Abstract
Soft tissue sarcomas of the esophagus represent an extremely rare cause of esophageal masses, and an even smaller proportion of these tumors represent dedifferentiated liposarcomas. We present a case of a 75-year-old gentleman presenting with dysphagia found to have a 5 cm pedunculated mass in the cervical esophagus, originating at the cricopharyngeus. This was found to have involvement limited to the superficial mucosa by endoscopic ultrasound, and the lesion was subsequently resected endoscopically. Pathology demonstrated an undifferentiated pleomorphic sarcoma later determined to represent dedifferentiated liposarcoma after fluorescence in situ hybridization analysis. The patient received no additional adjuvant therapy and remains disease free 20 months from the procedure. While treatment experience is limited, our case demonstrates that in selected patients, sustained local control can be obtained without radical resection.
Key words: Dedifferentiated liposarcoma, Undifferentiated pleomorphic sarcoma, Surgical resection, Esophageal mass
Introduction
Soft tissue sarcomas of the esophagus represent an extremely rare cause of esophageal masses, estimated at 0.1-1.5% of esophageal neoplasms.1,2 An even smaller proportion of these tumors represent dedifferentiated liposarcomas, representing 171 out of 26,758 cases of soft tissue sarcoma in one review.3,4 It is a malignant high grade sarcoma that most commonly occurs in the retroperitoneum.5 It is complicated by local recurrence in 40-60% of cases and metastasis in 15-20%, although it appears that local recurrence occurs less frequently in non-retroperitoneal locations.5,6 Given the rarity of its occurrence, there is a scarcity of data regarding the effective management of esophageal sarcomas, and case reports offer valuable insights into tumor behavior and treatment. In this report we describe a case of dedifferentiated liposarcoma of the esophagus treated with endoscopic resection, as well as a selected review of the literature.
Case Report
A 75 year old gentleman presented to the gastroenterology clinic for evaluation of dysphagia. He had a history of stage I (pT1N0Mx), moderately differentiated adenocarcinoma of the distal esophagus treated with surgical resection and intrathoracic esophagogastric anastomosis 10 years earlier. He had a ten pack-year smoking history, but stopped smoking around the time of his surgery, and does not drink alcohol. Since his surgery he had experienced no major associated symptoms until 6 months prior to his current presentation, at which time he described a sensation of something in the back of his throat. This progressed to frank dysphagia for both solids and liquids. He pursued evaluation at an outside facility, where CT of the neck demonstrated a 5.0×2.0×2.8 cm obstructing abnormality in the proximal esophagus (Figure 1). Upper endoscopy then revealed an impacted piece of steak which was removed, as well as a 2.0 cm polypoid lesion. A repeat endoscopy performed the next month redemonstrated the polyp as well as an area of fullness in the proximal esophagus. Biopsies were obtained at that time and were interpreted as sarcomatoid squamous cell carcinoma. After presentation to our facility the patient underwent upper endoscopy with endoscopic ultrasound (EUS), demonstrating a pedunculated mass in the cervical esophagus, originating at the cricopharyngeus (Figure 2). The mass measured 5.0 cm in length and up to 1.0 cm in thickness. On sonographic evaluation local invasion appeared limited to the superficial mucosal layer. The lesion was resected completely in piecemeal fashion with biopsy then obtained from the resection bed.
Figure 1.
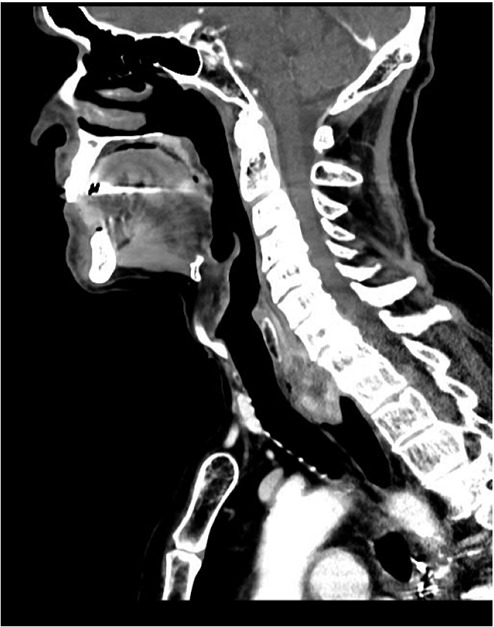
Sagittal computed tomography image demonstrating esophageal mass arising from the cervical esophagus.
Figure 2.
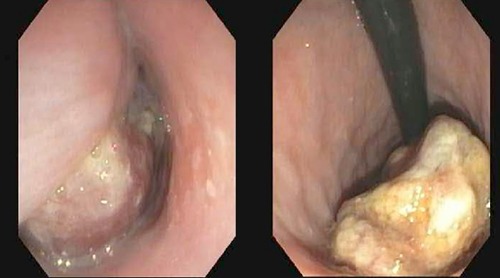
Endoscopic images of pedunculated esophageal mass.
Pathology examination revealed a high-grade spindle cell tumor in a whorled pattern. The tumor cells showed marked cytological atypia and brisk mitotic activity. Focal necrosis was also noted. Immunohistochemical studies showed that the spindle cells were negative for pancytokeratin, desmin, actin, p63, CD 34, CD 117, CAM 5.2 and CK 5/6. The proliferative rate was high by Ki-67 (80%). Fluorescent in situ hybridization study demonstrated amplification of the CPM gene in 80% of lesional cells. Although the entire specimen was comprised of high grade sarcoma and no well-differentiated liposarcoma was seen, the overall findings suggested the tumor likely represented a de-differentiated liposarcoma. The procedural bed biopsy was negative (Figure 3).
Figure 3.
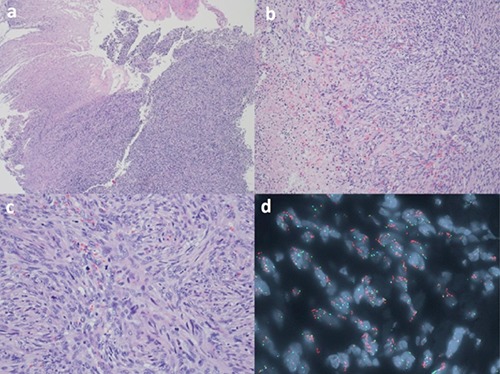
The Hematoxylin and Eosin (H&E) section of the tumor cells demonstrated the proliferation of spindle cells eroding the overlying squamous mucosa (a, H&E 4×). Focal necrosis is noted (b, H&E 10×). The spindle cells showed marked cytological atypia and brisk mitoses (c, H&E 20×). FISH study on the paraffin block demonstrated CPM gene amplification (orange) in tumor cells (d, green labeled the probe for chromosome 12 centromere; orange labeled the probe for CPM gene at 12q15; aqua labeled the probe for chromosome 4 centromere as internal control).
PET scan one month later demonstrated decreasing avidity at the surgical site and no increased uptake suggestive of recurrence or metastasis. Repeat endoscopy/EUS demonstrated reflux esophagitis with focal Barrett’s mucosa on biopsy, but no evidence of residual tumor or recurrence. Given this it was decided to withhold radiation therapy at that time and pursue close observation. The patient is now 20 months out from his procedure and has had no evidence of local recurrence or metastasis on most recent CT imaging and endoscopy.
Discussion and Conclusions
Dedifferentiated liposarcoma is an extremely rare cause of esophageal mass, and treatment experience with tumors of this histology is limited. This is reflected in a recent paper by Wu et al. which examined the data on esophageal sarcoma and carcinoma in the Surveillance, Epidemiology and End Results (SEER) database. Of the 63,726 cases identified, there were only 178 cases of esophageal sarcoma, most of which were of carcinosarcoma, leiomyosarcoma, or gastrointestinal stromal tumors.7 Outcome data from this analysis demonstrated that patients with esophageal sarcoma had more favorable outcomes, with lesions more likely to be localized and a higher median survival for locoregional disease (50 vs 24 mo).
Small case series have demonstrated a favorable response of esophageal sarcoma to radical resection, and the SEER data demonstrated improved overall survival for nonmetastatic disease that was treated with surgical resection (37 vs 14%).7,8 The question remains, however, regarding how extensive the surgical resection should be.8 Yang et al. recently published a review with bearing on our patient’s initial pathology that included eight cases of undifferentiated pleomorphic sarcoma of the esophagus.8 Six of these cases presented as a pedicled mass, ranging from 3.3 to 14.0 cm in size, and in all but one of these cases a total or partial esophagectomy was performed. Meanwhile, Xu et al. review a series of nineteen esophageal liposarcomas, 18 of which were polypoid in nature.9 Polypectomy was pursued in twelve of these lesions with good initial results, although it must be noted that these lesions were well differentiated or myxoid in nature.10 In our case we present a pedunculated dedifferentiated liposarcoma with sustained local control after endoscopic resection alone. While much more data is needed to determine the optimal management of these patients, this case highlights that there may be a certain subset of patients who can safely undergo local resection alone without the morbidity associated with radical resection. In addition to surgical resection, other modalities explored in the treatment of esophageal sarcomas include chemotherapy and radiotherapy. One interesting case described by Lokesh et al. describes a complete local response of a 7.2 cm ulcerated esophageal spindle cell sarcoma to radical radiotherapy to a total of 66 Gy. This raises interesting possibilities, especially for patients who are not considered operative candidates, although it is noted that the above analysis from the SEER database did not suggest improved survival with external beam radiation. While clearly there are a great many more questions left to be answered regarding the optimum treatment of these patients, our case demonstrates that in selected patients, sustained local control can be obtained with little associated morbidity through the implementation of endomucosal resection.
References
- 1.Lokesh V, Naveen T, Pawar YS. Spindle cell sarcoma of esophagus: a rare case presentation. J Can Res Ther 2010;6:100-1. [DOI] [PubMed] [Google Scholar]
- 2.Stout AP, Latters RL. Tumors of the esophagus. In: Atlas of tumor pathology. Sect V, fasc 20. Washington: Armed Forces Institute of Pathology; 1957. pp 1-95. [Google Scholar]
- 3.Wibmer C, Leithner A, Zielonke N, et al. Increasing incidence rates of soft tissue sarcomas? A population-based epidemio-logic study and literature review. Ann Oncol 2010;21:1106-11. [DOI] [PubMed] [Google Scholar]
- 4.Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer 2006;119:2922-30. [DOI] [PubMed] [Google Scholar]
- 5.Henricks WH, Chu YC, Goldblum JR, et al. Dedifferentiated liposarcoma. A clinicopathologic analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol 1997;21: 271-81. [DOI] [PubMed] [Google Scholar]
- 6.Coindre JM1, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch 2010;456: 167. [DOI] [PubMed] [Google Scholar]
- 7.Wu GX, Ituarte PH, Paz IB, et al. A population-based examination of the surgical outcomes for patients with esophageal sarcoma. Ann Surg Oncol 2015;22:S1310-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang CJ, Shai SE, Li WS, Hsu CP. A huge intraluminally growing polypoid tumor of the cervical esophagus: a case report and literature review of spindle cell (undifferentiated pleomorphic) sarcoma. Formosan J Surg 2013;46:56-60. [Google Scholar]
- 9.Xu S, Xu Z, Hou Y, Tan Y. Primary pedunculated giant esophageal liposarcoma: case report. Dysphagia 2008;23:327-30. [DOI] [PubMed] [Google Scholar]
- 10.Will U, Lorenz P, Urban H, Meyer F. Curative endoscopic resection of a huge pedunculated esophageal liposarcoma. Endoscopy 2007;39:E15-6. [DOI] [PubMed] [Google Scholar]


