Abstract
Metastasis is the leading cause of cancer mortality, resulting from changes in the tumor microenvironment which increases tumor cell migration, dispersal to distant organs, and subsequent survival. This is accompanied by changes in tumor collagen which may allow cells to travel more efficiently away from a primary tumor and invade the surrounding tissue. Second Harmonic generation (SHG) is an intrinsic optical signal that has expanded our understanding of collagen evolution throughout tumor progression. This article addresses current research into tumor progression using SHG, as well as the future prospects of using SHG to advance our understanding of the tumor microenvironment.
Keywords: Second Harmonic Generation, multiphoton microscopy, tumor progression, metastasis, collagen type I
Abbreviations
- SHG
Second Harmonic Generation
- PET
Positron Emission Tomogrophy
- MRI
Magnetic Resonance Imaging
- MPM
Multiphoton Microscopy
- ECM
Extracellular Matrix
- TACS
Tumor Associated Collagen Signatures
- TAFs
Tumor Associated Fibroblasts
Introduction
Tumor metastasis is a multistep, low-efficiency process that remains the leading cause of cancer mortality throughout the United States 1. Increasing our understanding of the dynamic changes that occur throughout tumor progression, and accompany the generation of metastases, may create opportunities to increase detection capabilities, personalize medical diagnostics, and develop new targeted therapies. As first suggested by Steven Paget with his “Seed and Soil Hypothesis” 2, understanding tumor progression not only involves studying the tumor cells themselves, but also the cells and structures surrounding them. Consequently, studying the stromal changes throughout tumor progression is critical for understanding microenvironmental changes that may lead to increased metastasis. Current clinical methods of monitoring tumor progression are often incapable of monitoring stromal changes with adequate temporal and spatial resolution: Imaging of H&E or immunohistochemically stained tissue sections provides high resolution visualization of stromal changes occurring in the tumor, but only provides a “snapshot” of tumor progression in time. Other methods of intravital imaging, such as Positron Emission Tomogrophy (PET), Magnetic Resonance Imaging (MRI), or ultrasound have improved tumor detection significantly over the course of the last few years, and can monitor temporal changes 3-5, but lack spatial resolution and/or molecular sensitivity. The gap existing between these techniques is filled by intravital microscopy and especially multiphoton microscopy (MPM). In this perspective we will provide insight into the development and current role of Second Harmonic Generation imaging in the study of the stroma during tumor progression.
Multiphoton and Second Harmonic Generation Imaging
In 1839 Rudolf Wagner pioneered the concept of in vivo imaging by using microscopy to study leukocyte interactions with vessel walls 6. This revolutionized the study of biological processes, allowing for the analysis of tissue dynamics in real time. Fluorescence microscopy was invented in the early 1900s, and its molecular specificity rendered it an important tool in the cancer biology field. However, this technique is not optimal for intravital imaging because of the high level of background fluorescence captured when imaging a field within thick tissue 7. This limits the signal to noise ratio as well as the imaging depth. The field of intravital microscopy took a significant step forward with the invention of the MPM, which enabled 3D imaging with high spatial resolution and significant depth penetration in tissue 8. MPM has been used to excite both exogenous and endogenous fluorescence signals to study tumor progression in vivo, including studies of angiogenesis 9, 10, metastasis 11-13, and extracellular matrix (ECM) modification 14, 15.
Within a few years of its development, MPM also became a tool for imaging intrinsic signals using the scattering phenomena of Second Harmonic Generation (SHG). In the process of SHG light is scattered by non-centrosymmetric structures to combine two incoming photons into one outgoing photon without the loss of energy due to a Stoke's shift as seen in fluorescence (Figure 1a). Hence the outgoing photon has the same total energy as the incoming photons, and half the incoming wavelength. SHG was first discovered in crystals in 1961 16 and later used to image collagen fibers in vitro 17. In 1999 Campagnola et al. imaged cell membranes with SHG to monitor physiological changes in cancerous cells and healthy fibroblasts 18. Soon after, SHG techniques were applied to human disease models of collagen rich tissue such as skin 19, as well as in three dimensional collagenous tissue culture models 20. Intravital SHG was subsequently exploited in vivo to image rat mammary adenocarcinoma progression through an acute skin flap 21 and to study collagen structure in human melanoma using a chronic mouse dorsal skin fold chamber, thereby initiating the use of SHG in the study of tumor progression 22.
Figure 1.
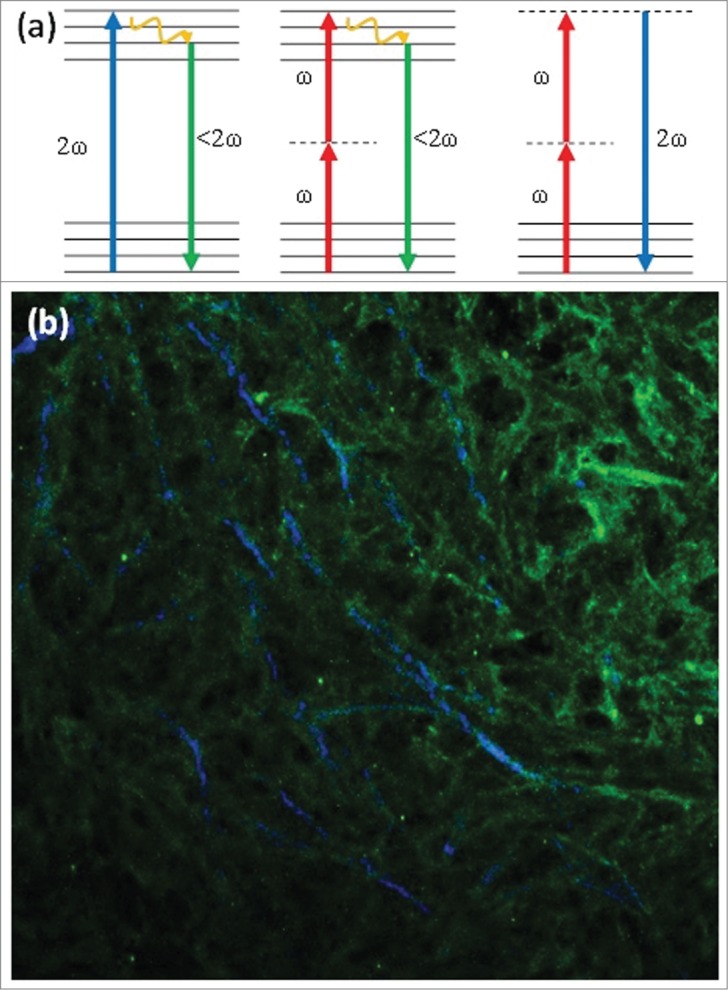
(a) Jablonski diagram of (from left to right) one-photon excited fluorescence, two-photon excited fluorescence and Second Harmonic Generation, depicting the differences in excitation processes between these 3 optical processes. (b) Sample image of Type I collagen antibody staining imaged with TPEF (green) overlapped with SHG imaging of collagen fibers (blue). This image demonstrates that SHG is produced by type I collagen, but not all type I collagen produces a significant SHG signal.
Current Applications of SHG Imaging in Tumor Progression
SHG is currently applied in vivo, in vitro and ex vivo, most commonly to understand mechanisms of tumor progression as well as to attempt to detect and diagnose cancer based upon optical signatures (Figure 2):
Figure 2.
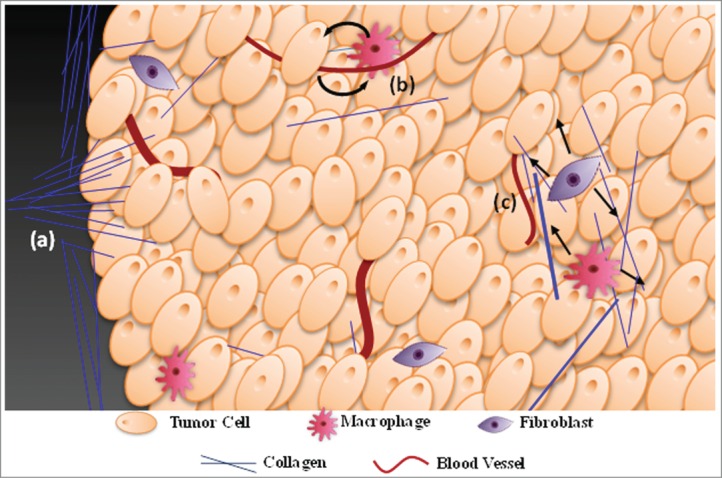
Summary of the major work being conducted in the application of SHG to tumor progression. (a) The tumor collagen framework undergoes significant restructuring which can increase the efficiency of cell travel away from the primary tumor. Morphological analysis of restructuring can subsequently be utilized to predict survival rates in breast cancer patients 28. Furthermore, analysis of scattering directionality can be used to understand how matrix microstructure changes with progression 63. (b) SHG is being used to monitor how tumor cells and host cells such as macrophages interact in the tumor microenvironment while using collagen as a framework to move towards blood vessels 52. (c) Many in vivo and ex vivo studies are underway to better understand the pathways connecting tumor cells, macrophages, fibroblasts and the reorganization of collagen in the ECM 44, 53.
Collagen Reorganization in Tumor Progression
Tumor metastasis is commonly divided into several stages of progression including: development of the primary tumor, invasion of the tumor cells into the surrounding tissue, intravasation into blood or lymphatic vessels, survival of tumor cells in the vessel, extravasation from the vessel, and the development of a secondary tumor 23-25. TPEF and SHG imaging of tumor progression often focus on the initial steps of this process, where SHG has the benefit of highlighting the changes in the stromal collagen structure throughout progression of the primary tumor towards metastasis. One of the pivotal findings in the field of SHG and tumor progression is that tumor cells travel along SHG+ fibers as a means of collective or individual cell migration towards blood or lymph vessels 21, 26, 27. Throughout breast tumor progression collagen fibers display a series of characteristic morphologies, entitled Tumor Associated Collagen Signatures (TACS) 26, 28, which may affect the efficiency of metastasis. In TACS-1 collagen density is increased surrounding the tumor/host interface. These fibers straighten out to form a border circumferentially surrounding the tumor in TACS-2. In TACS-3 tumor collagen fibers are reorganized so that they protrude out perpendicularly from the tumor border, allowing cells to travel along the fibers towards surrounding blood vessels 26. This SHG-based assessment of collagen morphology during breast cancer progression hints at a previously hidden dynamic control of collagen morphology and demonstrates the importance of studying the role of collagen in the tumor microenvironment to determine the mechanisms of tumor progression, with the goals of targeting this process as well as exploiting this in the clinic to assess tumor progression.
Collagen density, which correlates with tissue stiffness 29, 30, has been shown to affect the probability of developing breast cancer, as well as subsequently affecting the aggressiveness of that tumor 31, 32. Tumors exhibiting high stiffness show more metastatic tendencies, possibly as a function of their ability to reorganize the fibers to increase cell motility 26, 33-35 and the abilities of individual cells to travel along these fibers 36, 37. SHG imaging is playing a major role in elucidating the mechanistic processes of ECM remodeling in the tumor microenvironment. One key regulator of tumor matrix stiffness is lysyl oxidase, which causes specific collagen crosslinking that has been shown, through SHG imaging, to result in the linearization of collagen fibers 38. SHG imaging of tumor explants, xenografts, or individual cells seeded in collagen gels have shown that matrix reorganization is dependent on the ROCK 39, 40 and FAK 41 pathways. SHG imaging of relatively “clean” collagen gel systems allows for parsing out these pathways in great detail, showing that significant molecules in these pathways include upstream Caveolin-1, which aids in remodeling the tumor ECM through the Rho/ROCK pathway 36. In a mouse model of tumorigenesis in the involuting mammary gland microenvironment, inhibition of COX-2 reduces the collagen fibrillogenesis that is shown by SHG to be associated with involution, as well as the resultant tumor growth and metastasis 42. On the level of organism-wide signaling, mouse models of emotional stress reveal that α2-adrenergic receptor activation promotes breast tumor progression and this progression is associated with alterations in collagen structure as shown by SHG 43. Likewise, tumor collagen morphology can be altered by systemic application of the hormone relaxin, as demonstrated by in vivo SHG imaging 22. In vivo SHG imaging also revealed that relaxin treatment increased the interaction of tumor associated fibroblasts (TAFs) with collagen fibers via TAF expression of β1 integrin, and this integrin expression is necessary for TAF/SHG+ fiber association and subsequent fiber remodeling 44.
Matrix metalloproteinases are enzymes responsible for matrix degradation and have been shown to play a significant role in tumor cell/ collagen interactions 45, 46. Mammary tumors developing in MMP13 knockout mice, a stromal MMP capable of cleaving collagen types I - III, have significantly elevated numbers of distant metastases as well as altered orientation and structure, quantified through changes in the SHG signal directionality (see below). This revealed that MMP13 has a significant effect on tumor invasiveness and matrix structure 47. SHG imaging of tumor cells in collagen gels has shown that membrane bound MMPs play a significant and necessary role in collagen reorganization and invasion. Membrane-anchored proteases, i.e. types 1, 2, and 3 metalloproteinases, aid in tumor cell penetration through the basement membrane as well as invasion through conventionally cross-linked tissue 48, 49. Although it is not yet clear what other stromal components are necessary for tumor invasion, it is very clear what a significant role SHG imaging is having on this field.
In addition to providing insight into the key molecular players, SHG imaging has also helped elucidate the cellular players expressing or responding to these enzymes and signals. Intravital SHG imaging has produced key insights into the related processes of mammary gland development 50 and mammary tumor progression 51, and revealed a key role for the macrophage in both processes. The rate of tumor cell motility along SHG+ fibers, and the rate of intravasation into blood vessels, was shown to be dependent upon proximity to macrophages 52. Macrophages may also play a role in altering the structure of the fibers themselves: in a mouse mammary tumor model, ablation of stromal macrophages altered collagen structure as reported by SHG and reduced metastatic output, further implicating the macrophage and its collagen remodeling as a player in the metastatic process 53. The aforementioned demonstration that the hormone relaxin promotes TAF/SHG+ fiber association and fiber degradation suggests that macrophages may promote matrix remodeling via modulation of TAF behavior 44.
Differentiating Healthy and Tumor Tissue: Morphology and SHG Intensity
SHG signals have been used not only to study the molecular and cellular regulation of collagen structure as described above, but also to differentiate between healthy and tumor tissue for a variety of different cancer types in an attempt to improve clinical detection and diagnosis of cancer. For example, SHG intensity and polarization properties were used to distinguish osteosarcoma, breast carcinoma, and melanoma from normal tissues 54. SHG imaging of human tissue has been shown to be capable of differentiating between healthy and tumor tissue in breast cancer using collagen morphology 55. Likewise basal cell carcinoma was distinguished from normal skin using the ratio of the intensity of the SHG signal to autofluorescence intensity 56. Using the same ratio measurement healthy and tumor ovarian tissue have been differentiated in vivo through a laproscopic stick objective 57. By analyzing the orientation of collagen fibers, as embodied in the Tumor Associated Collagen Signatures discussed above, one can predict survival rates of breast cancer patients 28. The current field of SHG imaging has shown significant potential to increase clinical cancer diagnostic capabilities, by being able to locate areas of tumor presence within various tissue types, as well as to distinguish tumors by grade and metastatic ability.
Differentiating Healthy and Tumor Tissue: SHG Directionality
The methods described above face potential challenges in reproducibly exciting the same SHG intensity (for intensity based measurements) or in requiring trained observers or robust image analysis algorithms (to consistently outline fibers or analyze fiber orientation). An alternative method is to measure the directionality of the SHG signal. SHG emission is coherent, hence the directionality, intensity, and polarization of the outgoing light is sensitive to properties of the scatterers including scatterer order, spacing, angle, and the overall spatial extent of the scatterer distribution along the laser axis 58-60. In collagen this translates to “microstructural” properties of the fibers, including: fibril diameter, spacing, and order versus disorder in fibril packing within the fiber 58, 59, 61, 62. These properties affect the directionality of SHG signals, which can be measured through the ratio of forward to backward propagating light (F/B ratio), which is inherently insensitive to variations in excitation efficiency. Measurement of F/B has been used to differentiate healthy and tumor tissue in ovarian cancer 61. Recently, F/B ratio was used to differentiate invasive breast cancer from in situ breast cancer and healthy breast tissue, and was shown to vary with tumor stage and grade, revealing the power of this technique in a clinical setting 63.
Measuring the true emitted F/B ratio in vivo is difficult due to the thickness of the tissue, because scattering of emitted SHG photons within the tissue affects the measured signals, and capturing a forward propagating signal intravitally can be essentially impossible due to the presence of tissue in between the plane of interest and the detection optics. Consequently direct measurement of the emitted F/B ratio is usually conducted using the direct capture of signals from thin tissue sections. Therefore it has been of interest in the field to explore methods to measure the F/B ratio from thick tissue sections, or even intact tissue. One method is to use collagen gels to study in detail how the measured F/B ratio changes as a function of depth, how different types of collagen types fibers affect the F/B measurements throughout gels, and hence how best to interpret forward and backward propagating detected SHG signals 64. After measuring the F/B ratio as a function of depth into a tissue with known scattering properties, Monte Carlo simulations can be used to extrapolate to the true F/B ratio 59, 61. As an alternative solution Han et al. developed a method to measure the F/B ratio using just the backward scattering signal by incorporating confocal pinholes of different diameters to determine the amount of forward propagating signal that is being scattered backwards into the epi-detection lens 65. This allows for the intravital measurement of the emitted F/B ratio, from the surface of intact tumor tissue, over time. The application of either thick tissue technique to intravital systems would aid in measuring microstructural changes in collagen fibers throughout tumor progression, in vivo or in explants.
The Future of SHG in Tumor Progression
Based on the current progress in the field there are a variety of possible future applications of SHG in the study of tumor progression. Three of the most promising are using SHG in chronic window models to create and evaluate therapeutics that target the ECM, implementing SHG with molecular diagnostic techniques to produce more complete clinical diagnostics, and using SHG methods to find positive tumor borders on tumor biopsies.
Creation and Evaluation of Drug Treatments
The study of the cellular interactions and molecular pathways affecting matrix structure, as revealed by SHG and described above, has created a new window into the matrix and hence an opportunity to discover new therapeutic targets to inhibit metastasis. SHG has already been used to monitor tumor progression in animal models, consequently candidate therapeutic drugs can be administered in one of these models, and monitored with SHG, to determine how the candidate changes those aspects of collagen structure that influence SHG. This process is already starting, as demonstrated by the testing of Losartan treatment in mouse dorsal skin-fold chambers and the use of SHG to monitor changes in collagen structure, as well as subsequent therapeutic invasion into the tumor 66. One possible class of therapeutic targets to explore with SHG is the membrane-bound MMPs. MMPs have been a potential drug target for over thirty years due to their obvious connections to matrix remodeling, but have thus far failed to produce efficient solutions for decreasing tumor invasiveness 67. Through imaging of changes in type I collagen, membrane type 1 metalloprotease has been shown to play a necessary role in tumor invasion into the surrounding matrix ECM in covalently crosslinked collagen networks 49. Membrane bound MMPs therefore provide a new target to explore for possible therapeutics using SHG, with the goal of inhibiting tumor invasion through the ECM through the use of these enzymes. Other alternatives to targeting MMPs include inhibiting the ROCK and FAK pathways, or their effectors, which have been shown to play significant roles in matrix reorganization throughout tumor progression 39-41.
Clinical Evaluation Through SHG and Molecular Diagnostics
With the increased capabilities of molecular diagnostics and genetic analysis, protein and gene expression profiles are beginning to play a more significant role in differentiating tumor characteristics and determining the best prospective treatments for a specific patient. As an example, OncotypeDX is a 21-gene screen used to help decide which patients will receive chemotherapy after removal of their primary breast cancer 68. However, this is still an expensive and time consuming process. Many of the gene expression profiles known to indicate higher metastatic efficiency involve networks and pathways that control extracellular matrix structure 69. Hence it is possible that SHG-based quantification of matrix structure may provide a readout that integrates the contribution of many of these networks and pathways. Consequently, correlating SHG properties with some of these genetic profiles could allow for a complementary, and possibly cheaper and quicker, method of providing predictions of tumor progression. For example, genetic testing has shown that the expression level of genes such as Snai1, and other genes related to epithelial to mesenchymal transition, are predictive of metastatic breast cancer, resulting from increased epithelial to mesenchymal transition of the cells 70. Snail induces the expression of membrane anchored type 1 and type 2 MMPs on tumor cells, facilitating invasion through the surrounding collagen membrane 71, a process that could be detected through SHG imaging. An SHG interrogation could integrate changes in gene expression without having to perform genetic testing, providing a quicker method of determining similar or complimentary information. Combining genetic research such as this with SHG imaging could increase diagnostic capabilities and aid in understanding the true physical consequences of the changes in genetic expression in the context of tumor development.
Role of SHG Imaging in Tumor Margin Evaluation
As the clinical aim of breast cancer surgery transitions from mastectomy to lumpectomy for maximum preservation of healthy tissue, precisely defining the tumor margins and ensuring full removal of the primary tumor during the initial surgery have become increasingly important concerns in order to avoid secondary surgeries and cancer reoccurrence. The current standard for analyzing tumor margins involves removing and staining sections of the tumor, so a pathologist may analyze multiple sections to quantify negative, close, and positive tumor margins. This process, which requires several days, means patients will need to return for secondary surgeries if the borders are found to be positive for the presence of tumor tissue. Efforts to improve this process include various intraoperative techniques such as imprint cytology 72, gross examination 73, and ultrasound imaging 74. However no extant method balances the accuracy, speed and ease of use required to achieve an ideal method of intraoperative analysis, and all present methods still result in 20-55% of removals requiring a secondary surgery 75-77. The ability of SHG F/B to readily distinguish tumor from healthy tissue 61, 63 coupled with the fact that it is a quantitative, intrinsic signal, suggests that it may provide useful information to assist with margin assessment. Implementing F/B SHG techniques to detect different collagen profiles between healthy and tumor tissue along the border of the removed tissue would allow for the immediate analysis of the biopsy upon removal from the patient without the extensive staining and sectioning required for traditional pathological analysis. It has previously been demonstrated that fresh, human ovarian biopsies imaged with an MPM system showed differences in collagen morphology between healthy and abnormal tissue 78. By combining this with quantitative methods of differentiating ovarian and breast tumor tissue from healthy tissue 61, 63, this could create an automated system of analyzing tumor biopsies for positive margins. The creation of a system that could rapidly image the surface of a tumor biopsy would hold the potential to significantly decrease the amount of secondary surgeries necessary after tumor removal, saving patients from unnecessary physical and emotional stress.
Conclusion
Over the past 15 years Second Harmonic Generation imaging has advanced from its first imaging of cellular structures to routine use as an intravital imaging technique that can monitor cellular processes throughout tumor progression. Advancements in this field have illuminated necessary molecular pathways for collagen reorganization, increased knowledge about stromal evolution in cellular interactions, and created a new potential tool for cancer diagnosis. Many challenges still remain in regards to expanding our understanding of the tumor “soil”, perfecting in vivo imaging methods, and translating this research to clinical applications. Overall SHG has proven to be a viable technique for identifying tumor location while providing a means of monitoring tumor progression intravitally with high spatial and temporal resolution.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. American Cancer Society Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2. Paget S. The Distribution of Secondary Growths in Cancer of the Breast. The Lancet 1889; 133:571–3. [PubMed] [Google Scholar]
- 3. Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nature reviews Cancer 2002; 2:11–8. [DOI] [PubMed] [Google Scholar]
- 4. Pearlman JD, Laham RJ, Post M, Leiner T, Simons M. Medical imaging techniques in the evaluation of strategies for therapeutic angiogenesis. Current pharmaceutical design 2002; 8:1467–96. [DOI] [PubMed] [Google Scholar]
- 5. Blankenberg FG. In vivo detection of apoptosis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2008; 49 Suppl 2:81S–95S. [DOI] [PubMed] [Google Scholar]
- 6. Wagner R. Erlauterungstafeln zur Physiologie und Entwicklungsgeschichte.. Leipzig: Leopold Voss 1839. [Google Scholar]
- 7. Conchello JA, Lichtman JW. Optical sectioning microscopy. Nature methods 2005; 2:920–31. [DOI] [PubMed] [Google Scholar]
- 8. Denk W, Strickler J, Webb W. Two-photon laser scanning fluorescence microscopy. Science 1990; 248:73–6. [DOI] [PubMed] [Google Scholar]
- 9. Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation (New York, NY : 1994) 2010; 17:206–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lunt SJ, Gray C, Reyes-Aldasoro CC, Matcher SJ, Tozer GM. Application of intravital microscopy in studies of tumor microcirculation. Journal of biomedical optics 2010; 15:011113. [DOI] [PubMed] [Google Scholar]
- 11. Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. Journal of cell science 2011; 124:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nature reviews Cancer 2003; 3:921–30. [DOI] [PubMed] [Google Scholar]
- 13. Kedrin D, Wyckoff J, Sahai E, Condeelis J, Segall JE. Imaging tumor cell movement in vivo. Current protocols in cell biology / editorial board, Juan S Bonifacino [et al] 2007; Chapter 19:Unit 19 7. [DOI] [PubMed] [Google Scholar]
- 14. Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochemistry and cell biology 2008; 130:1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lohela M, Werb Z. Intravital imaging of stromal cell dynamics in tumors. Current opinion in genetics & development 2010; 20:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franken PA, Hill AE, Peters CW, Weinreich G. Generation of Optical Harmonics. Physical Review Letters 1961; 7:118–9. [Google Scholar]
- 17. Freund I, Deutsch M, Sprecher A. Connective tissue polarity. Optical second-harmonic microscopy, crossed-beam summation, and small-angle scattering in rat-tail tendon. Biophysical journal 1986; 50:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campagnola PJ, Wei M-d, Lewis A, Loew LM. High-Resolution Nonlinear Optical Imaging of Live Cells by Second Harmonic Generation. Biophysical journal 1999; 77:3341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Current Opinion in Chemical Biology 2001; 5:603–8. [DOI] [PubMed] [Google Scholar]
- 20. Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:11014–9. [DOI] [PMC free article] [PubMed]
- 21. Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer research 2002; 62:6278–88. [PubMed] [Google Scholar]
- 22. Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nature medicine 2003; 9:796–800. [DOI] [PubMed] [Google Scholar]
- 23. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer 2002; 2:563–72. [DOI] [PubMed] [Google Scholar]
- 24. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews Cancer 2003; 3:453–8. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature reviews Cancer 2009; 9:274–84. [DOI] [PubMed] [Google Scholar]
- 26. Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC medicine 2006; 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nature reviews Molecular cell biology 2009; 10:445–57. [DOI] [PubMed] [Google Scholar]
- 28. Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American journal of pathology 2011; 178:1221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature reviews Cancer 2009; 9:108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices With Varied Microstructure. Journal of Biomechanical Engineering 2002; 124:214–22. [DOI] [PubMed] [Google Scholar]
- 31. Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. The Lancet Oncology 2005; 6:798–808. [DOI] [PubMed] [Google Scholar]
- 32. Aiello EJ, Buist DS, White E, Porter PL. Association between mammographic breast density and breast cancer tumor characteristics. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005; 14:662–8. [DOI] [PubMed]
- 33. Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. The Journal of biological chemistry 2013; 288:12722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of Cell Biology 2003; 163:583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Discher DE, Janmey P, Wang Y-l. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005; 310:1139–43. [DOI] [PubMed] [Google Scholar]
- 36. Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011; 146:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nature cell biology 2013; 15:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophysical journal 2008; 95:5374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and Myosin-Dependent Matrix Deformation Enables Protease-Independent Tumor-Cell Invasion In Vivo. Current Biology 2006; 16:1515–23. [DOI] [PubMed] [Google Scholar]
- 41. Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 2009; 28:4326–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nature medicine 2011; 17:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer prevention research (Philadelphia, Pa) 2013; 6:1262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perentes JY, McKee TD, Ley CD, Mathiew H, Dawson M, Padera TP, Munn LL, Jain RK, Boucher Y. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nature methods 2009; 6:143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic Acid Promotes Matrix Metalloproteinase (MMP) Activation and MMP-dependent Invasion in Ovarian Cancer Cells. Cancer research 2001; 61:3194–9. [PubMed] [Google Scholar]
- 46. Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer research 2005; 65:130–6. [PubMed] [Google Scholar]
- 47. Perry SW, Schueckler JM, Burke K, Arcuri GL, Brown EB. Stromal matrix metalloprotease-13 knockout alters Collagen I structure at the tumor-host interface and increases lung metastasis of C57BL/6 syngeneic E0771 mammary tumor cells. BMC cancer 2013; 13:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes & development 2006; 20:2673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol 2009; 185:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Developmental dynamics : an official publication of the American Association of Anatomists 2006; 235:3222–9. [DOI] [PubMed] [Google Scholar]
- 51. Pollard JW. Macrophages define the invasive microenvironment in breast cancer. Journal of leukocyte biology 2008; 84:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer research 2007; 67:2649–56. [DOI] [PubMed] [Google Scholar]
- 53. Burke R, Madden K, Perry S, Zettel M, Brown M. Tumor-associated macrophages and stromal TNF-α play central roles in the regulation of collagen structure in breast tumor models as visualized by second harmonic generation. Submitted 2012. [DOI] [PMC free article] [PubMed]
- 54. Hompland T, Erikson A, Lindgren M, Lindmo T, de Lange Davies C. Second-harmonic generation in collagen as a potential cancer diagnostic parameter. Journal of biomedical optics 2008; 13:054050. [DOI] [PubMed] [Google Scholar]
- 55. Falzon G, Pearson S, Murison R. Analysis of collagen fibre shape changes in breast cancer. Physics in medicine and biology 2008; 53:6641–52. [DOI] [PubMed] [Google Scholar]
- 56. Lin SJ, Jee SH, Kuo CJ, Wu RJ, Lin WC, Chen JS, Liao YH, Hsu CJ, Tsai TF, Chen YF, et al. Discrimination of basal cell carcinoma from normal dermal stroma by quantitative multiphoton imaging. Optics letters 2006; 31:2756–8. [DOI] [PubMed] [Google Scholar]
- 57. Williams RM, Flesken-Nikitin A, Ellenson LH, Connolly DC, Hamilton TC, Nikitin AY, Zipfel WR. Strategies for High-Resolution Imaging of Epithelial Ovarian Cancer by Laparoscopic Nonlinear Microscopy. Transl Oncol 2010; 3:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han X, Burke RM, Zettel ML, Tang P, Brown EB. Second harmonic properties of tumor collagen: determining the structural relationship between reactive stroma and healthy stroma. Optics express 2008; 16:1846–59. [DOI] [PubMed] [Google Scholar]
- 59. Lacomb R, Nadiarnykh O, Townsend SS, Campagnola PJ. Phase Matching considerations in Second Harmonic Generation from tissues: Effects on emission directionality, conversion efficiency and observed morphology. Optics communications 2008; 281:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mertz J, Moreaux L. Second-harmonic generation by focused excitation of inhomogeneously distributed scatterers. Optics communications 2001; 196:325–30. [Google Scholar]
- 61. Nadiarnykh O, LaComb RB, Brewer MA, Campagnola PJ. Alterations of the extracellular matrix in ovarian cancer studied by Second Harmonic Generation imaging microscopy. BMC cancer 2010; 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophysical journal 2005; 88:1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burke K, Tang P, Brown E. Second harmonic generation reveals matrix alterations during breast tumor progression. Journal of biomedical optics 2013; 18:31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ajeti V, Nadiarnykh O, Ponik SM, Keely PJ, Eliceiri KW, Campagnola PJ. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: implications for probing stromal alterations in human breast cancer. Biomedical optics express 2011; 2:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Han X, Brown E. Measurement of the ratio of forward-propagating to back-propagating second harmonic signal using a single objective. Optics express 2010; 18:10538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences 2011; 108:2909–14. [DOI] [PMC free article] [PubMed]
- 67. Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends in Pharmacological Sciences 2013; 34:233–42. [DOI] [PubMed] [Google Scholar]
- 68. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. New England Journal of Medicine 2004; 351:2817–26. [DOI] [PubMed] [Google Scholar]
- 69. Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn JG, Sleijfer S, Foekens JA, et al. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clinical cancer research : an official journal of the American Association for Cancer Research 2008; 14:5555–64. [DOI] [PubMed] [Google Scholar]
- 70. Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences 2010; 107:15449–54. [DOI] [PMC free article] [PubMed]
- 71. Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proceedings of the National Academy of Sciences of the United States of America 2009; 106:20318–23. [DOI] [PMC free article] [PubMed]
- 72. Muttalib M, Tai CC, Briant-Evans T, Maheswaran I, Livni N, Shousha S, Sinnett HD. Intra-operative assessment of excision margins using breast imprint and scrape cytology. Breast (Edinburgh, Scotland) 2005; 14:42–50. [DOI] [PubMed] [Google Scholar]
- 73. Balch GC, Mithani SK, Simpson JF, Kelley MC. Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy. The American surgeon 2005; 71:22–7; discussion 7-8. [PubMed] [Google Scholar]
- 74. Tafra L, Fine R, Whitworth P, Berry M, Woods J, Ekbom G, Gass J, Beitsch P, Dodge D, Han L, et al. Prospective randomized study comparing cryo-assisted and needle-wire localization of ultrasound-visible breast tumors. American journal of surgery 2006; 192:462–70. [DOI] [PubMed] [Google Scholar]
- 75. Atkins J, Mushawah FA, Appleton CM, Cyr AE, Gillanders WE, Aft RL, Eberlein TJ, Gao F, Margenthaler JA. Positive margin rates following breast-conserving surgery for stage I–III breast cancer: palpable versus nonpalpable tumors. Journal of Surgical Research 2012; 177:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lovrics PJ, Cornacchi SD, Farrokhyar F, Garnett A, Chen V, Franic S, Simunovic M. The relationship between surgical factors and margin status after breast-conservation surgery for early stage breast cancer. American journal of surgery 2009; 197:740–6. [DOI] [PubMed] [Google Scholar]
- 77. Klimberg VS, Jr, Harms S, Jr, Korourian S., Jr Assessing margin status. Surgical Oncology 1999; 8:77–84. [DOI] [PubMed] [Google Scholar]
- 78. Kirkpatrick ND, Brewer MA, Utzinger U. Endogenous optical biomarkers of ovarian cancer evaluated with multiphoton microscopy. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2007; 16:2048–57. [DOI] [PubMed]


