishable to that of the nylon suture and untreated controls. There were no obvious signs of neovascularization or inflammation in the control, nylon, or antibiotic-eluting suture conditions. However, immune cell infiltration was apparent in each of the rat eyes containing a Vicryl suture.
Figure 4.
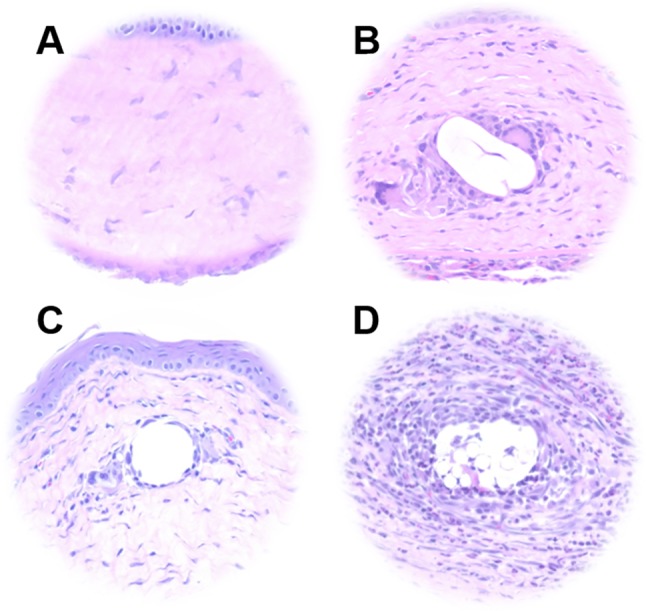
Histological analysis of suture biocompatibility. Representative images of hematoxylin and eosin stained sections of (A) untreated corneal tissue and tissue surrounding (B) 4% PEG/PLLA/levofloxacin, (C) 8-0 permanent nylon (Ethilon), and (D) 8-0 absorbable PLGA (Vicryl) sutures following implantation for 1 week in Sprague-Dawley rat corneas. The electrospun suture elicited a tissue reaction comparable to that of nylon, while immune cell infiltration was observed in the vicinity of the Vicryl suture.
Discussion
In this study, we demonstrated the manufacture, via wet electrospinning, of an absorbable, antibiotic-eluting suture composed of PLLA, PEG, and levofloxacin. The drug-eluting sutures provided sustained antibiotic release for more than 60 days in vitro, demonstrated activity against S. epidermidis for at least 1 week in vitro, and demonstrated similar biocompatibility to standard nylon sutures when implanted into rat corneas. To our knowledge, this is the first study to describe ophthalmic-grade antibiotic-eluting sutures prepared by electrospinning. Future studies to further preclinical development will include evaluation of duration of in vivo antibiotic release and prevention of ocular infection in vivo using varied suture lengths in the range of lengths typically used for ophthalmic procedures (a few mm to several cm).38
Wet electrospinning proved to be a versatile and scalable platform for the manufacture of drug-loaded sutures. Filament size was easily modified by changing equipment parameters such as flow rate, and a single run was able to manufacture meters of uniform filament material.37 As with other electrospinning setups, the system is compatible with a wide range of polymer, drug, and solvent combinations.35 There is potential for this system to be used to tune suture size (2-0 to 10-0), degradation time (weeks to years), and drug release (burst and/or sustained) to allow for myriad clinical applications.
Similar to previous studies using electrospinning to manufacture drug-eluting sutures, the breaking strength of the 8-0 sized 4% PEG/PLLA/levofloxacin was below what is required by United State Pharmacopeia (USP) specifications.1,5,8,11,39 Although the suture maintained integrity when implanted in vivo, additional optimization to further improve the mechanical properties of electrospun sutures is necessary. The literature has shown that voltage and polymer concentration can influence PLLA crystallinity and, therefore, breaking strength.40 Additionally, it is possible that certain polymer and drug combinations would be stronger than others due to physicochemical interactions. Interestingly, although levofloxacin and PLLA are both hydrophobic, increasing the concentration of hydrophilic PEG did not significantly modify suture tensile strength.36,41 In addition to varying electrospinning parameters and modifying polymer/drug choice, electrostretching and other postmodifications may serve to improve tensile strength.37 Residual solvent may also have contributed to the reduced tensile strength of the sutures.1
While optimizing tensile strength, consideration must be given to maintaining an ideal drug release profile. In this study, we observed a burst release in the first 48 hours followed by sustained release for more than 30 days, which is ideal for eliminating and preventing infection. However, increasing the PLLA molecular weight to improve strength may further slow the second period of drug release to the point where antibiotic release is below the minimum inhibitory concentration.
Of critical importance in this study was the in vivo safety profile of the 4% PEG/PLLA/levofloxacin suture. While residual solvent is a concern in the use of electrospinning for biomedical applications, there were no signs of local or systemic toxicity due to any residual chloroform that may have remained in the suture after desiccation. Furthermore, the local inflammation observed via histology was comparable to nylon and less than that of commercially available sutures composed of PLGA. This may be due to the quicker degradation of PLGA into acidic byproducts.1 PLLA is known to be a biodegradable, biocompatible polymer with good crystallinity and strength, and has been shown not to elicit negative cellular or tissue reactions in other electrospun suture applications.12
Nylon sutures can harbor bacteria and lead to postoperative ocular infections that are vision-threatening if not properly cared for. While antibiotic eye drops are effective, they are rarely used as prescribed. Hermann and coworkers29 observed topical antibiotic eye drop compliance in patients following cataract surgery and found that no patient followed the protocol exactly. Of patients, 50% took less than half of the prescribed doses over the course of the study.29 Lack of compliance with antibiotic drops may not only allow for an infection to occur and to progress, but will also provide for the development of antibiotic resistance.42 Ophthalmic-grade sutures capable of sustained antibiotic delivery may provide a solution to this significant clinical need.
Conclusions
This study demonstrated the manufacture of an absorbable, ophthalmic-sized suture capable of sustained in vitro release of levofloxacin and inhibition of S. epidermidis, a common cause of postoperative ocular infection. The suture composed of PLLA, PEG, and levofloxacin was histologically similar after implantation in the rat cornea to commercially available nylon, whereas Vicryl, a commercially available, absorbable suture composed of PLGA, demonstrated immune cell infiltration. With improved strength, this platform has the potential to prevent postoperative infection and preclude issues with compliance that may result in the development of ulcers, endophthalmitis, and antibiotic resistance.
Acknowledgments
Supported by grants from the Robert H. Smith Family Foundation, National Science Foundation Graduate Research Fellowship BGE-1232825, and an unrestricted grant from Research to Prevent Blindness, Inc. to the Johns Hopkins Department of Ophthalmology.
Disclosure: F. Kashiwabuchi, inventor on a patent associated with the technology described here (P); K.S. Parikh, inventor on a patent associated with the technology described here (P); R. Omiadze, None; S. Zhang, inventor on a patent associated with the technology described here (P); L. Luo, None; H.V. Patel, inventor on a patent associated with the technology described here (P); Q. Xu, inventor on a patent associated with the technology described here (P); L. M. Ensign, inventor on a patent associated with the technology described here (P); H.-Q. Mao, inventor on a patent associated with the technology described here (P); J. Hanes, inventor on a patent associated with the technology described here (P); P.J. McDonnell, inventor on a patent associated with the technology described here (P)
References
- 1. Weldon CB,, Tsui JH,, Shankarappa SA,, et al. Electrospun drug-eluting sutures for local anesthesia. J Control Release. 2012. ; 161: 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neligan PC. Bioactive sutures. Plast Reconstr Surg 2006. ; 118: 1645–1647. [DOI] [PubMed] [Google Scholar]
- 3. Lee JE,, Park S,, Park M,, et al. Surgical suture assembled with polymeric drug-delivery sheet for sustained, local pain relief. Acta Biomater. 2013. ; 9: 8318–8327. [DOI] [PubMed] [Google Scholar]
- 4. Casalini T,, Masi M,, Perale G. Drug eluting sutures: a model for in vivo estimations. Int J Pharm. 2012. ; 429: 148–157. [DOI] [PubMed] [Google Scholar]
- 5. Wen Hu, Zheng-Ming Huang, Liu X-Y. Development of braided drug-nanofiber sutures. Nanotechnology. 2010. ; 21: 1–11. [DOI] [PubMed] [Google Scholar]
- 6. Pasternak B,, Rehn M,, Andersen L,, et al. Doxycycline-coated sutures improve mechanical strength of intestinal anastomoses. Int J Colorectal Dis. 2008. ; 23: 271–276. [DOI] [PubMed] [Google Scholar]
- 7. Obermeier A,, Schneider J,, Wehner S,, et al. Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS One. 2014. ; 9: e101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morizumi S,, Suematsu Y,, Gon S,, Shimizu T. Inhibition of neointimal hyperplasia with a novel tacrolimus-eluting suture. J Am Coll Cardiol. 2011. ; 58: 441–442. [DOI] [PubMed] [Google Scholar]
- 9. Mack BC,, Wright KW,, Davis ME. A biodegradable filament for controlled drug delivery. J Control Release. 2009. ; 139: 205–211. [DOI] [PubMed] [Google Scholar]
- 10. Joseph J,, Nair SV,, Menon D. Integrating substrateless electrospinning with textile technology for creating biodegradable three-dimensional structures. Nano Letters. 2015. ; 15: 5420–5426. [DOI] [PubMed] [Google Scholar]
- 11. Hu W,, Huang ZM,, Liu XY. Development of braided drug-loaded nanofiber sutures. Nanotechnology. 2010. ; 21: 315104. [DOI] [PubMed] [Google Scholar]
- 12. Hu W,, Huang Z-M. Biocompatibility of braided poly(L-lactic acid) nanofiber wires applied as tissue sutures. Society of Chemical Industry. 2010. ; 59: 92–99. [Google Scholar]
- 13. He CL,, Huang ZM,, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2009. ; 89: 80–95. [DOI] [PubMed] [Google Scholar]
- 14. Catanzanoa O,, Aciernob S,, Russo P,, et al. Melt-spun bioactive sutures containing nanohybrids for local delivery of anti-inflammatory drugs. Mater Sci Eng C Mater Biol Appl. 2014; 43: 300–309. [DOI] [PubMed] [Google Scholar]
- 15. Choudhury AJ,, Gogoi D,, Chutia J,, et al. Controlled antibiotic-releasing antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery. 2016;129:539. –547. [DOI] [PubMed]
- 16. Hughes WL,, Castroviejo R,, Blaydes JE,, et al. The evolution of ophthalmic sutures. Ann Plast Surg. 1981; 6: 48–65. [DOI] [PubMed] [Google Scholar]
- 17. Grinstaff MW. Designing hydrogel adhesives for corneal wound repair. Biomaterials. 2007. ; 28: 5205–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skilbeck CJ. Sutures, ligatures and knots. Surgery (Oxford). 2011. ; 29: 63–66. [Google Scholar]
- 19. Pillai CK,, Sharma CP. Review paper: absorbable polymeric surgical sutures: chemistry, production, properties, biodegradability, and performance. J Biomater Appl. 2010. ; 25: 291–366. [DOI] [PubMed] [Google Scholar]
- 20. Pruitt LA,, Chakravartula AM. Mechanics of biomaterials: fundamental principles for implant design. MRS Bull. 2012. ; 37: 698. [Google Scholar]
- 21. Marco F,, Vallez R,, Gonzalez P,, Ortega L,, de la Lama J,, Lopez-Duran L. Study of the efficacy of coated Vicryl plus antibacterial suture in an animal model of orthopedic surgery. Surg Infect (Larchmt). 2007; 8: 359–365. [DOI] [PubMed] [Google Scholar]
- 22. Ming X,, Nichols M,, Rothenburger S. In vivo antibacterial efficacy of MONOCRYL plus antibacterial suture (Poliglecaprone 25 with triclosan). Surg Infect (Larchmt). 2007. ; 8: 209–214. [DOI] [PubMed] [Google Scholar]
- 23. Ming X,, Rothenburger S,, Yang D. In vitro antibacterial efficacy of MONOCRYL plus antibacterial suture (Poliglecaprone 25 with triclosan). Surg Infect (Larchmt). 2007. ; 8: 201–208. [DOI] [PubMed] [Google Scholar]
- 24. Xintian Ming, Stephen Rothenberger, Nichols M. In vivo and in vitro antibacterial efficacy of PDS* plus (polidioxanone with triclosan) suture. Surg Infect (Larchmt). 2008. ; 9: 451–458. [DOI] [PubMed] [Google Scholar]
- 25. Brian J, Lee M,, Scott D,, Smith M. Suture-related corneal infections after clear corneal cataract surgery. J Cataract Refract Surg. 2009. ; 35: 939–942. [DOI] [PubMed] [Google Scholar]
- 26. Katz S,, Izhar M,, Mirelman D. Bacterial adherence to surgical sutures. A possible factor in suture induced infection. Ann Surg. Jul 1981. ; 194: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leaper D,, McBain AJ,, Kramer A,, et al. Healthcare associated infection: novel strategies and antimicrobial implants to prevent surgical site infection. Ann R Coll Surg Engl. 2010. ; 92: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heaven CJ,, Davison CR,, Cockcroft PM. Bacterial contamination of nylon corneal sutures. Eye (Lond). 1995. ; 9 (pt 1): 116–118. [DOI] [PubMed] [Google Scholar]
- 29. Hermann MM,, Ustundag C,, Diestelhorst M. Compliance with topical therapy after cataract surgery using a new microprocessor–controlled eye drop monitor. Invest Ophthalmol Vis Sci. 2005. ; 46: 3832–3832. [Google Scholar]
- 30. Winfield AJ,, Jessiman D,, Williams A,, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990; 74: 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burns E,, Mulley GP. Practical problems with eye-drops among elderly ophthalmology outpatients. Age Ageing. 1992; 21: 168–170. [DOI] [PubMed] [Google Scholar]
- 32. Diren Sarisaltik, Teksin ZS. Bioavailability file: levofloxacin. J Pharm Sci. 2007. ; 32: 197–208. [Google Scholar]
- 33. Raizman MB,, Rubin JM,, Graves AL,, Rinehart M. Tear concentrations of levofloxacin following topical administration of a single dose of 0.5% levofloxacin ophthalmic solution in healthy volunteers. Clin Ther 2002. ; 24: 1439–1450. [DOI] [PubMed] [Google Scholar]
- 34. Bhardwaj N,, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010. ; 28: 325–347. [DOI] [PubMed] [Google Scholar]
- 35. Li D,, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mat. 2004. ; 16: 1151–1170. [Google Scholar]
- 36. Ulery BD,, Nair LS,, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys 2011. ; 49: 832–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang S,, Liu X,, Barreto-Ortiz SF,, et al. Creating polymer hydrogel microfibres with internal alignment via electrical and mechanical stretching. Biomaterials. 2014. ; 35: 3243–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dunn JP,, Langer PD. Basic Techniques of Ophthalmic Surgery: San Francisco, CA: American Academy of Ophthalmology; 2009. [Google Scholar]
- 39. Chuan-Long He, Zheng-Ming Huang, Han X-J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2008: 80–95. [DOI] [PubMed]
- 40. Ero-Phillips O,, Jenkins M,, Stamboulis A. Tailoring crystallinity of electrospun plla fibres by control of electrospinning parameters. Polymers. 2012. ; 4: 1331. [Google Scholar]
- 41. Aeschlimann JR,, Dresser LD,, Kaatz GW,, Rybak MJ. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of staphylococcus aureus. Antimicrob Agents Chemother. 1999; 43: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bremond-Gignac D,, Chiambaretta F,, Milazzo SA. European perspective on topical ophthalmic antibiotics: current and evolving options. Ophthalmol Eye Dis. 2011. ; 3: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]