Abstract
Little is known about how individual cells can organize themselves to form structures of a given size. During development, Dictyostelium discoideum aggregates in dendritic streams and forms groups of ∼20,000 cells. D. discoideum regulates group size by secreting and simultaneously sensing a multiprotein complex called counting factor (CF). If there are too many cells in a stream, the associated high concentration of CF will decrease cell-cell adhesion and increase cell motility, causing aggregation streams to break up. The pulses of cyclic AMP (cAMP) that mediate aggregation cause a transient translocation of Akt/protein kinase B (Akt/PKB) to the leading edge of the plasma membrane and a concomitant activation of the kinase activity, which in turn stimulates motility. We found that countin− cells (which lack bioactive CF) and wild-type cells starved in the presence of anticountin antibodies (which block CF activity) showed a decreased level of cAMP-stimulated Akt/PKB membrane translocation and kinase activity compared to parental wild-type cells. Recombinant countin has the bioactivity of CF, and a 1-min treatment of cells with recombinant countin potentiated Akt/PKB translocation to membranes and Akt/PKB activity. Western blotting of total cell lysates indicated that countin does not affect the total level of Akt/PKB. Fluorescence microscopy of cells expressing an Akt/PKB pleckstrin homology domain-green fluorescent protein (PH-GFP) fusion protein indicated that recombinant countin and anti-countin antibodies do not obviously alter the distribution of Akt/PKB PH-GFP when it translocates to the membrane. Our data indicate that CF increases motility by potentiating the cAMP-stimulated activation and translocation of Akt/PKB.
One of the fascinating questions in biology is how an organism can regulate the size of a multicellular structure. A simple model system that exhibits size regulation is the eukaryote Dictyostelium discoideum, where development results in the formation of groups of up to 100,000 cells, depending on the strain used, with our laboratory strains forming groups of ∼20,000 cells. D. discoideum normally lives as individual amoebae that feed on bacteria on soil surfaces. When the cells overgrow their food supply, they use relayed pulses of extracellular cyclic AMP (cAMP) as a chemoattractant to aggregate in dendritic streams. The aggregated cells form a fruiting body consisting of a thin column of stalk cells supporting a mass of spore cells, and dispersal of the spores allows new colonies to form (28, 33). Because an excessively large fruiting body will collapse (5), the cells sense the number of cells in a stream and cause the stream to break into groups if there are too many cells in a stream (24, 29, 45).
The number of cells in a stream appears to be sensed by counting factor (CF), a ∼450-kDa complex of proteins (5). High extracellular levels of CF cause streams to break up and form smaller groups (3, 63). Disrupting the genes encoding either countin, CF45-1, or CF50, three components of CF, causes cells to secrete virtually undetectable levels of CF activity (5-7). The streams formed by developing countin−, cf45-1−, or cf50− cells do not break up, and as a result the streams coalesce into large groups which form large fruiting bodies that either collapse or fall over (5-7). Adding anti-countin antibodies to developing wild-type cells also causes cells to form larger groups (5). Adding recombinant countin or recombinant CF50 to developing cells mimics the effect of adding highly purified CF and causes cells to form smaller groups (6, 21). These data suggested that CF is part of a negative feedback loop that limits group size.
Computer simulations indicate that if the cell-cell adhesion in a stream is low and/or the random motility of the cells is high, the stream will begin to dissipate. If the adhesion then increases and/or the random motility decreases, the dissipated cells will coalesce into groups rather than reform a stream (42). We found that, as predicted, CF decreases cell-cell adhesion and also increases cell motility (42, 51). Wild-type cells starved in the presence of either countin− conditioned starvation medium (CM) or anti-countin antibodies move more slowly than wild-type cells starved in wild-type CM, and wild-type cells starved in the presence of smlA− (a transformant that oversecretes CF) CM or recombinant countin move faster than control cells (21, 51). It is important to note that the computer simulations predicted, and the observations verified, that CF causes small changes in motility rather than all-or-none changes in order to modulate stream breakup and thus regulate group size.
Motility in D. discoideum is mediated by actin-driven protrusion of the leading edge and by cell body translocation driven at least in part by myosin II-mediated contraction of the actin cortex in areas away from the leading edge (for review, see references 10, 17, and 35). Myosin heavy chain kinase is recruited to these new protrusions and phosphorylates myosin II in order to prevent minifilament assembly (2, 34, 43, 47). Cells which are morphologically polarized (for instance due to exposure to a cAMP gradient) have a clear cytoskeletal polarity, with actin-filled protrusions at the front and myosin II accumulation at the rear (12, 14, 36, 39). CF increases the percentage of polarized cells in a population and increases the amount of phosphorylated myosin II heavy chains; CF decreases the amount of polymerized myosin II without affecting total levels of myosin II (51). CF also increases the amount of F-actin in cells without affecting the total levels of actin (51). A 1-min exposure of cells to recombinant countin increases the levels of F-actin, increases myosin II heavy chain phosphorylation, and decreases the amount of polymerized myosin without affecting total levels of myosin II heavy chain (21).
Myosin II heavy chain phosphorylation and assembly are regulated by myosin II heavy chain kinases (13, 30). A kinase called PAKa regulates myosin II assembly, cell polarity, and cell motility via the myosin II heavy chain kinases (9, 10). PAKa is, in turn, phosphorylated and regulated by Akt/protein kinase B (Akt/PKB) (10). Akt/PKB binds to phosphatidylinositol 3,4,5-trisphosphate (PIP3) via a pleckstrin-homology (PH) domain (49). The pulses of cAMP that mediate chemotaxis activate phosphatidylinositol 3 (PI3) kinases that cause a transient appearance of phosphatidylinositol(3,4,5)P3 (PIP3) and phosphatidylinositol(3,4)P2 at the leading edge of cells; this in turn causes Akt/PKB to transiently bind to the leading edge of the cell and become activated by another two 3′ phosphoinositide-regulated kinases named PDK1 and PDK2 (20, 25, 37). Another protein that binds to PIP3 at the leading edge of cells is PhdA (20). PhdA regulates the assembly of actin at the leading edge of cells (20), suggesting that the activation of PI3 kinase at the leading edge of cells causes both the disassembly of myosin and the appearance of an actin pseudopod at the leading edge. The pulses of cAMP that mediate chemotaxis activate a serpentine cAMP receptor, cAR1, which in turn activates a heterotrimeric G protein whose α subunit is Gα2 (37). This in turn causes a transient activation of PI3 kinase (25).
CF regulates the pathway between cAMP stimulation and motility (51). CF does not appear to affect cAMP binding to cells, cAMP binding to membranes, the GTPγS-induced inhibition of cAMP binding to membranes, GTP binding to membranes, cAMP-stimulated GTP binding to membranes, or cAMP-stimulated membrane GTPase, suggesting that CF does not affect the cAMP receptor, the associated G proteins, or their interaction (50). Since CF does affect myosin phosphorylation, myosin assembly, cell polarity, and motility, we have examined whether CF regulates Akt/PKB, a key step in the pathway between the cAMP receptor and motility, and found that CF modulates the cAMP-induced translocation and activity of Akt/PKB.
MATERIALS AND METHODS
Antibodies.
The peptide GFTYVAESEHLR, corresponding to amino acids 433 through 444 from D. discoideum Akt/PKB (GenBank accession no. U15210), was synthesized and used by Bethyl Laboratories (Montgomery, Tex.) to produce affinity-purified rabbit polyclonal antibodies. Anti-green fluorescent protein (GFP) antibodies (no. 632382) were obtained from BD Biosciences (Palo Alto, Calif.).
Cell culture.
Cell culture and development of cells were done as previously described (5). GFP-CRAC/crac− cells (a kind gift from Carole Parent) were cultured with 20 μg of G418 per ml. The preparation of CM and filter pad assays were performed as described elsewhere (6). Treatment of cells with 200 ng of recombinant countin per ml or 0.9 μg of affinity-purified anti-countin antibodies per ml followed the method of Gao et al. (21). Approximately once a week during the course of the experiments, we verified that countin− cells, wild-type cells, and wild-type cells treated with anti-countin antibodies or recombinant countin formed normal aggregation territory sizes; that countin− cells and wild-type cells treated with anti-countin antibodies formed large groups (less breakup of aggregation streams); and that wild-type cells treated with recombinant countin formed streams that broke into many small groups.
Akt/PKB activity assay.
Akt/PKB activity was measured according to the method of Meili et al. (37) with the following modifications: cells were starved in PBM (20 mM KH2PO4, 10 μM CaCl2, 1 mM MgCl2 [pH 6.1 with KOH]) in shaking culture (3) at a density of 5 × 106 cells/ml for 6 h without cAMP pulsing. Cells were then harvested by centrifugation and resuspended to a density of 3 × 107 cells/ml in PBM. A 200-μl sample was taken right before the cells were stimulated with cAMP, and the rest of the samples were taken at the indicated times after cAMP stimulation. Akt/PKB was immunoprecipitated with 1 μl of affinity-purified anti-Akt/PKB antibody (1.1 μg/μl). Akt/PKB kinase activity was assayed by incubating protein A beads (Roche, Indianapolis, Ind.) containing the immunocomplex with kinase buffer (37), [γ-32P]ATP (NEN, Boston, Mass.) and histone H2B (Roche). The samples were separated by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS-15% PAGE). The gel was then washed for 1 h with six changes of wash solution (5% trichloroacetic acid, 3% sodium pyrophosphate) and exposed to X-ray film to examine the extent to which the histone H2B was labeled with 32P.
Akt/PKB and CRAC translocation assay.
The translocation of Akt/PKB or cytosolic regulator of adenylyl cyclase (CRAC)-GFP to membranes was performed by the method of Parent et al. (41) with the following modifications: log-phase vegetative cells were harvested by centrifugation, resuspended in PBM to a density of 5 × 106 cells/ml, and allowed to starve in shaking culture for 6 h without exogenous pulses of cAMP, and the subsequent caffeine treatment was omitted. The cells were then harvested and resuspended to a density of 8 × 107 cells/ml in PM (41). A 200-μl sample was taken before the cells were stimulated with cAMP, mixed with an equal volume of lysis buffer (20 mM Tris-Cl [pH 8.0], 2 mM MgSO4), and lysed by filtration through a 5-μm-pore-size Cameo 17N nylon syringe filter (Osmonics, Minnetonka, Minn.) into a 1.5-ml Eppendorf tube containing 1 ml of ice-cold PM buffer. Microscopic examination of the filter-lysed cells indicated that less than 0.1% were not lysed, and this percentage was not affected by the cell strain used or the presence of recombinant countin or anti-countin antibodies. The rest of the samples were taken at the indicated times after cAMP stimulation and were similarly lysed. Membranes were collected by centrifugation for 4 min at 15,000 × g. Each pellet was dissolved in 60 μl of SDS sample buffer, and 15 μl of each sample was separated by SDS-7.5% PAGE and then blotted onto Immobilon-P membranes (Millipore, Bedford, Mass.). Western blotting was done by using an ECL Western blotting system following the manufacturer's directions (Amersham Biosciences, Piscataway, N.J.). The level of Akt/PKB translocation to membranes was assayed by staining the blotted membrane with 0.5 μg of affinity-purified anti-Akt/PKB antibodies per ml. CRAC-GFP/crac− cells were used for the CRAC PH domain translocation assay and Western blots were stained with 1:10,000 anti-GFP antibodies.
Visualization of Akt/PKB PH-GFP translocation.
Ax4 parental cells were electroporated according to the method of Pang et al. (40) with a vector in which expression of an AKT/PKB PH domain-GFP fusion protein is under control of the actin 15 promoter (37). The cells were selected for resistance to 10 μg of G418 per ml, and then fluorescent cells were selected by flow cytometry by using a FACScalibur (Becton Dickson Inc., Mountain View, Calif.). For visualization of Akt/PKB PH-GFP localization during pulsatile cAMP signaling, the cells were imaged by using an under-agarose development assay. Cells were harvested during vegetative growth, washed twice with MCPB buffer (10 mM Na2HPO4, 10 mM KH2PO4, 2 mM MgCl2, 0.2 mM CaCl2 [pH 6.5]) and resuspended in MCPB, and 2 ml of a 1:10 mixture of Akt/PKB PH-GFP cells and Ax2 cells at a final density of 106 cells/ml was plated into each of three 30-mm glass-bottom petri dishes (Willco Wells, Amsterdam, The Netherlands). In a second set of three dishes, an agarose gel was poured consisting of 2 ml of 1.5% DNA-grade agarose (Invitrogen, Carlsbad, Calif.) dissolved in MCPB plus either no addition, 2 μl of recombinant countin (at a concentration of 0.2 mg/ml), or 5 μl of affinity-purified anti-countin antibodies (at a concentration of 0.9 mg/ml). The recombinant countin and anti-countin antibodies were added just before the cooled agarose was added to the dish. Once the cells had attached to the first set of dishes, the buffer was removed, and the agarose gels were transferred to overlay the cells. The agarose flattens the aggregation streams so that the cAMP wave response is essentially two dimensional, making it much easier to visualize cell movement. The dishes were then incubated in a humid box until aggregation streams formed (6 to 10 h). Imaging was performed on a Leica SP2 laser scanning confocal microscope with a 488-nm argon laser line. Images were captured every 10 s in an area of broad flat aggregation streams by using a 100× objective with a numerical aperture of 1.4. For each treatment, data were collected for multiple pulses in the same region and in multiple regions. All data were collected with the same laser power and photomultiplier tube gain settings. Images were processed and analyzed by using ImageJ (developed at the National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/imagej).
RESULTS
Countin increases the cAMP-stimulated activation of Akt/PKB within 1 min.
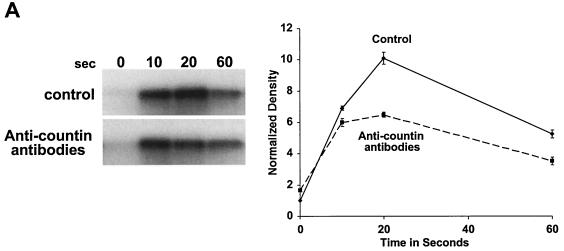
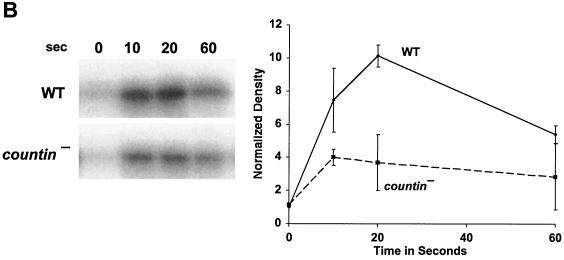
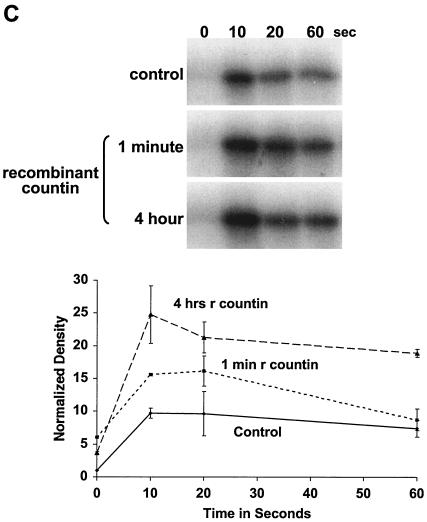
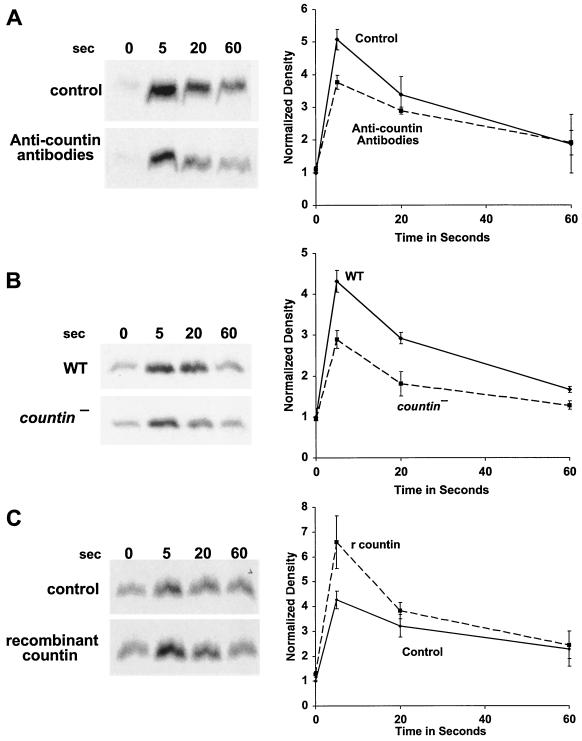
CF regulates group size in D. discoideum in part by regulating motility during stream formation (51), and starving cells in the presence of anti-countin antibodies increases group size and decreases motility (5, 51). Motility is regulated in turn by Akt/PKB (37). As the streams are forming and coalescing, Akt/PKB kinase activity is rapidly and transiently stimulated by each pulse of cAMP. The peak of Akt/PKB kinase activity is at 10 to 20 s after stimulation and then returns to the basal level at 60 s after cAMP stimulation (37). The Akt/PKB activity of our strain Ax4 wild-type cells was similarly stimulated by cAMP, and the peak was also reached at 10 to 20 s (Fig. 1A). In experiments done with wild-type cells, the peak of activity and the extent of the increase varied between 10 and 20 s. When Ax4 cells were starved in the presence of affinity-purified anti-countin antibodies, there was less of an increase in Akt/PKB activity at 20 s (Fig. 1A). There was also less of an increase in the level of cAMP-stimulated Akt/PKB activity in countin− cells than in parental Ax4 cells (Fig. 1B). However, when Ax4 cells were starved in the presence of 200 ng of recombinant countin per ml for 4 h, the cAMP-stimulated Akt/PKB activity of these cells increased (Fig. 1C). In cultures of starving D. discoideum cells, recombinant countin potentiates actin polymerization and inhibits myosin assembly within 1 min (21). Since Akt/PKB is involved in myosin assembly (10), we examined the effect on Akt/PKB activity of a 1-min treatment with recombinant countin. As shown in Fig. 1C, a 60-s exposure of cells to recombinant countin enhanced cAMP-stimulated Akt/PKB activity.
FIG. 1.
Countin potentiates cAMP-stimulated Akt/PKB activity. (A) Anti-countin antibodies decrease Akt/PKB activity. Ax4 cells were starved in the absence (control) or in the presence (4 h) of anti-countin antibodies. After 6 h of starvation, the cells were collected, stimulated with cAMP, and lysed at the indicated times after stimulation. Akt/PKB was immunoprecipitated from the NP-40-soluble fraction with anti-Akt/PKB antibodies, and Akt/PKB kinase activity was assayed by using [γ-32P]ATP and histone H2B as substrates. The samples were then subjected to SDS-PAGE, and the gel was exposed to X-ray film. The heavy band is phosphorylated histone H2B. The autoradiograms were scanned, and the integrated density in each band was calculated. All of the densities, including the time zero experimental value, were normalized to the time zero control value, which was set to 1. The graph shows the means ± standard errors of the means from three separate experiments. The difference at 20 s was significant (P < 0.01; t test). (B) cAMP-stimulated Akt/PKB activity is decreased in countin− cells. Akt/PKB activities were examined in 6-h-starved countin− cells and parental Ax4 cells (WT). Cells were stimulated with cAMP and processed as described for panel A. The graph shows data normalized as for panel A from three separate experiments. The difference at 20 s was significant (P < 0.05; t test). (C) Recombinant countin increases Akt/PKB activity. Ax4 cells were starved and exposed to 200 ng of recombinant countin per ml for 4 h, 1 min, or never (control) prior to harvesting at 6 h. Cells were stimulated with cAMP and assayed for Akt/PKB activity as described for panel A. The graph shows data normalized as for panel A from four separate experiments. The differences between control and either countin treatment at 10 s were significant (P < 0.01; t tests).
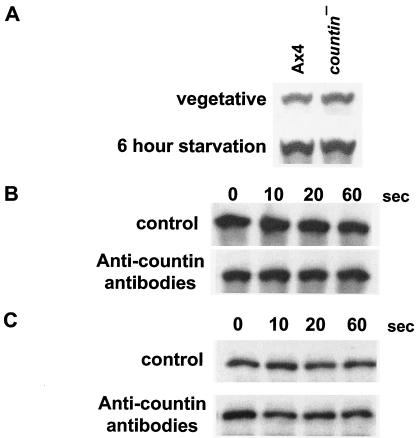
To determine if countin regulates the expression of Akt/PKB during growth and development, Western blots of vegetative and 6-h-starved countin− and Ax4 cells were stained with anti-Akt/PKB antibodies. There was no apparent difference in Akt/PKB protein levels in countin− and Ax4 cells (Fig. 2A). The exposure of cells to the anti-countin antibodies had no discernible effect on the total amount of Akt/PKB in cells (Fig. 2B) or the amount of Akt/PKB in the NP-40 extracts prior to immunoprecipitation (Fig. 2C). Treatment of cells with recombinant countin also had no observable effect on the amount of Akt/PKB in cells (data not shown). Together, our data suggest that countin potentiates cAMP-stimulated Akt/PKB activity within 1 min without affecting Akt/PKB protein levels.
FIG. 2.
Countin does not affect the level of Akt/PKB protein during development. (A) Vegetative and 6-h-starved Ax4 and countin− cells were collected, and Western blots of the total cell lysates were stained with anti-Akt/PKB antibodies. The results are representative of three independent experiments. (B) Western blots of total cell lysates from cells treated without (control) or with anti-countin antibodies were stained with anti-Akt/PKB antibodies. (C) Western blots of the NP-40-soluble fractions of the cell lysates were stained with anti-Akt/PKB antibodies. The difference in band intensities compared to bands shown in panel B is due to a difference in the exposure of the X-ray film to the chemiluminescence-stained Western blot.
Countin enhances the cAMP-stimulated transient translocation of Akt/PKB and CRAC from the cytosol to membranes.
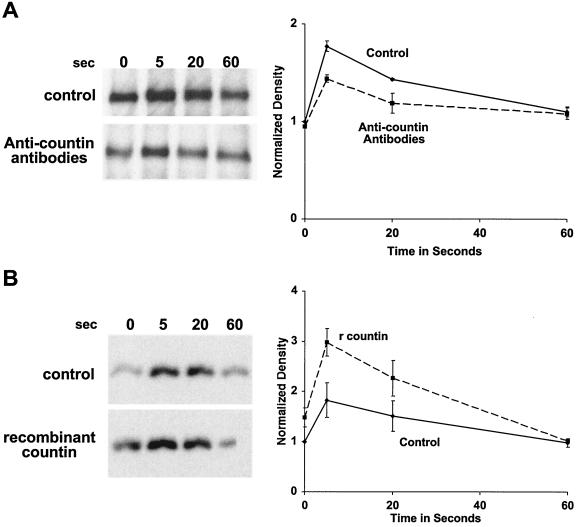
There are several possible ways in which countin could potentiate Akt/PKB activity. First, countin could stimulate PI3 kinase activity, and the increased level of PI(3,4,5)P3 and PI(3,4)P2 would cause an increased Akt/PKB binding to the membrane. Second, countin could inhibit the activity of the PI3-phosphatase, PTEN (11, 23). Third, countin could increase Akt/PKB activity through the induction of PDK1 and PKD2 activity. Using fluorescence microscopy of cells expressing the PH domain of Akt/PKB fused to GFP (Akt/PKB PH-GFP), Meili et al. (37) demonstrated that there is a transient membrane translocation of Akt/PKB PH-GFP with a peak between 3 and 17 s after cAMP stimulation. To determine if CF affects the translocation of Akt/PKB to membranes, strain Ax4 cells were starved for 6 h and stimulated with a saturating dose of cAMP (0.1 μM). After stimulation, cells were lysed, and the membrane fraction was collected by centrifugation. Western blots stained with anti-Akt/PKB antibodies showed that there was a transient translocation of Akt/PKB from the cytosol to membranes, with a peak between 5 and 20 s after cAMP stimulation (Fig. 3). When Ax4 cells were starved in the presence of anti-countin antibodies and were then stimulated with cAMP, there was reduced Akt/PKB translocation at 5 s (Fig. 3A). Compared to parental Ax4 cells, countin− cells also had a reduced level of Akt/PKB translocation (Fig. 3B). However, when Ax4 cells that had been starved for 6 h were exposed to 200 ng of recombinant countin per ml for 1 min, the levels of Akt/PKB translocation to membranes were increased at 5 s (Fig. 3C). Together, these results suggest that countin increases the cAMP-stimulated translocation of Akt/PKB from the cytosol to membranes.
FIG. 3.
Countin potentiates the cAMP-stimulated translocation of Akt/PKB to membranes. (A) Anti-countin antibodies inhibit Akt/PKB translocation to the membrane. Ax4 cells were developed in the presence or absence of anti-countin antibodies, collected at 6 h, and stimulated with cAMP, and then aliquots of cells were filter lysed at the indicated times after stimulation. The membrane fraction was collected and isolated by SDS-PAGE. Western blots stained with anti-Akt/PKB antibodies are shown. The graph shows densitometry of the X-ray films normalized as described for Fig. 1 from four separate experiments. The difference at 5 s was significant (P < 0.05; t test). (B) countin− cells have a reduced Akt/PKB translocation. Akt/PKB translocation to membranes was examined in 6-h-starved countin− cells and parental Ax4 cells (WT). Cells were stimulated with cAMP and processed as described for panel A. The graph shows data normalized as for panel A from four separate experiments. The difference at 5 s was significant (P < 0.01; t test). (C) A 1-min exposure of cells to recombinant countin potentiates Akt/PKB translocation to the membrane. After 6 h of starvation, Ax4 cells were exposed to 200 ng of recombinant countin per ml for 1 min, and then cells were stimulated by cAMP. Samples were treated as above, and Western blots were stained with anti-Akt/PKB antibodies. The graph shows data normalized as described for panel A from four separate experiments. The difference at 5 s was significant (P < 0.05; t test).
In D. discoideum, CRAC is another PH domain-containing protein, which translocates to the plasma membrane and plays an important role in directing cell movement and chemotaxis. cAMP causes a transient translocation of CRAC to the membrane, and the peak is within 5 to 10 s after cAMP stimulation (41). To determine if countin potentiates the translocation of not only Akt/PKB but also other PH domain-containing proteins, CRAC-GFP/crac− cells were starved for 6 h in the presence or absence of anti-countin antibodies and then stimulated with cAMP. Cells were lysed and membranes were isolated. Western blotting showed that CRAC-GFP associated with membranes at 5 to 20 s after stimulation and then dissociated from the membrane fraction at 60 s after cAMP stimulation, as previously observed (Fig. 4A) (41). When CRAC-GFP/crac−cells were starved in the presence of anti-countin antibodies and were then stimulated with cAMP, the levels of CRAC-GFP associated with the membrane fraction were reduced compared to levels in the control at 5 to 20 s (Fig. 4A). When 6-h-starved CRAC-GFP/crac− cells were incubated with 200 ng of recombinant countin per ml for 1 min, the levels of CRAC-GFP were increased compared to levels in untreated cells both immediately before and 5 s after the addition of cAMP (Fig. 4B). These results suggest that countin potentiates binding of CRAC-GFP to membranes after cells are exposed to a cAMP pulse.
FIG. 4.
Countin enhances cAMP-stimulated GFP-CRAC binding to membranes. (A) Treatment of cells with anti-countin antibodies inhibits CRAC translocation to the membrane. CRAC-GFP/crac− cells were treated as described in the legend of Fig. 3A, and Western blots were stained with anti-GFP antibodies. The graph shows data normalized as described for Fig. 3 from three separate experiments. The difference at 5 s was significant (P < 0.05; t test). (B) A 1-min treatment of cells with recombinant countin enhances cAMP-stimulated CRAC-GFP binding to membranes. CRAC-GFP/crac− cells were treated as described in the legend of Fig. 3C, and Western blots of the membranes were stained with anti-GFP antibodies. The graph shows data normalized as described for panel A from three separate experiments. The difference at 5 s was significant (P < 0.05; t test).
Countin does not affect Akt/PKB PH-GFP localization on the membrane.
Cells expressing the PH domain of Akt/PKB fused to GFP (Akt/PKB PH-GFP) show a transient cAMP-induced translocation of the fusion protein from the cytosol to the leading edge of the membrane (37). To visualize the effect of countin on this translocation, wild-type cells expressing Akt/PKB PH-GFP were starved in the presence of anti-countin antibodies or recombinant countin. Confocal fluorescence microscopy of cells in aggregation streams showed that control cells, cells starved in the presence of anti-countin antibodies, and cells starved in the presence of recombinant countin all had a transient translocation of Akt/PKB PH-GFP to the leading edge (data not shown). Under all three conditions, there was a considerable amount of variability from cell to cell and pulse to pulse in the intensity and size of the region to which the Akt/PKB PH-GFP translocated. In some cells, it was a narrow region at the anterior, while in other cells the Akt/PKB PH-GFP translocated to the anterior plus one or both lateral membranes of the cell. Although there appeared to be slightly more Akt/PKB PH-GFP translocating to the membrane in cells treated with recombinant countin and slightly less Akt/PKB PH-GFP translocating to the membrane in cells treated with anti-countin antibodies, there was no obvious effect of either treatment on the size of the domain at the leading edge that Akt/PKB PH-GFP translocated to (data not shown).
DISCUSSION
CF regulates group size in part by increasing cell polarity and cell motility (51). CF enhances actin polymerization and decreases myosin II assembly, and recombinant countin can do this within 1 min (21). Since actin polymerization provides the major driving force to mediate cell motility (22, 27, 44) and myosin II is also a key component of cell motility (46), it seems that CF increases cell motility by potentiating actin polymerization and repressing myosin II assembly. We found here that CF regulates Akt/PKB, a key regulator of motility and myosin polymerization.
CF appears to enhance both the translocation and the total activity of Akt/PKB. Since the first step of the Akt/PKB activation is membrane translocation (1, 18), it is possible that the increased translocation caused by CF leads to the observed increase in Akt/PKB activity. Both the translocation and the activity of Akt/PKB are potentiated by a 1-min treatment of cells with recombinant countin, and we previously observed that a 1-min treatment of cells with recombinant countin increases actin polymerization and myosin heavy chain phosphorylation and decreases myosin heavy chain polymerization (21). Since Akt/PKB is upstream of myosin in the cAMP-motility signal transduction pathway (8), our working hypothesis is that Akt/PKB is part of the fast signal transduction pathway whereby CF modulates the effect of cAMP on cell motility.
It has previously been observed that a 1-min exposure of cells to purified CF increases the cAMP-stimulated cAMP pulse (50) and a 1-min exposure of cells to recombinant countin increases GTPγS-stimulated adenylyl cyclase activity without affecting the basal activity or the Mn2+-stimulated activity (21). CRAC translocation to membranes is required for the GTPγS stimulation of adenylyl cyclase activity (31). A 1-min treatment of cells with recombinant countin causes a small increase in the translocation of CRAC to membranes, so one possibility is that CF regulates GTPγS stimulation of adenylyl cyclase activity by regulating CRAC translocation. We observed a smaller percent increase in CRAC translocation than was observed by Parent et al. (41). This may be due to differences in how the cells were treated, since we starved vegetative cells that were at a density of 2 ×106 cells/ml while Parent et al. used cells that were at a density of 5 × 106 cells/ml; we starved cells for 6 h in PBM at a density of 107 cells/ml without exogenous cAMP pulses or a caffeine treatment while Parent et al. starved cells in DB (5 mM Na2HPO4, 5 mM KH2PO4, 2 mM MgSO4, 0.2 mM CaCl2) at a density of 2 × 107 cells/ml with exogenous cAMP pulses and with a caffeine treatment. Using confocal microscopy of cells expressing GFP-CRAC to assay translocation, our laboratory previously reported that CF did not appear to affect cAMP-induced CRAC translocation (50). We observed here by using cell fractionation that although there is a quantitative change in the translocation of CRAC in the presence of anti-countin antibodies or recombinant countin, the changes are relatively small, and we doubt that we would have been able to see these changes by using imaging of cells expressing CRAC-GFP. We thus conclude that a previous report by our laboratory that CF does not affect CRAC translocation was incorrect.
Since CF appears to regulate both CRAC and Akt/PKB translocation, this suggests that CF either regulates the translocation of the two proteins independently or through a common mechanism. Both proteins bind to PIP3 on membranes (15, 16, 31, 37, 41, 48, 52, 53). If there is a common mechanism, a good candidate for regulation by CF would be the appearance of PIP3 on membranes. Each pulse of cAMP causes a transient translocation to and activation of PI3 kinase at the leading edge of cells (19, 25, 38) and a transient removal of the PI3 phosphatase PTEN from the leading edge of cells (11, 19, 26), and so one possibility is that the CF signal (which changes relatively slowly with time) modulates the relatively rapid pulsatile activity and/or translocation of PI3 kinase and/or PTEN.
We found that CF does not have an obvious effect on the area of the plasma membrane that the Akt/PKB PH domain localizes to. This localization is due to the binding of Akt/PKB to PIP3 (25, 37, 49). Disruption of the PI3 phosphatase PTEN leads to cells that show an abnormally broad region of Akt/PKB PH-GFP localization (26). However, the pi3k1/2− (cells lacking the two PI3 kinases that appear to be regulated by pulses of cAMP) and pten− cells do not aggregate. This is in contrast to smlA− cells and cells treated with recombinant countin, recombinant CF45-1, recombinant CF50, or combinations of these proteins, where in all cases examined the cells do aggregate (4, 6, 7, 21). Thus, if CF does regulate Akt/PKB translocation and activation by regulating either PI3 kinase or PTEN, there is likely to be CF-independent basal and cAMP-regulated activity.
The cAMP-stimulated activation of PI3 kinase and, thus, the translocation and activation of Akt/PKB require the presence of the cAMP receptor-activated G protein components Gα2 and Gβ, as well as the starvation response kinase YakA (25). The cAR1 cAMP receptor, Gα2, Gβ, the Ras subfamily protein RasC, and the Ras guanine exchange factor AleA are also required for Akt/PKB activation (32, 37). CF does not appear to affect the cAR2 cAMP receptor or its interaction with G proteins (50). Thus, possible candidates in the cAMP to motility pathway that might mediate the regulation of myosin II polymerization and assembly by CF would include Akt/PKB itself, one or more of the PI3 kinases, PTEN, YakA, RasC, and AleA. Our working hypothesis is, thus, that by modulating the activity of a preexisting pathway that mediates chemotaxis, a secreted factor is able to regulate group size in D. discoideum.
Acknowledgments
We thank Carole Parent for the gift of GFP-CRAC/crac− cells and an anonymous reviewer for suggesting the Akt/PKB PH-GFP translocation experiment.
R.H.G. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grant C-1555 from the Robert A. Welch Foundation. D.A.K. was supported by a grant from the National Institutes of Health (GM40599).
REFERENCES
- 1.Alessi, D., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Berlot, C. H., J. A. Spudich, and P. N. Devreotes. 1985. Chemoattractant-elicited increases in myosin phosphorylation in Dictyostelium. Cell 43:307-314. [DOI] [PubMed] [Google Scholar]
- 3.Brock, D. A., F. Buczynski, T. P. Spann, S. A. Wood, J. Cardelli, and R. H. Gomer. 1996. A Dictyostelium mutant with defective aggregate size determination. Development 122:2569-2578. [DOI] [PubMed] [Google Scholar]
- 4.Brock, D. A., K. Ehrenman, R. Ammann, Y. Tang, and R. H. Gomer. 2003. Two components of a secreted cell-number counting factor bind to cells and have opposing effects on cAMP signal transduction in Dictyostelium. J. Biol. Chem. 278:52262-52272. [DOI] [PubMed] [Google Scholar]
- 5.Brock, D. A., and R. H. Gomer. 1999. A cell-counting factor regulating structure size in Dictyostelium. Genes Dev. 13:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock, D. A., R. D. Hatton, D.-V. Giurgiutiu, B. Scott, R. Ammann, and R. H. Gomer. 2002. The different components of a multisubunit cell number-counting factor have both unique and overlapping functions. Development 129:3657-3668. [DOI] [PubMed] [Google Scholar]
- 7.Brock, D. A., R. D. Hatton, D.-V. Giurgiutiu, B. Scott, W. Jang, R. Ammann, and R. H. Gomer. 2003. CF45-1, a secreted protein which participates in group size regulation in Dictyostelium. Eukaryot. Cell 2:788-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung, C., G. Potikyan, and R. Firtel. 2001. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell 7:937-947. [DOI] [PubMed] [Google Scholar]
- 9.Chung, C. Y., and R. A. Firtel. 1999. PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J. Cell Biol. 147:559-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung, C. Y., S. Funamoto, and R. A. Firtel. 2001. Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26:557-566. [DOI] [PubMed] [Google Scholar]
- 11.Comer, F. I., and C. A. Parent. 2002. PI 3-kinases and PTEN: how opposites chemoattract. Cell 109:541-544. [DOI] [PubMed] [Google Scholar]
- 12.Condeelis, J. 1992. Are all pseudopods created equal? Cell Motil. Cytoskeleton 22:1-6. [DOI] [PubMed] [Google Scholar]
- 13.de la Roche, M., and G. Cote. 2001. Regulation of Dictyostelium myosin I and II. Biochim. Biophys. Acta 1525:245-261. [DOI] [PubMed] [Google Scholar]
- 14.Dharmawardhane, S., V. Warren, A. L. Hall, and J. Condeelis. 1989. Changes in the association of actin-binding proteins with the actin cytoskeleton during chemotactic stimulation of Dictyostelium discoideum. Cell Motil. Cytoskeleton 13:57-63. [DOI] [PubMed] [Google Scholar]
- 15.Dormann, D., T. Libotte, C. J. Weijer, and T. Bretschneider. 2002. Simultaneous quantification of cell motility and protein-membrane association using active contours. Cell Motil. Cytoskeleton 52:221-230. [DOI] [PubMed] [Google Scholar]
- 16.Dormann, D., G. Weijer, C. Parent, P. Devreotes, and C. Weijer. 2002. Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr. Biol. 12:1178. [DOI] [PubMed] [Google Scholar]
- 17.Elson, E. L., S. F. Felder, P. Y. Jay, M. S. Kolodney, and C. Pasternak. 1999. Forces in cell locomotion. Biochem. Soc. Symp. 65:299-314. [PubMed] [Google Scholar]
- 18.Frech, M., M. Andjelkovich, E. Ingley, K. Reddy, J. Falck, and B. Hemmings. 1997. High affinity binding of inositol phosphatase and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J. Biol. Chem. 272:8474-8481. [DOI] [PubMed] [Google Scholar]
- 19.Funamoto, S., R. Meili, S. Lee, L. Parry, and R. A. Firtel. 2002. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109:611-623. [DOI] [PubMed] [Google Scholar]
- 20.Funamoto, S., K. Milan, R. Meili, and R. Firtel. 2001. Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J. Cell Biol. 153:795-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, T., K. Ehrenman, L. Tang, M. Leippe, D. A. Brock, and R. H. Gomer. 2002. Cells respond to and bind countin, a component of a multisubunit cell number counting factor. J. Biol. Chem. 277:32596-32605. [DOI] [PubMed] [Google Scholar]
- 22.Higgs, H., and T. Pollard. 2001. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70:649-676. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi, M., N. Masuyama, Y. Fukui, A. Suzuki, and Y. Gotoh. 2001. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr. Biol. 11:1958-1962. [DOI] [PubMed] [Google Scholar]
- 24.Hohl, H. R., and K. B. Raper. 1964. Control of sorocarp size in the cellular slime mold Dictyostelium discoideum. Dev. Biol. 9:137-153. [Google Scholar]
- 25.Huang, Y. E., M. Iijima, C. A. Parent, S. Funamoto, R. A. Firtel, and P. Devreotes. 2003. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell 14:1913-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima, M., and P. Devreotes. 2002. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109:599-610. [DOI] [PubMed] [Google Scholar]
- 27.Iijima, M., Y. E. Huang, and P. Devreotes. 2002. Temporal and spatial regulation of chemotaxis. Dev. Cell 3:469-478. [DOI] [PubMed] [Google Scholar]
- 28.Kessin, R. H. 2001. Dictyostelium evolution, cell biology, and the development of multicellularity. Cambridge University Press, New York, N.Y.
- 29.Kopachik, W. J. 1982. Size regulation in Dictyostelium. J. Embryol. Exp. Morphol. 68:23-35. [PubMed] [Google Scholar]
- 30.Liang, W., L. Licate, H. Warrick, J. Spudich, and T. Egelhoff. 2002. Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biol. 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilly, P. J., and P. N. Devreotes. 1995. Chemoattractant and GTP gamma S-mediated stimulation of adenylyl cyclase in Dictyostelium requires translocation of CRAC to membranes. J. Cell Biol. 129:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim, C., G. Spiegelman, and G. Weeks. 2001. RasC is required for optimal activation of adenylyl cyclase and Akt/PKB during aggregation. EMBO J. 20:4490-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loomis, W. F. 1975. Dictyostelium discoideum: a developmental system. Academic Press, New York, N.Y.
- 34.Luck-Vielmetter, D., M. Schleicher, B. Grabatin, J. Wippler, and G. Gerisch. 1990. Replacement of threonine residues by serine and alanine in a phosphorylatable heavy chain fragment of Dictyostelium myosin-II. FEBS Lett. 269:239-243. [DOI] [PubMed] [Google Scholar]
- 35.Manstein, D. J. 1993. Myosin function in the motile behaviour of cells. Symp. Soc. Exp. Biol. 47:375-381. [PubMed] [Google Scholar]
- 36.McRobbie, S. J., and P. C. Newell. 1983. Changes in actin associated with cytoskeleton following chemotactic stimulation of Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 115:351-359. [DOI] [PubMed] [Google Scholar]
- 37.Meili, R., C. Ellsworth, S. Lee, T. B. Reddy, H. Ma, and R. A. Firtel. 1999. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18:2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merlot, S., and R. A. Firtel. 2003. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 116:3471-3478. [DOI] [PubMed] [Google Scholar]
- 39.Noegel, A. A., and J. E. Luna. 1995. The Dictyostelium cytoskeleton. Experientia 51:1135-1143. [DOI] [PubMed] [Google Scholar]
- 40.Pang, K. M., M. A. Lynes, and D. A. Knecht. 1999. Variables controlling the expression level of the exogenous genes in Dictyostelium. Plasmid 41:187-197. [DOI] [PubMed] [Google Scholar]
- 41.Parent, C. A., B. J. Blacklock, W. M. Froehlich, D. B. Murphy, and P. N. Devreotes. 1998. G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95:81-91. [DOI] [PubMed] [Google Scholar]
- 42.Roisin-Bouffay, C., W. Jang, and R. H. Gomer. 2000. A precise group size in Dictyostelium is generated by a cell-counting factor modulating cell-cell adhesion. Mol. Cell 6:953-959. [PubMed] [Google Scholar]
- 43.Sabry, J. H., S. L. Moores, S. Ryan, J. H. Zang, and J. A. Spudich. 1997. Myosin heavy chain phosphorylation sites regulate myosin localization during cytokinesis in live cells. Mol. Biol. Cell 8:2605-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt, A., and M. Hall. 1998. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14:305-338. [DOI] [PubMed] [Google Scholar]
- 45.Shaffer, B. M. 1957. Variability of behavior of aggregating cellular slime moulds. Q. J. Microsc. Sci. 98:393-405. [Google Scholar]
- 46.Spudich, J. A., J. Finer, B. Simmons, K. Ruppel, B. Patterson, and T. Uyeda. 1995. Myosin structure and function. Cold Spring Harbor Symp. Quant. Biol. 60:783-791. [DOI] [PubMed] [Google Scholar]
- 47.Steimle, P., S. Yumura, G. Cote, Q. Medley, M. Polyakov, B. Leppert, and T. Egelhoff. 2001. Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr. Biol. 11:708-713. [DOI] [PubMed] [Google Scholar]
- 48.Sun, B., and R. A. Firtel. 2003. A regulator of G protein signaling-containing kinase is important for chemotaxis and multicellular development in Dictyostelium. Mol. Biol. Cell 14:1727-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka, K., H. Adachi, H. Konishi, A. Iwamatsu, K. Ohkawa, T. Shirai, S. Nagata, U. Kikkawa, and Y. Fukui. 1999. Identification of protein kinase B (PKB) as a phosphatidylinositol 3,4,5-trisphosphate binding protein in Dictyostelium discoideum. Biosci. Biotechnol. Biochem. 63:368-372. [DOI] [PubMed] [Google Scholar]
- 50.Tang, L., R. Ammann, T. Gao, and R. H. Gomer. 2001. A cell number-counting factor regulates group size in Dictyostelium by differentially modulating cAMP-induced cAMP and cGMP pulse sizes. J. Biol. Chem. 276:27663-27669. [DOI] [PubMed] [Google Scholar]
- 51.Tang, L., T. Gao, C. McCollum, W. Jang, M. G. Vickers, R. Ammann, and R. H. Gomer. 2002. A cell number-counting factor regulates the cytoskeleton and cell motility in Dictyostelium. Proc. Natl. Acad. Sci. USA 99:1371-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsujioka, M., M. Yokoyama, K. Nishio, H. Kuwayama, T. Morio, M. Katoh, H. Urushihara, T. Saito, H. Ochiai, Y. Tanaka, I. Takeuchi, and M. Maeda. 2001. Spatial expression patterns of genes involved in cyclic AMP responses in Dictyostelium discoideum development. Dev. Growth Differ. 43:275-283. [DOI] [PubMed] [Google Scholar]
- 53.Wang, B., G. Shaulsky, and A. Kuspa. 1999. Multiple developmental roles for CRAC, a cytosolic regulator of adenylyl cyclase. Dev. Biol. 208:1-13. [DOI] [PubMed] [Google Scholar]