Abstract
Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase required for Saccharomyces cerevisiae to adapt to a variety of environmental stresses. Once activated, calcineurin dephosphorylates the Zn-finger transcription factor Crz1p/Tcn1p, causing it to accumulate in the nucleus where it activates gene expression. Here we show that cyclic AMP-dependent protein kinase A (PKA) phosphorylates and negatively regulates Crz1p activity by inhibiting its nuclear import. Activation of PKA in vivo decreases Crz1p-dependent transcription. PKA phosphorylates Crz1p in vitro, and we identify specific residues required for this phosphorylation, all of which reside in or adjacent to the nuclear localization signal. Mutation of these residues to alanine results in increased nuclear import of Crz1p and results in higher levels of both basal and Ca2+-induced Crz1p transcriptional activity. PKA regulates the general stress response in yeast and coordinates this response with nutrient availability. In contrast, calcineurin regulates the cellular response to a restricted set of environmental insults. Thus, these studies identify a specific biochemical mechanism through which the activities of multiple stress-activated signaling pathways are integrated in vivo.
Activation of Ca2+-dependent signal transduction pathways is critical for many cellular responses. In the yeast Saccharomyces cerevisiae, Ca2+ promotes cell survival under a variety of environmental stress conditions (9). Calcineurin, a conserved Ca2+/calmodulin-dependent protein phosphatase is crucial for these responses. Yeast cells growing under standard laboratory conditions exhibit low levels of cytosolic Ca2+ and basal levels of calcineurin activity. Thus, cells lacking calcineurin activity are viable under these conditions. In contrast, exposure of cells to stresses such as high salt, alkaline pH, and cell wall damage leads to elevated cytosolic Ca2+ and promotes signaling through calcineurin, which is required for survival under these conditions (9).
A primary function of calcineurin in yeast is the dephosphorylation and activation of the transcription factor Crz1p/Tcn1p/Hal8p (23, 25, 34). Under standard growth conditions, Crz1p is phosphorylated and localizes to the cytosol. Dephosphorylation by calcineurin results in the rapid translocation of Crz1p to the nucleus, where it activates the transcription of a variety of genes whose products promote adaptation to stress (35, 41). Nuclear accumulation of Crz1p is the result of an increase in nuclear import and a concomitant decrease in nuclear export, processes that are both calcineurin dependent (3, 28). Once in the nucleus, Crz1p binds a DNA element known as the CDRE (for calcineurin-dependent response element), through which it activates gene transcription (34). Crz1p targets include genes whose products are involved in ion homeostasis, cell wall maintenance, vesicle transport, lipid synthesis, and small molecule transport (8, 15, 24, 26, 29, 41). All known calcineurin-dependent transcriptional changes are believed to be mediated through Crz1p (41).
We have previously shown that Crz1p is phosphorylated and negatively regulated by the casein kinase I homolog Hrr25p (18). However, we also demonstrated that Hrr25p is insufficient to fully inhibit Crz1p activity, indicating the existence of additional Crz1p kinases. We show here that cyclic AMP (cAMP)-dependent protein kinase A (PKA) is one such kinase. PKA is an essential component of the general stress response in yeast and functions to coordinate metabolism, cell growth, and stress resistance with nutrient availability (36). The PKA holoenzyme is a heterotetramer composed of two catalytic subunits and two regulatory subunits. Binding of cAMP to the regulatory subunits results in dissociation and activation of the catalytic subunits (20). The yeast genome encodes three homologous catalytic subunits (TPK1, TPK2, and TPK3) and a single regulatory subunit (BCY1) (37, 38). Any single catalytic subunit is sufficient for viability, but deletion of all three subunits results in lethality (38). Yeast cells lacking PKA arrest in G0 and share many characteristics with nutrient-starved cells. In contrast, cells with constitutively high levels of PKA activity fail to enter stationary phase under nutrient deprivation. These observations have led to the model that levels of cAMP and PKA activity signal nutrient availability to the cell (36).
Previous work has suggested that the PKA and calcineurin signaling pathways function antagonistically in yeast, since overexpression of the low-affinity cAMP phosphodiesterase PDE1 suppresses the Na+ sensitivity of calcineurin-deficient cells (16). Based on this observation and because both PKA and calcineurin are important mediators of stress response pathways, we investigated whether Crz1p is a target of PKA. In the present study, we demonstrate that PKA opposes calcineurin signaling by directly phosphorylating Crz1p and inhibiting its activity. We also provide evidence that PKA phosphorylates the Crz1p nuclear localization signal (NLS), thereby preventing the nuclear import of Crz1p. These studies define a novel role for PKA in regulation of Crz1p signaling and provide a mechanism by which two critical stress response pathways, calcineurin and PKA, are coordinated.
MATERIALS AND METHODS
Yeast media and general methods.
Standard media and culture conditions were used (31) except that twice the level of amino acids and nucleotides were added to synthetic media. Selective media used for Ca2+ induction were made with 3.5% NH4Cl instead of NH4SO4. Yeast extract-peptone-dextrose (YPD) media used for Ca2+ induction was buffered to pH 5.5 with 40 mM succinate. FK506 was used at 2 μg/ml and generously provided by Fujisawa Corp. Yeast cells were transformed by the lithium acetate method, and bacterial cells were transformed by electroporation (2). All recombinant DNA manipulations were performed according to standard protocols (2). Plasmids were prepared by using Qiagen spin miniprep kits (Qiagen).
Yeast strains.
The yeast strains used in the present study are described in Table 1. Strain KKY385 was created by deletion of BCY1 in ASY832 (TRP1-4xCDRE-lacZ::ura3-52; A. Stathopoulos, unpublished data) by using a BCY1::loxP-KanMX-loxP cassette generated by PCR (14). KKY420 was created by generating a NMD5-GST::LoxP-KanMX-LoxP insertion cassette by PCR from pFA6a-GST-kanMX6 (21), followed by transformation into BJ5459. KKY436 was created by integrating a 4xCDRE::LacZ reporter fusion (pAMS367) (34) into a cdc35 pde2 yak1 strain (12).
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| YPH499 | MATaura3-52 lys2-801 ade2-101 trp-Δ63 his3-Δ200 leu2-Δ1 | 32 |
| ASY832 | YPH499, but TRP1-4xCDRE-LacZ::ura3-52 | A. Stathopoulos, unpublished |
| BJ5459 | MATaura3-52 trp1 lys2-801 leu2-Δ1 his3-Δ200 pep4::HIS3 prb1-Δ1.6R can1 GAL | 17 |
| KKY271 | BJ5459, except CRZ1::ZZ-tev-S::kanMX | 18 |
| KKY385 | ASY832, but bcy1Δ::loxP-kanMX-loxP | This study |
| KKY420 | BJ5459, except NMD5::GST::kanMX | This study |
| W303-1A | MATaleu2 his3 trp1 ade2 can1 | 12 |
| W303 msn2 msn4 | W303, except msn2::HIS3 msn4::TRP1 | 12 |
| W303 tpk1,2,3 msn2 msn4 | W303, except tpk1::URA tpk2::HIS3 tpk3::TRP1 msn2::HIS3 msn4::TRP1 | 12 |
| W303 cdc35 pde2 yak1 | W303, except cdc35::KanMX pde2::TRP1 yak1::LEU2 | 12 |
| KKY436 | cdc35 pde2 yak1 URA3-4xCDRE-LacZ::ura3-52 | This study |
| KWY251 | ASY832, but crz1Δ::loxP-kanMX-loxP | K. Williams, unpublished |
Plasmids.
The plasmids used here are listed in Table 2. pKK209 was created by PCR amplification of TPK1 flanked by BamHI and XhoI sites and ligated into pGEX4T-3 (Stratagene). To create pKK220, pKK227, pKK228, and pKK229, the respective fragments of CRZ1 were amplified from pRSP121 flanked by BamHI and XhoI sites and cloned into pGEX4T-3. pKK245 was generated in two steps with the QuikChange site-directed mutagenesis kit (Stratagene): first, serines 409 and 410 of CRZ1 were mutated to alanine in pRSP121 to create pKK240; second, serine 423 of CRZ1 was altered to alanine in pKK240 to create pKK245. pKK258 was created by mutation of serines 427 and 429 to alanine by using the QuikChange site-directed mutagenesis kit (Stratagene) and pKK245 as a template. CRZ1 flanked by BamHI and SalI sites was generated by PCR with pRSP121 as a template and cloned into pUG36 (CEN URA3 pMET25-yEGFP3; gift from U. Guldener and J. H. Hegemann, Institute for Microbiology, Heinrich-Heine University, Dusseldorf, Germany) to create pKK249. pKK251 and pKK267 were generated by amplifying CRZ1 flanked by BamHI and SalI sites from pKK245 and pKK258, respectively, and ligated into pUG36. To generate pKK260 and pKK261, CRZ1341-440 was amplified from pRSP121 and pKK245, respectively, flanked by HindIII and SalI sites and cloned into pOM4 (28). CRZ1341-440 was amplified by PCR from pKK245 and pKK258 flanked by BamHI and XhoI sites and cloned into pGEX4T-3 to create pKK255 and pKK265, respectively.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| pKK209 | TPK1 in pGEX4T-3 | This study |
| pKK220 | CRZ1341-440 in pGEX4T-3 | This study |
| pKK227 | CRZ11-180 in pGEX4T-3 | This study |
| pKK228 | CRZ1181-340 in pGEX4T-3 | This study |
| pKK229 | CRZ1441-678 in pGEX4T-3 | This study |
| pKK245 | CRZ1S409,410,423A in pGEX4T-3 | This study |
| pKK249 | CRZ1 in pUG36 | This study |
| pKK251 | CRZ1S409,410,423A in pUG36 | This study |
| pKK255 | CRZ1341-440 in pGEX4T-3 S409,410,423A | This study |
| pKK258 | CRZ1S409,410,423,427,429A pGEX4T-3 | This study |
| pKK267 | CRZ1S409,410,423,427,429A in pUG36 | This study |
| pKK260 | CRZ1341-440 in pOM4 | This study |
| pKK261 | CRZ1341-440 in pOM4 S409,410,423A | This study |
| pKK265 | CRZ1341-440 in pGEX4T-3 S409,410,423, 427,429A | This study |
| pRSP121 | GST-CRZ1 in pGEX4T-3 | 28 |
| pGST-BCY1 | BCY1 yeast expression vector | 42 |
| pGST-TPK1 | TPK1 yeast expression vector | 42 |
In vitro analysis of Crz1p phosphorylation and dephosphorylation.
glutathione S-transferase (GST) fusions of Crz1p and its fragments and Tpk1p were isolated from extracts by affinity purification as described previously (18). In vitro phosphorylation reactions were performed as follows: 2 μg of each GST-Crz1p fusion was incubated with 2 μg of GST-Tpk1p, 100 μM ATP, and 10 μCi of [γ-32P]ATP (Amersham) in a total volume of 20 μl of kinase buffer (50 mM Tris [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol) with a standard cocktail of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 2 μg of leupeptin/ml, 2 μg of pepstatin/ml, 2 μg of aprotinin/ml) added for 40 min at 30°C. The reactions were passed over Centri-Sep columns (Princeton Separations) to remove unincorporated label and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. For dephosphorylation reactions, 1 μg of GST-Crz1p was phosphorylated by 2 μg of GST-Tpk1p as described above, except in a total volume of 10 μl. For dephosphorylation reactions, we added the following to the kinase reactions: (i) 10 μl of distilled H2O; (ii) 500 U of calmodulin (Sigma), 1 U of calcineurin (Sigma), and 20 mM CaCl2; or (iii) 500 U of calmodulin, 1 U of calcineurin, and 20 mM EGTA. The reaction volume was adjusted to 20 μl, followed by incubation at 30°C for 40 min. The reactions were terminated by the addition of loading dye, and samples were analyzed by SDS-PAGE and autoradiography.
Phosphorylation of GST-Crz1p by yeast extracts.
Extracts were made from log-phase msn2Δ msn4Δ and tpk1Δ tpk2Δ tpk3Δ msn2Δ msn4Δ cells. A total of 5 μg of recombinant GST-Crz1p was immobilized on glutathione beads, followed by incubation with 250 μg of each extract, 10 μCi of [γ-32P]ATP, an ATP-regenerating system (25 U of pyruvate kinase [Calbiochem], 4 mM ATP, 5 mM phosphoenolpyruvate), 2 μg of FK506/ml, phosphatase inhibitors, and 3 mM cAMP in kinase buffer; the volume was then adjusted to 200 μl. The reactions were incubated on a rotator at 30°C for 40 min, the beads were washed once with Buffer 88 (20 mM HEPES [pH 6.8], 150 mM potassium acetate, 250 mM sorbitol, 2 mM magnesium acetate, 1 mM dithiothreitol) plus 0.1% Triton X-100, and SDS sample buffer was added. Samples were analyzed by SDS-PAGE and imaged by using a phosphor screen and Typhoon Imager (Amersham). Quantitation was performed with ImageQuant v.5.0 software.
Quantitative β-galactosidase assays.
For comparison of Crz1pWT, Crz1pS409,410,423A, and Crz1pS409,410,423,427,429A activity, KWY251 strains expressing pKK249, pKK251, or pKK267, respectively, were grown overnight in synthetic medium lacking uracil. Cells were then diluted to an optical density at 600 nm (OD600) of 0.25 into medium lacking uracil and methionine and grown for 4 h. CaCl2 was added as indicated for an additional 1 h. For PKA activation, strain KKY436 was grown to log phase, 100 mM CaCl2 and 3 mM cAMP were added as indicated, and cells were grown for an additional 2 h. For PKA overexpression assays, ASY832 or KKY385 cells were grown to log phase and induced with 100 mM CaCl2 where indicated for 3 h. Extracts were made, and β-galactosidase asssays were performed as described previously (18). The β-galactosidase activity is reported as the maximal rate of OD415 change/minute/microgram of protein.
Analysis of Crz1p interactions.
Yeast cultures were grown to log phase and harvested by centrifugation. Cells expressing pRD56, GST-TPK1, and GST-BCY1 were grown overnight in medium containing raffinose to an OD600 of 0.5 and then induced with 2% galactose for 4 h. KKY420 cells expressing pUG36, green fluorescent protein (GFP)-CRZ1WT (pKK249), or GFP-CRZ1S409,410,423A (pKK251) were grown in synthetic medium lacking uracil and containing 0.5× methionine, and 2 μg of FK506/ml was added for the final 30 min of culture time where indicated. Cells were resuspended in 100 μl of Buffer 88 with protease inhibitors and 2 μg of FK506/ml where indicated. Cells were lysed by glass bead disruption as described previously (40). For pulldowns, 20 μl of packed, equilibrated immunoglobulin G (IgG) or glutathione-Sepharose (Amersham) was added to the extracts, and the total volume brought up to 200 μl in Buffer 88. The reactions were placed on a rotator at 4°C for 1 h and then washed three times in Buffer 88 plus 0.1% NP-40. The beads were resuspended in loading dye and loaded onto a 7% polyacrylamide gel. Crz1p-ZZ, GST-Nmd5p, GST-Tpk1p, and GST-Bcy1 were visualized by using a rabbit α-Crz1p polyclonal antiserum (which also recognizes GST) and an anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham). GFP-Crz1p was visualized by using a monoclonal anti-GFP JL-8 antibody (BD Biosciences) and an anti-mouse horseradish peroxidase-conjugated secondary antibody (Amersham). Immunoblots were developed by using enhanced chemiluminescence (Amersham).
Fluorescence microscopy.
Cells expressing GFP fusions were visualized as described previously (3) by using a Nikon Eclipse E600 microscope with fluorescence optics and an HB100 mercury lamp. Fluorescein filter sets (Chroma) were used to visualize GFP. Photos were taken by using a Hamamatsu digital charge-coupled device 47420-95 camera and QED software (QED Imaging). BJ5459 cells expressing pKK260 or pKK261 were grown to log phase at room temperature in synthetic medium lacking uracil and methionine and then visualized.
RESULTS
PKA phosphorylates and physically interacts with Crz1p in vitro and in vivo.
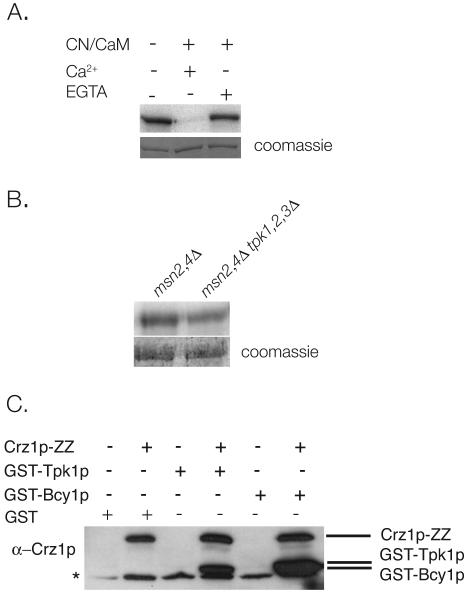
To determine whether PKA affects calcineurin signaling through Crz1p, we first investigated whether Crz1p is a direct target of PKA. Crz1p and Tpk1p were purified from E. coli as GST fusions and incubated together in the presence of [γ-32P]ATP. Incubation of Crz1p with Tpk1p results in Crz1p phosphorylation, indicating that Tpk1p directly phosphorylates Crz1p in vitro (Fig. 1A). We noted that the electrophoretic mobility of Tpk1p-phosphorylated Crz1p was indistinguishable from that of nonphosphorylated Crz1p. In contrast, fully phosphorylated Crz1p isolated from calcineurin -deficient cells exhibits a significant decrease in mobility (35).
FIG. 1.
PKA and Crz1p interact in vivo and in vitro. (A) Crz1p is phosphorylated by Tpk1p in vitro and can subsequently be dephosphorylated by calcineurin. Recombinant GST-Crz1p and GST-Tpk1p were incubated with ATP and [γ-32P]ATP for 40 min at 30°C, followed by incubation with buffer, calcineurin-calmodulin-Ca2+, or calcineurin-calmodulin-EGTA. Samples were analyzed by SDS-PAGE and autoradiography. (B) Crz1p phosphorylation is reduced in extracts lacking PKA. A total of 5 μg of GST-Crz1p was immobilized on glutathione-Sepharose and then incubated with extracts of msn2Δ msn4Δ cells or msn2Δ msn4Δ tpk1Δ tpk2Δ tpk3Δ cells (12), [γ-32P]ATP, and an ATP-regenerating system for 40 min at 30°C. Samples were analyzed by SDS-PAGE and imaged on a Typhoon scanner for quantitation. (C) Crz1p interacts with Tpk1p and Bcy1p in vivo. IgG-Sepharose was used to isolate Crz1p-ZZ from extracts of cells (BJ5459 and KKY271) expressing GST (pRD56), GST-Tpk1p, or GST-Bcy1p (42). Samples were analyzed by SDS-PAGE and Western blotting. Proteins were visualized with an α-Crz1p antibody that also recognizes GST. The asterisk marks a nonspecific band that cross-reacts with the α-Crz1p antibody.
Next we tested the ability of calcineurin to dephosphorylate Crz1p phosphorylated by Tpk1p. Crz1p was phosphorylated by Tpk1p in vitro and incubated with calcineurin in the presence of calmodulin and Ca2+. Under these conditions, Crz1p is specifically dephosphorylated by calcineurin, as this reaction was inhibited by the addition of the Ca2+ chelator EGTA (Fig. 1A). These results suggest that the phosphorylation of Crz1p by Tpk1p may be physiologically significant.
To determine whether Crz1p phosphorylation is reduced in the absence of PKA activity, we examined the effect of PKA on Crz1p phosphorylation in cell extracts. Recombinant GST-Crz1p immobilized on glutathione beads was incubated with extracts from control or PKA mutant cells. Deletion of all three PKA catalytic subunits is lethal; however, viability is restored by the deletion of two downstream targets of PKA: Msn2p and Msn4p (33). We therefore examined the phosphorylation of Crz1p after incubation in extracts of msn2Δ msn4Δ and tpk1Δ tpk2Δ tpk3Δ msn2Δ msn4Δ cells. As shown in Fig. 1B, in extracts lacking PKA, 32P incorporation into GST-Crz1p is 64% of that observed for GST-Crz1p phosphorylated by control extracts (Fig. 1B). This finding suggests that PKA contributes to Crz1p phosphorylation in vivo.
To determine whether Crz1p and PKA physically interact in vivo, GST-Tpk1p, GST-Bcy1p, or GST (pRD56) were expressed in cells whose endogenous Crz1p is C terminally tagged with the IgG-binding domain of protein A (ZZ) (KKY271). Crz1p-ZZ was isolated from cells, and its ability to bind GST-Tpk1p and GST-Bcy1p was examined by Western blotting. We found that Crz1p-ZZ specifically interacts with both GST-Tpk1p and GST-Bcy1p, suggesting that Crz1p and PKA form a stable complex in vivo (Fig. 1C).
PKA phosphorylates the Crz1p NLS.
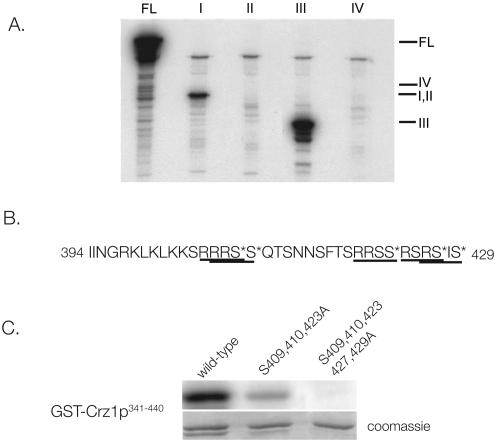
We next investigated which regions of Crz1p are phosphorylated by PKA. To this end, full-length and four consecutive fragments of Crz1p were isolated from E. coli as GST fusions (amino acids 1 to 180, 181 to 340, 341 to 440, and 441 to 678) and incubated with GST-Tpk1p and [γ-32P]ATP, and their phosphorylation state was determined by autoradiography. Tpk1p predominantly phosphorylates Crz1p341-440; Crz1p1-180 is also phosphorylated but to a lesser extent. The remaining fragments are not detectably phosphorylated (Fig. 2A). That Tpk1p predominantly phosphorylates GST-Crz1p341-440 is of particular interest since this region contains the Crz1p NLS (amino acids 394 to 422) (28). Previous work showed that phosphorylation of Crz1p is critical to proper regulation of its localization. The Crz1p import receptor, Nmd5p, binds Crz1p only in its nonphosphorylated form, promoting Crz1p nuclear import upon activation by calcineurin (28).
FIG. 2.
Tpk1p phosphorylates residues within the Crz1p NLS. (A) Phosphorylation of Crz1p fragments by Tpk1p. Recombinant GST-Tpk1p was combined with 2 μg each of full-length GST-Crz1p (FL), GST-Crz1p1-180 (I), GST-Crz1p181-340 (II), GST-Crz1p341-440 (III), or GST-Crz1p441-678 (IV) and then incubated with ATP and [γ-32P]ATP for 40 min at 30°C. Samples were analyzed by SDS-PAGE and autoradiography. Fragment III (residues 341 to 440) contains the NLS. (B) Sequence of the Crz1p NLS, extended from residues 422 to 429 (28). Putative PKA phosphorylation sites are underlined (19). Residues mutated to alanine are marked with an asterisk. (C) Mutation of residues 409, 410, 423, 427, and 429 disrupts Tpk1p phosphorylation of Crz1p in vitro. Wild-type and mutant versions of recombinant GST-Crz1p341-440 were incubated with GST-Tpk1p, ATP, and [γ-32P]ATP. Samples were analyzed by SDS-PAGE and imaged on a Typhoon scanner for quantitation.
The optimal sequence for PKA phosphorylation in vitro is R-R/K-X-S/T, although the enzyme will also phosphorylate R-X-X-S/T and R-X-S/T with lower efficiency (19). We examined Crz1p341-440 and found six PKA potential phosphorylation sites, all of which are in or immediately adjacent to the NLS. Three of these sites (containing serines 409, 410, and 423) fit the ideal consensus sequence (19), and three additional serines (422, 427, and 429) are present within lower efficiency consensus sites (Fig. 2B). We first mutated serines 409, 410, and 423 to alanine in GST-Crz1p341-440 and found that these mutations significantly reduced the ability of Tpk1p to phosphorylate GST-Crz1p341-440 in vitro (Fig. 2C). Quantification of these results revealed that incorporation of 32P into this mutant fragment is 17% that of wild-type. When two additional serines, i.e., serines 427 and 429, located just beyond the defined NLS, were mutated to alanine, in vitro phosphorylation of GST-Crz1p341-440 by Tpk1p was virtually undetectable (Fig. 2C). Mutation of serine 422 had no discernible effect on phosphorylation by Tpk1p (data not shown).
Phosphorylation by PKA affects Crz1p activity.
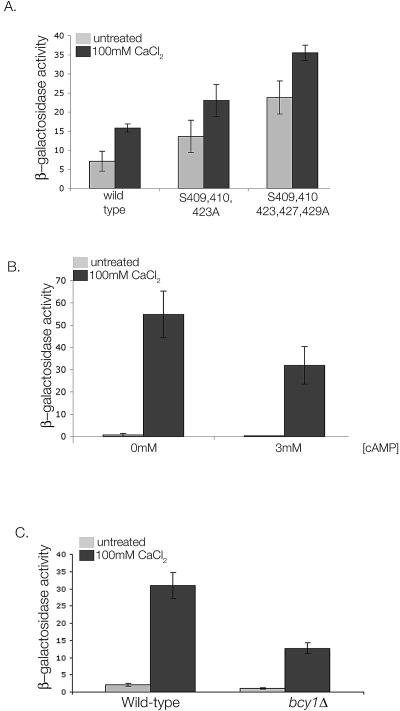
To investigate the effects of PKA on Crz1p in vivo, we monitored the transcriptional activity of full-length wild-type Crz1p (pKK249), Crz1pS409,410,423A (pKK251), and Crz1pS409,410,423,427,429A(pKK267) in a strain lacking endogenous CRZ1 and carrying a reporter gene containing four tandem copies of the Crz1p DNA-binding site placed upstream of the β-galactosidase gene (4xCDRE::LacZ; KWY251; K. E. Williams, unpublished data). Cells carrying Crz1pS409,410,423A exhibited 1.9- and 1.5-fold increases in basal and Ca2+-induced CDRE activation, respectively, compared to wild-type (Fig. 3A). Cells expressing Crz1pS409,410,423,427,429A showed a further increase in activity, with 3.3- and 2.2-fold increases in basal and induced activities, respectively (Fig. 3A). All three versions of Crz1p were expressed at equal levels, as determined by Western blotting analysis (data not shown). Thus, mutation of these serines to alanine both eliminates PKA phosphorylation of the Crz1p NLS in vitro and leads to Crz1p activation in vivo.
FIG. 3.
PKA regulates Crz1p activity in vivo. (A) Mutation of serines 409, 410, 423, 427, and 429 to alanine results in increased Crz1p activity. KWY251 cells (crz1Δ 4xCDRE::LacZ) expressing wild-type GFP-Crz1p (pKK249), GFP-Crz1pS409,410,423A (pKK251), or GFP-Crz1pS409,410,423,427,429A (pKK267) were treated with 100 mM CaCl2 where indicated. β-Galactosidase activity was measured and is reported as the maximum rate OD415 change/minute/microgram of protein. (B) PKA activation reduces Ca2+-dependent Crz1p activation. KKY436 cells (cdc35Δ pde2Δ yak1Δ 4xCDRE::LacZ) were incubated with 100 mM CaCl2 in the absence or presence of 3 mM cAMP as indicated, and the β-galactosidase activity was determined. (C) Constitutive PKA activity results in decreased Crz1p activity. Strains ASY832 (4xCDRE::LacZ) and KKY385 (bcy1Δ 4xCDRE::LacZ) were treated with 100 mM CaCl2 as indicated, and the β-galactosidase activity was determined.
To verify that the effects on Crz1p activity described above are dependent on PKA activity, we examined CDRE activation in a strain that allows manipulation of PKA activity by exogenous addition of cAMP. This strain (cdc35 pde2 yak1) lacks the ability to both synthesize and degrade cAMP; therefore, PKA is inactive unless cAMP is supplied exogenously (12). Strain KKY436 (cdc35 pde2 yak1 4xCDRE::LacZ) was grown to log phase and treated with or without Ca2+ in the absence or presence of cAMP. As shown in Fig. 3B, activation of the 4xCDRE reporter construct by Ca2+ is reduced 1.7-fold in the presence of 3 mM cAMP. (Fig. 3B). Increased concentrations of cAMP had no further effect on Crz1p activity (data not shown). These data indicate that activation of PKA by addition of cAMP inhibits Crz1p activation by Ca2+.
We next examined CDRE::LacZ activation in a bcy1Δ strain. This strain lacks the negative regulatory subunit of PKA and therefore displays constitutively high levels of PKA activity. As shown in Fig. 3C, deletion of BCY1 decreases Ca2+-induced CDRE activation twofold, a finding consistent with the hypothesis that Crz1p is inhibited by PKA phosphorylation (Fig. 3C).
PKA regulates Crz1p nuclear import.
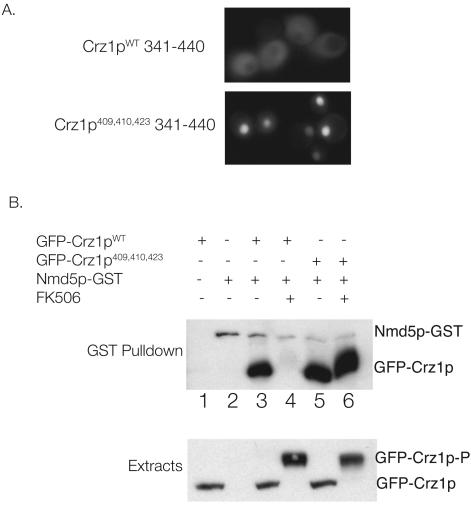
Phosphorylation of Crz1p prevents Crz1p nuclear import (28). To examine whether the sites phosphorylated by PKA in vitro contribute to the regulation of Crz1p nuclear import, we investigated the localization of GFP-Crz1p341-440. Residues 341 to 440 from wild-type Crz1p and Crz1pS409,410,423A were fused to three tandem copies of GFP (3× GFP) and transformed into strain BJ5459. Crz1p341-440 contains the NLS (amino acids 394 to 422 28) but lacks the nuclear export sequence; thus, its localization is a direct reflection of the efficiency with which it is imported into the nucleus. This fusion protein also lacks a motif required for calcineurin binding and is therefore not dephosphorylated by calcineurin (3). As shown in Fig. 4A, wild-type GFP-Crz1p341-440 is cytosolic. However, the triple-mutant version of GFP-Crz1p341-440 is strongly nuclear, supporting the hypothesis that phosphorylation of these residues inhibits Crz1p nuclear localization (Fig. 4A). Additional mutation of serines 427 and 429 had no further effects on the localization of GFP-Crz1p341-440 (data not shown).
FIG. 4.
PKA regulates Crz1p nuclear import (A) Localization of 3× GFP-Crz1p341-440 in BJ5459 cells is affected by the mutation of serines 409, 410, and 423 to alanine. The wild-type fragment (pKK260) is cytosolic, whereas the S409,410,423A mutant (pKK261) is strongly nuclear. (B) Full-length GFP-Crz1pS409,410,423A constitutively binds the importin Nmd5p. (Top) Extracts were prepared from cells (BJ5459 or KKY420) treated with or without FK506 from which GST-Nmd5p was isolated by using glutathione-Sepharose. Association of wild-type GFP-Crz1p (pKK249) and GFP-Crz1pS409,410,423A (pKK251) was analyzed by GST-pulldown, SDS-PAGE, and Western blot analysis with an α-Crz1p antibody, which also recognizes GST. Lane 1, BJ5459 with pKK249; lane 2, KKY420 with pUG36; lanes 3 and 4, KKY420 with pKK249 (wild-type GFP-Crz1p) ± FK506; lanes 5 and 6, KKY420 with pKK251 (GFP-Crz1pS409,410,423A) with or without FK506. (Bottom panel) Western blot analysis of extracts showing equal expression of GFP-Crz1p fusions. A total of 40 μg of each extract was analyzed by SDS-PAGE and Western blot analysis with an α-GFP antibody.
We then used a sensitive biochemical assay to examine the effect of mutations in serines 409, 410, and 423 on the nuclear import of full-length Crz1p. The interaction between Crz1p and its import receptor, Nmd5p, is regulated by phosphorylation. Previously, NMD5p was shown to bind Crz1p only in it nonphosphorylated state, although the particular phosphorylated residues that regulate this interaction were not identified (28). We investigated whether mutation of serines 409, 410, and 423 to alanine affects the ability of Nmd5p to bind Crz1p. Full-length wild-type GFP-Crz1p (pKK249) or GFP-Crz1pS409,410,423A (pKK251) was expressed in cells whose genomic copy of NMD5 is tagged with GST (KKY420), and extracts were prepared from cells that were untreated or treated with the calcineurin inhibitor FK506. Nmd5p-GST was isolated by using glutathione-Sepharose, and the ability of GFP-Crz1p to bind Nmd5p was analyzed by Western blotting. As expected, the association of wild-type GFP-Crz1p with Nmd5p-GST is dependent on calcineurin; Nmd5-GST is unable to interact with wild-type GFP-Crz1p from cells treated with FK506, i.e., in its fully phosphorylated form (Fig. 4B, lanes 3 and 4). In contrast, Nmd5-GST interacts with GFP-Crz1pS409,410,423A regardless of calcineurin activity (Fig. 4B, lanes 5 and 6). Note that the overall phosphorylation state of GFP-Crz1pS409,410,423A is still regulated by calcineurin, as evidenced by its decreased electrophoretic mobility in extracts of FK506-treated cells. This represents phosphorylation at residues other than serines 409, 410, and 423. However, the fact that GFP-Crz1pS409,410,423A associates with Nmd5-GST in extracts of FK506-treated cells indicates that the critical determinant for Crz1p-Nmd5p binding, and thus for regulation of Crz1p nuclear import, is the phosphorylation state of these specific residues within the NLS.
DISCUSSION
The ability of yeast cells to adapt to a wide variety of environmental conditions is dependent on the activation of multiple signaling pathways that sense and respond to changes in their surroundings. Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase that is activated in response to high salinity, alkaline pH, elevated temperature, and damage to the cell wall (9). Calcineurin effects changes in transcription through the dephosphorylation and activation of Crz1p. Upon dephosphorylation, Crz1p accumulates in the nucleus where it activates a transcriptional program enabling the cell to adapt to stress (9). We have previously shown that calcineurin-dependent activation of Crz1p is antagonized by the casein kinase I homolog Hrr25p (18). However, these studies also indicated that Hrr25p is not the sole Crz1p kinase (18). A previous finding that overexpression of the low-affinity cAMP phosphodiesterase PDE1 suppresses the salt sensitivity of calcineurin-deficient cells suggested a possible connection between the calcineurin and PKA pathways in yeast (16). Here we identify a specific mechanism of cross talk between calcineurin and PKA and demonstrate that Crz1p is an integration point of these two essential signaling pathways.
We show here that PKA phosphorylates Crz1p in vitro and that phosphorylation of Crz1p is reduced in extracts lacking PKA. Once phosphorylated by PKA, Crz1p can be efficiently dephosphorylated by calcineurin. In addition, we identified five serines in Crz1p, all of which lie within or adjacent to its NLS, that are required for Crz1p phosphorylation by PKA. These observations indicate that PKA phosphorylates the Crz1p NLS. We also demonstrate that PKA affects Crz1p activity. Crz1pS409,410,423A and Crz1pS409,410,423,427,429A have increased basal and induced CDRE::LacZ activity relative to the wild type, and cells with increased levels of PKA activity exhibit decreased CDRE::LacZ activation. These data indicate that phosphorylation of Crz1p by PKA decreases Ca2+-induced Crz1p activity. Finally, we present evidence that phosphorylation of the Crz1p NLS by PKA inhibits Crz1p nuclear import. A fragment of Crz1p (amino acids 341 to 440) containing the NLS fused to three copies of GFP is cytosolic; however, mutation of the PKA phosphorylation sites to alanine results in strong nuclear localization of the NLS. In addition, whereas wild-type Crz1p binds the importin Nmd5p only upon dephosphorylation by calcineurin, Crz1pS409,410,423A interacts with Nmd5p regardless of calcineurin activity. Therefore, we conclude from these studies that PKA antagonizes calcineurin signaling by phosphorylating the Crz1p NLS and thus inhibiting Crz1p nuclear import.
Multiple kinases regulate Crz1p nuclear localization.
Crz1p localization in vivo is the net result of its rate of nuclear import and its rate of nuclear export (3, 28). Both of these processes are dependent on Crz1p phosphorylation and are regulated by calcineurin. We provide evidence that PKA inhibits Crz1p nuclear accumulation by specifically affecting its nuclear import. Thus, additional kinases are clearly required to explain fully the different aspects of Crz1p regulation observed in vivo, in particular, the regulation of Crz1p nuclear export. One such kinase is Hrr25p, a casein kinase I homologue (18). We previously showed that Hrr25p phosphorylates Crz1p extensively and alters its localization, activity, and electrophoretic mobility (18). Preliminary evidence indicates that phosphorylation of Crz1p by these two kinases is independent. Hrr25p phosphorylates portions of Crz1p in vitro that do not serve as substrates for PKA. Furthermore, mutation of serines 409, 410, 423, 427, and 429 to alanine completely eliminates in vitro phosphorylation of Crz1p341-440 by PKA but has no effect on its phosphorylation by Hrr25p (data not shown). Finally, incubation of Crz1p with both kinases in vitro results in additive, not synergistic, levels of 32P incorporation (data not shown). It is likely that other, as-yet-unidentified kinases phosphorylate Crz1p in vivo and that differential regulation of all of these kinases contributes to the physiological modulation of Crz1p/calcineurin signaling.
PKA and Crz1p physically interact.
Physical association of regulatory and effector proteins results in rapid and finely modulated responses to stimuli. We found that Crz1p interacts in vivo with both Tpk1p and Bcy1p but were unable to observe direct binding between Crz1p and PKA by using purified proteins. This suggests that Crz1p and PKA may be part of a larger multiprotein complex (K. A. Kafadar and M. S. Cyert, unpublished results). Crz1p also binds calcineurin and Hrr25p (3, 18), and it is possible that multiple Crz1p-containing complexes exist in vivo, each playing a different role in modulating Crz1p activity.
Integration of PKA and calcineurin signaling.
In yeast, PKA activity is required for multiple cellular functions, including growth, carbohydrate synthesis, and the response to stress (36). Cells deficient in PKA arrest in G0, accumulate glycogen and trehalose, and become stress resistant, properties associated with nutrient-deprived cells (39). In contrast, cells with elevated PKA activity fail to store carbohydrates or enter stationary phase under nutrient-poor conditions and are extremely stress sensitive (4, 5). These observations suggest that yeast PKA is active under optimal conditions and functions to repress the stress response, thereby integrating cell growth and metabolism with environmental stimuli (36).
We have demonstrated that the PKA and calcineurin signaling pathways are connected through their regulation of a common effector protein, Crz1p. When cells are grown in optimal conditions and PKA activity is elevated, high calcineurin activity is required to activate Crz1p. In contrast, in a nutrient-poor environment, PKA activity is low, and cells are sensitized such that lower calcineurin activity is sufficient for Crz1p activation. The activity of PKA therefore plays a role in setting the threshold for calcineurin-dependent Crz1p activation and enables the general nutritional environment of the cell to affect other specific stress response pathways, such as calcineurin.
Two additional targets of PKA are the transcription factors Msn2p and Msn4p (33). Msn2/4p activate transcription through stress-response elements and upregulate the expression of ∼180 genes in response to environmental stress (6, 10, 22). As with Crz1p, Msn2/4p are regulated primarily through their subcellular localization. Under optimal conditions, both proteins are phosphorylated by PKA and are cytosolic. Upon inactivation of PKA, Msn2/4p rapidly accumulate in the nucleus, where they activate stress-induced gene transcription. Indeed, the stress sensitivity and resistance of PKA mutants can be attributed to effects on Msn2/4p activity (11, 12).
PKA and calcineurin are both conserved signaling molecules that transmit a multitude of signals in eukaryotes from fungi to mammals (1, 13). We show that calcineurin and PKA act as positive and negative regulators, respectively, of Crz1p signaling in yeast. Interestingly, in mammals, calcineurin and PKA also function antagonistically in multiple processes. In particular, nuclear factor of activated T cells (NFAT), in a manner analogous to that of Crz1p, translocates from the cytosol to the nucleus upon dephosphorylation by calcineurin, where it activates a transcriptional program essential for T-cell activation (7). Rephosphorylation of NFAT by PKA and other kinases results in the return of NFAT to the cytosol and termination of signaling (7, 27, 30).
In summary, we have identified here a novel physiological role for PKA in the modulation of calcineurin signaling in yeast and have shown that these pathways converge in their regulation of Crz1p. These data further demonstrate the complex integration of different signaling pathways in vivo that allows yeast to respond appropriately to their changing environment.
Acknowledgments
We thank Erich Durchschlag and Christoph Schüller for strains (W303 msn2 msn4, tpk1,2,3 msn2 msn4, and cdc35 pde2 yak1). We also thank Jagoree Roy for critical reading of the manuscript.
This study was supported by NIH research grant GM-48729 (M.S.C.) and by NIH training grant 5T32GM07276 (K.A.K.).
REFERENCES
- 1.Aramburu, J., A. Rao, and C. B. Klee. 2000. Calcineurin: from structure to function. Curr. Top. Cell. Reg. 36:237-295. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. Wiley, New York, N.Y.
- 3.Boustany, L. M., and M. S. Cyert. 2002. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 16:608-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, S., L. Levin, M. Zoller, and M. Wigler. 1988. cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in Saccharomyces cerevisiae. Cell 53:555-566. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, J. F., and K. Tatchell. 1987. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 7:2653-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Causton, H. C., B. Ren, S. S. Koh, C. T. Harbison, E. Kanin, E. G. Jennings, T. I. Lee, H. L. True, E. S. Lander, and R. A. Young. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.):S67-S79. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham, K. W., and G. R. Fink. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143-1150. [DOI] [PubMed] [Google Scholar]
- 10.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schèuller. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorner, W., E. Durchschlag, J. Wolf, E. L. Brown, G. Ammerer, H. Ruis, and C. Schèuller. 2002. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 21:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffioen, G., and J. M. Thevelein. 2002. Molecular mechanisms controlling the localization of protein kinase A. Curr. Genet. 41:199-207. [DOI] [PubMed] [Google Scholar]
- 14.Guldener, U., S. Heck, T. Fielder, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haro, R., B. Garciadeblas, and A. RodrÂiguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 16.Hirata, D., S. Harada, H. Namba, and T. Miyakawa. 1995. Adaptation to high-salt stress in Saccharomyces cerevisiae is regulated by Ca2+/calmodulin-dependent phosphoprotein phosphatase (calcineurin) and cAMP-dependent protein kinase. Mol. Gen. Genet. 249:257-264. [DOI] [PubMed] [Google Scholar]
- 17.Jones, E. W. 1991. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 194:428-453. [DOI] [PubMed] [Google Scholar]
- 18.Kafadar, K. A., H. Zhu, M. Snyder, and M. S. Cyert. 2003. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 17:2698-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennelly, P. J., and E. G. Krebs. 1991. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266:15555-15558. [PubMed] [Google Scholar]
- 20.Krebs, E. G., and J. A. Beavo. 1979. Phosphorylation-dephosphorylation of enzymes. Annu. Rev. Biochem. 48:923-959. [DOI] [PubMed] [Google Scholar]
- 21.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Pastor, M. T., G. Marchler, C. Schuller, A. Marchler-Bauer, H. Ruis, and F. Estruch. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15:2227-2235. [PMC free article] [PubMed] [Google Scholar]
- 23.Matheos, D. P., T. J. Kingsbury, U. S. Ahsan, and K. W. Cunningham. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11:3445-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazur, P., N. Morin, W. Baginsky, M. el-Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendizabal, I., G. Rios, J. M. Mulet, R. Serrano, and I. F. de Larrinoa. 1998. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 425:323-328. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza, I., F. Rubio, A. Rodriguez-Navarro, and J. M. Pardo. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792-8796. [PubMed] [Google Scholar]
- 27.Okamura, H., C. Garcia-Rodriguez, H. Martinson, J. Qin, D. M. Virshup, and A. Rao. 2004. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol. Cell. Biol. 24:4184-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polizotto, R. S., and M. S. Cyert. 2001. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J. Cell Biol. 154:951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman, J. LeVitre, L. S. Davidow, J. I. Mao, and D. T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133-145. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan, C. M., E. K. Heist, C. R. Beals, G. R. Crabtree, and P. Gardner. 2002. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J. Biol. Chem. 277:48664-48676. [DOI] [PubMed] [Google Scholar]
- 31.Sherman, F. 1991. Getting started with yeast, p. 3-22. In C. Guthrie and G. R. Fink (ed.), Guide to yeast genetics and molecular biology. Academic Press, Inc., San Diego, Calif.
- 32.Silorski, R. S. a. H., P. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, A., M. P. Ward, and S. Garrett. 1998. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stathopoulos-Gerontides, A., J. J. Guo, and M. S. Cyert. 1999. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13:798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 37.Toda, T., S. Cameron, P. Sass, M. Zoller, J. D. Scott, B. McMullen, M. Hurwitz, E. G. Krebs, and M. Wigler. 1987. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 7:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toda, T., S. Cameron, P. Sass, M. Zoller, and M. Wigler. 1987. Three different genes in Saccharomyces cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50:277-287. [DOI] [PubMed] [Google Scholar]
- 39.Wigler, M., J. Field, S. Powers, D. Broek, T. Toda, S. Cameron, J. Nikawa, T. Michaeli, J. Colicelli, and K. Ferguson. 1988. Studies of RAS function in the yeast Saccharomyces cerevisiae. Cold Spring Harbor Symp. Quant. Biol. 53:649-655. [DOI] [PubMed] [Google Scholar]
- 40.Withee, J. L., R. Sen, and M. S. Cyert. 1998. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA1. Genetics 149:865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:31079-31088. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, H., M. Bilgin, R. Bangham, D. Hall, A. Casamayor, P. Bertone, N. Lan, R. Jansen, S. Bidlingmaier, T. Houfek, T. Mitchell, P. Miller, R. A. Dean, M. Gerstein, and M. Snyder. 2001. Global analysis of protein activities using proteome chips. Science 293:2101-2105. [DOI] [PubMed] [Google Scholar]