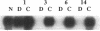Abstract
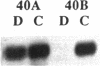
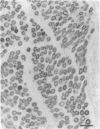
Striking features of the cellular response to sciatic nerve injury are the proliferation of Schwann cells in the distal nerve stump and the downregulation of myelin-specific gene expression. Once the axons regrow, the Schwann cells differentiate again to reform the myelin sheaths. We have isolated a rat cDNA, SR13, which is strongly downregulated in the initial phase after sciatic nerve injury. This cDNA encodes a glycoprotein that shares striking amino acid similarity with a purified myelin protein and is specifically precipitated by a myelin-specific antiserum. Immunohistochemistry experiments using peptide-specific polyclonal antibodies localize the SR13 protein to the myelin sheath of the sciatic nerve. Computer-aided sequence analysis identified a pronounced homology of SR13 to a growth arrest-specific mRNA (Gas-3) that is expressed in resting but not in proliferating 3T3 mouse fibroblasts. SR13 is similarly downregulated during Schwann cell proliferation in the rat sciatic nerve. The association of the SR13 as well as the Gas-3 mRNA with nonproliferating cells in two different experimental systems suggests a common role for these molecules in maintaining the quiescent cell state.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilofsky H. S., Burks C., Fickett J. W., Goad W. B., Lewitter F. I., Rindone W. P., Swindell C. D., Tung C. S. The GenBank genetic sequence databank. Nucleic Acids Res. 1986 Jan 10;14(1):1–4. doi: 10.1093/nar/14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. M. Regeneration in the peripheral nervous system. Neuropathol Appl Neurobiol. 1989 Nov-Dec;15(6):513–529. doi: 10.1111/j.1365-2990.1989.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Bandtlow C., Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987 Jun;104(6):1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Suzuki M., Uyemura K. Purification and partial characterization of two glycoproteins in bovine peripheral nerve myelin membrane. Biochim Biophys Acta. 1976 Dec 14;455(3):806–816. doi: 10.1016/0005-2736(76)90050-x. [DOI] [PubMed] [Google Scholar]
- LeBlanc A. C., Poduslo J. F., Mezei C. Gene expression in the presence or absence of myelin assembly. Brain Res. 1987 Apr;388(1):57–67. doi: 10.1016/0169-328x(87)90021-0. [DOI] [PubMed] [Google Scholar]
- Manfioletti G., Ruaro M. E., Del Sal G., Philipson L., Schneider C. A growth arrest-specific (gas) gene codes for a membrane protein. Mol Cell Biol. 1990 Jun;10(6):2924–2930. doi: 10.1128/mcb.10.6.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R., Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986 Dec;103(6 Pt 1):2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J Cell Biol. 1988 Jul;107(1):279–286. doi: 10.1083/jcb.107.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieke J., Schachner M. Expression of the neural cell adhesion molecules L1 and N-CAM and their common carbohydrate epitope L2/HNK-1 during development and after transection of the mouse sciatic nerve. Differentiation. 1985;30(2):141–151. doi: 10.1111/j.1432-0436.1985.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Kitamura K., Yoshimura K., Nishijima T., Uyemura K. Complete amino acid sequence of PO protein in bovine peripheral nerve myelin. J Biol Chem. 1987 Mar 25;262(9):4208–4214. [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980 Mar;84(3):739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Perret V. Immunological nonidentity of 19K protein and TP0 in peripheral nervous system myelin. J Neurochem. 1986 Sep;47(3):924–929. doi: 10.1111/j.1471-4159.1986.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Hauer P., Lemke G. Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells. J Neurosci. 1988 Sep;8(9):3515–3521. doi: 10.1523/JNEUROSCI.08-09-03515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyemura K., Horie K., Kitamura K., Suzuki M., Uehara S. Developmental changes of myelin proteins in the chick peripheral nerve. J Neurochem. 1979 Mar;32(3):779–788. doi: 10.1111/j.1471-4159.1979.tb04561.x. [DOI] [PubMed] [Google Scholar]
- Welcher A. A., Bitler C. M., Radeke M. J., Shooter E. M. Nerve growth factor binding domain of the nerve growth factor receptor. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):159–163. doi: 10.1073/pnas.88.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]