Abstract
Autoimmune diseases arise due to the loss of immunological self-tolerance. Regulatory T cells (Tregs) are important mediators of immunologic self-tolerance. Tregs represent about 5 - 10% of the mature CD4+ T cell subpopulation in mice and humans, with about 1 - 2% of those Tregs circulating in the peripheral blood. Induced pluripotent stem cells (iPSCs) can be differentiated into functional Tregs, which have a potential to be used for cell-based therapies of autoimmune diseases. Here, we present a method to develop antigen (Ag)-specific Tregs from iPSCs (i.e., iPSC-Tregs). The method is based on incorporating the transcription factor FoxP3 and an Ag-specific T cell receptor (TCR) into iPSCs and then differentiating on OP9 stromal cells expressing Notch ligands delta-like (DL) 1 and DL4. Following in vitro differentiation, the iPSC-Tregs express CD4, CD8, CD3, CD25, FoxP3, and Ag-specific TCR and are able to respond to Ag stimulation. This method has been successfully applied to cell-based therapy of autoimmune arthritis in a murine model. Adoptive transfer of these Ag-specific iPSC-Tregs into Ag-induced arthritis (AIA)-bearing mice has the ability to reduce joint inflammation and swelling and to prevent bone loss.
Keywords: Immunology, Issue 117, regulatory T cell, pluripotent stem cell, cell differentiation, antigen-induced arthritis, mouse, autoimmune diseases
Introduction
Autoimmune arthritis is a systemic disease characterized by hyperplasia of synovial tissue and progressive destruction of articular cartilage, bone, and ligaments1. The defective generation or function of Tregs in autoimmune arthritis contributes to chronic inflammation and tissue injury because Tregs play a crucial role in preventing the development of auto-reactive immune cells.
Manipulation of Tregs is an ideal strategy for the development of therapies to suppress inflammation in an Ag-dependent manner. For Treg-based immunotherapy, the specificity of the transferred Tregs is important for the treatment of ongoing autoimmunity2. To exhibit the suppressive activity, Tregs need to migrate and be retained at the afflicted region, which can be directed by the specificity of the TCR for the Ag at that location3. Although polyclonal Tregs may contain a small population containing this Ag specificity from their TCRs, the numbers of these Ag-specific Tregs are usually low. Consequently, cell-based therapies using polyclonal Tregs against autoimmune disorders require adoptive transfers of a large number of Tregs4,5. Because pluripotent stem cells (PSCs) have the ability to develop into any type of cell, Ag-specific PSC-Tregs may prove to be good candidates for Treg-based immunotherapy. Previous studies have shown the successful development of PSC-derived T cells, including Tregs6-8.
Here, we describe a protocol to develop Ag-specific iPSC-Tregs. We further describe a cell-based therapy of autoimmune arthritis in a murine model using such Tregs. This method is based upon genetically modifying murine iPSCs with Ag-specific TCRs and the transcriptional factor FoxP3. The engineered iPSCs then differentiate into Ag-specific Tregs on the OP9 stromal cells expressing Notch ligands DL1, DL4, and MHC-II (I-Ab) molecules in the presence of cytokines mFlt3L and mIL-7. These Ag-specific iPSC-Tregs can produce suppressive cytokines, such as TGF-β and IL-10, when stimulated with the Ag, and adoptive transfer of such Tregs has the ability to suppress AIA development in a murine model. The described protocol can be used to develop stem cell-derived Ag-specific Tregs for potential therapeutic interventions.
Protocol
All animal experiments are approved by the Pennsylvania State University College of Medicine Animal Care Committee (IACUC protocol #45470) and are conducted in compliance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
1. Stem Cell Culture
Incubate a 10 cm dish with 10 ml of 0.1% gelatin for at least 30 min at 37 °C (incubator) in order to coat the plate.
Remove gelatin from the dish and plate 3 x 106 irradiated SNL76/7 cells and incubate for one day at 37 °C (incubator) using 10% DMEM media (10% FBS and 1% penicillin streptomycin).
Remove the media from the plate and culture thawed iPSCs over the SNL76/7 cell feeder layer using iPSC media (DMEM media containing 15% fetal calf serum, 0.1 mmol/L non-essential amino acids, 1 mmol/L L-glutamine, and 0.1 mmol/L β-mercaptoethanol)9. The mouse iPS-MEF-Ng-20D-17 cell line, which was induced from mouse embryonic fibroblasts by retro viral transfection of Oct3/4, Sox2, Klf4, and c-Myc, was obtained from Dr. Shinya Yamanaka (Institute for Frontier Medical Sciences, Kyoto University, Kyoto, Japan).
Change the media on day 3 with fresh media and observe iPSC colonies under the microscope. NOTE: These colonies are small, shiny clusters or round cells with a clear demarcation showing GFP expression under a fluorescent microscope.
2. In Vitro Differentiation of Ag-specific iPSC-Tregs
Generate the constructs to be used in retroviral transduction by sub-cloning the OT-II TCR genes and FoxP3 into the MiDR plasmid10 linked with self-cleaving peptide 2A to make the MiDR-TCRα-2A-TCRβ-2A-FoxP3 construct.
Perform retroviral transduction9 of iPSCs using Plat E cells as the packaging cell line.
On day 0, seed OP9-DL1-DL4-I-Ab cells at a minimum density of 104 cells/cm2 by using OP-9 media (α-MEM media containing 20% FCS and 2.2 g/L sodium bicarbonate. Plate 1 x 106 cells per 10 cm dish.
On day 3, when OP9-DL1-DL4-I-Ab cells become 80-90% confluent, remove the media and seed 0.5 - 1 x 105 iPSCs over OP9-DL1-DL4--I-Ab cells in OP-9 media. NOTE: This establishes the co-culture of iPSCs on OP9-DL1-DL4-I-Ab cells and is considered to be day 0 of differentiation9.
- On day 5, remove the media from the 10 cm dish by aspiration, wash the cells with 10 ml of 1x PBS, and aspirate the PBS. Add 4 ml of 0.25% trypsin and incubate at 37 °C for 10 min. Add another 8 ml of iPSC media to the cells, re-suspend them, and centrifuge at 400 x g for 5 min at room temperature.
- Aspirate the supernatant and re-suspend the cells in 10 ml of iPSC media. Incubate these re-suspended cells on a fresh 10 cm dish and return to the incubator for 30 min. NOTE: Remove the OP9-DL1-DL4-I-Ab feeder cells and keep the differentiating iPSCs floating in the media. Maintain OP9-DL1-DL4-I-Ab cells continuously to achieve 80 - 90% confluency for further co-culture.
- After 30 min, collect the floating cells, filter them through a 70 µm cell strainer, and count the cells with a hemocytometer.
Seed 5 x 105 of iPSCs to a fresh 80 - 90% confluent of OP9-DL1-DL4-I-Ab cells in OP9 media. Supplement the media with mFlt-3L at a final concentration of 5 ng/ml.
- On day 8, collect partially-differentiated iPSCs by washing the plate with the media from the dish itself using a 10 ml pipette. Use another 5 ml of OP-9 media in the dish to gently wash off semi-adherent cells by careful, forceful pipetting so as not to break the OP9 monolayer at the bottom of the dish. Repeat the wash with 10 ml of PBS to harvest all semi-adherent differentiating cells.
- Combine both washes, centrifuge at 400 x g for 5 min at room temperature, re-suspend in 10 ml of OP9 media containing mFlt-3L (5 ng/ml) and mIL-7 (1 ng/ml), and filter through a 70 µm cell strainer.
Transfer the cells into a 6-well culture plate containing 80 - 90% confluent OP9-DL1-DL4-I-Ab cells, as in step 1.1. Transfer cells harvested from one 10 cm dish into one well of the 6-well plate.
On day 10, change half of the media from cells to fresh OP9 media supplemented with mFlt-3L (5 ng/ml) and mIL-7 (1 ng/ml). Repeat this step every two days.
Every 4-6 days, depending on growth, re-seed the differentiating iPSCs onto plates with a fresh layer of OP9-DL1-DL4-I-Ab cells, as described in step 1.1.
3. Evaluation of In Vitro Treg Differentiation and Maturation
- Morphological changes of differentiating iPSCs.
- Monitor the co-culture of iPSCs with OP9-DL1-DL4-I-Ab cells everyday by observing live cells under a conventional brightfield microscope (20X). By day 5, observe the colonies with mesoderm-like characteristics, such as flattened cells. By day 8, observe small, round clusters of cells, which represent differentiating cells.
- Use the Trypan Blue Exclusion Method to count the cells to detect the number and percentage of live cells. Calculate cell viability as the number of viable cells divided by the total number of cells within the four grids on the hemocytometer. If cells take up trypan blue, consider them dead or non-viable. Record the number of live cells harvested from the culture.
- Flow cytometry analysis of differentiating iPSCs.
- On days 5, 7, 11, 15, 19, 21, and 28 of co-culture, remove the cells with 0.25% trypsin as in step 25.
- Prior to the surface staining with different fluorochrome-conjugated antibodies, incubate 1 x 106 cells with 100 µl of Fc blocker 2.4G2 diluted in PBS (final concentration 10 µg/ml) at 4 °C for 20 min to prevent the binding of antibody to the Fc receptor on the cell surface.
- On days 5, 7, 11, 15, 19, 21, and 28, use 1 x 106 cells for flow cytometry with different fluorochrome-conjugated antibodies to detect the cell surface markers, including CD3, TCRβ, CD4, CD8, CD25, and CTLA4.
- Following Fc block, wash cells with 10 ml of PBS and stain with different fluorochrome-conjugated antibodies for 20 min at 4 °C, and then perform the flow cytometric analysis with the 15-color flow cytometer. Use 0.200 µg of antibody per 106 cells diluted in 100 µl of PBS.
- On day 28, analyze the cells by flow cytometry11 and gate on CD4+ CD8+ cells using CD4 and CD8 fluorochrome-conjugated staining; analyze for the expression of TCRVα2, TCRVβ5, CD25, CTLA-4, and FoxP3 (Figure 1). For FoxP3 staining, fix the cells and permealize them and then perform antibody staining11.
- In vitro antigen stimulation of iPSCs.
- On day 28 of co-culture, harvest iPSC-Tregs from cultures by collecting the floating cells. Trypsinize the rest of the cells with 0.25% trypsin (as in step 2.5) and re-suspend the cells in 8 ml of iPSC media. Centrifuge for 5 min at 400 x g at room temperature, aspirate the media, and again re-suspend the cells in 10 ml of media.
- Incubate the re-suspended cells in a new 10 cm dish at 37 °C for 30 min and collect the floating cells. Wash the cells with cold PBS.
- Incubate 3 x 106 splenocytes from spleens of C57BL/6 Rag1-/- mice with 5 μM ovalbumin peptide in 200 µl of media (OVA323-339) at 4 °C for 30 min in a 96 well plate11.
- Mix Tregs with OVA323-339-pulsedsplenocytes at a 1:4 ratio (use 0.75 x 106 Tregs). Incubate at 37 °C in a CO2 incubator for 40 hr. In the last 4 hr, add 4 µl of diluted Brefeldin A into the culture (actual concentration 1,000x, diluted in 1x culture media).
- Harvest cells with 0.25% trypsin (as in step 2.5), wash with 10% NaN3 and 0.5 M EDTA solution (FACS buffer), block with 100 µl of diluted (final concentration 10 µg/ml) Fc blocker 2.4G2, and stain for the surface markers, CD4, CD8, TCRVα2, and TCRVβ5 as in steps 3.2.3-3.2.4.
- Fix the cells with a 4% paraformaldehyde solution in PBS and permeabilize with 100 µl of 1x permeabilization buffer after cell surface staining. Caution! Paraformaldehyde is allergenic, carcinogenic, and toxic.
- Stain the cells with fluorochrome-conjugated TGF-β and IL-10 for 20 min in the dark. Use 0.200 µg of antibody/106 cells diluted in 100 µl of PBS. Detect the intracellular production of TGF-β and IL-10 with the 15-color flow cytometer (Figure 2).
- Wash the cells three times with cold FACS buffer prior to the flow cytometric analysis11.
- In vivo persistence of Ag-specific iPSC-Tregs.
- After 8 days of co-culture on OP9-DL1-DL4-I-Ab cells, transfer iPSC-derived pre-Tregs (Thy1.2) as in step 3.3.1 into Thy 1.1 congenic mice (4-6 weeks old) via a tail vein injection without anesthesia. Prior to performing the tail vein injection, dilate the tail vein using an infrared lamp for 5 min. After tail vein dilation, place the mouse in a restrainer and adoptively transfer 3 x 106 cells resuspended in 200 μl of PBS by injecting through the tail vein.
- Six weeks after the Treg transfer, euthanize mice by CO2 asphyxiation followed by cervical dislocation. Ensure that the mice are not breathing. Open the peritoneal cavity by cutting the outer skin of the peritoneum using scissors and forceps and gently pulling it back to expose the inner skin lining the peritoneal cavity. Isolate the spleen and peripheral lymph nodes of injected Thy 1.1 congenic mice.
- Collect the cells from the spleen and lymph nodes using mechanical breaking to yield a single cell suspension. Incubate the cells with 5 ml of ACK lysis buffer for 5 min at RT to lyse red blood cells. Collect and wash remaining splenocytes or lymphocytes with 10 ml of cold FACS buffer.
- Following the wash, treat cells with the Fc blocker 2.4G2 at 4 °C for 20 min as in step 3.2.2 and stain with 100 µl of diluted fluorochrome-conjugated anti-Thy 1.2 antibodies.
- Wash cells with 10 ml of FACS buffer and proceed to flow cytometric analysis11.
4. In Vivo Maturation and Suppression of Autoimmune Arthritis
Generate the constructs as in step 2.1.
Perform retroviral transduction9 as in step 2.2.
- In vivo differentiation and arthritis induction in mice.
- Differentiate OT-II TCR/FoxP3-transduced iPSCs (OT-II-FoxP3/iPSCs), FoxP3-transduced iPSCs, and DsRed transduced iPSCs on the OP9-DL1-DL4-I-Ab stromal cells in the presence of cytokines mFlt-3L and mIL-7 for 8 days as described in steps 2.1 - 2.6.
- Trypisinize all three kinds of cells from the 10 cm plate and re-suspend cells from each 10 cm plate in 10 ml of fresh media. Add the cells to a fresh 10 cm plate and return to incubator for 30 min. After 30 min, collect floating cells.
- Pass cells through a 70 µm cell strainer to remove cell clusters and count using a hemocytometer. Adjust the cells to a concentration of 1.5 x 107 cells/ml in cold PBS and filter again if needed. Keep cells on ice until adoptive cell transfer into mice.
- Inject 200 µl of the cell suspension (3 x 106 cells) into three different groups of 4 - 6 week-old female C57BL/6 mice through the tail vein as described in 3.4.1
- On day 10 after the cell transfer, inject mice with 100 µg of mBSA emulsified in Freund's complete adjuvant at the base of the tail by using a 1 ml syringe.
- On day 17, anesthetize mice using an isoflurane vaporizer (according to IACUC guidelines). Utilize 4 - 5% isoflurane for induction and 1 - 2% for maintenance. Induce arthritis by intra-articular injection of 20 µg mBSA in 10 µl PBS in the left knee joint and of 20 µg mBSA and 100 µg whole OVA in 10 µl PBS in the right knee joint. Food and a gelpack can be placed on the bedding floor after arthritis has developed. Mice will be euthanized: Body Condition Score (BCS) of 2/5 or moribund, severe cachexia and/or persistent recumbency (a 24-hr period), and severity score 4. In score 4, erythema and severe swelling encompass the ankle, foot and digits, or ankyloses of the limb.
- Characterization of the OT-II TCR/FoxP3-transduced iPSCs.
- Use a fluorescent microscope (20X) to visualize unfixed live DsRed+ GFP+ cells.
- Examine gene integration and expression by both Western blot and flow cytometry11.
- Treg development and maturation.
- At weeks 2, 4, and 6 after the cell transfer, euthanize the mice. For euthanasia, in each cage use 1 - 2 L of CO2 in first stage. Once the animal has lost consciousness, increase the flow rate of CO2 to 4 - 5 L/min. Euthanize mice by CO2 inhalation. Isolate the spleen and drain the lymph nodes by cutting the outer skin of the peritoneum using scissors and forceps and gently pulling it back to expose the inner skin lining the peritoneal cavity.
- Collect the single cell suspension from the spleen and lymph nodes by mechanical breakdown using a cell strainer and a 1 ml syringe in 1% iPSC media. Centrifuge the conical tube containing the media at 400 g for 5 min. Re-suspend the cell pellet in 5 ml of ACK lysis buffer to lyse red blood cells. Re-centrifuge at 1,000 rpm for 5 min. Re-suspend the cell pellet and wash the remaining splenocytes or lymphocytes with cold FACS buffer. Follow steps 3.4.4 - 3.4.5 and proceed to flow cytometric analysis.
- Intracellular staining.
- On day 50 post-challenge, isolate the spleen and drain the lymph nodes of the mice (as in step 4.5.1) after euthanization by CO2 inhalation followed by cervical dislocation.
- Collect the single cell suspension from the spleen and lymph nodes by mechanical breakdown using a cell strainer and a 1 ml syringe. Incubate the splenocytes at room temperature for 5 min with 5 ml of ACK lysis buffer to lyse red blood cells. Collect and wash the remaining splenocytes or lymphocytes with cold FACS buffer. Follow steps 3.2.3 - 3.2.4, but stain with surface markers CD4, CD8, CD25, and TCRVβ5.
- Proceed to fix cells for intracellular staining as described in 3.3.5 - 3.3.7 and analyze intracellular cytokine production (TGF-β and IL-10) of iPSC-derived Tregs and gate on live CD4+ CD25+ cells.
- Infiltration of Ag-specific Tregs in the knees and suppression of arthritis.
- Following steps (4.3 - 4.3.6) on days 7 - 14 post-arthritis induction, euthanize mice by CO2 inhalation followed by cervical dislocation (according to IACUC guidelines). Remove hair from the knees with an electric clipper and excise the knees by surgical procedure.
- Remove the skin from the legs by making an incision in the hind leg with blunt-end scissors and cut the skin across the thigh and down to the ankle. Gently peel the skin over the leg and foot to expose the muscle. Remove the muscle from the leg carefully without damaging the knee joint.
- Cut the hind leg just above the pelvic/hip joint and cut the tibia using sharp dissecting scissors.
- Fix the knees in 4% formalin for 48 hr. Decalcify the knees by using 2.5 M formic acid; rinse thrice in xylene for 3 min, rinse twice in 100% ethanol, rinse twice in 95% ethanol, rinse twice in deionized water for 2 min, and decalcify in 1 mM EDTA. Treat at a sub-boiling temperature (90 °C) for 20 min.
- Cool the fixed tissue for 30 min. Rinse it with 1x PBS for 4 min and embed it in paraffin. Dehydrate the tissues through a series of ethanol baths to displace the water, and then infiltrate them with wax. Then embed the infiltrated tissues into wax blocks. Perform both vertical and horizontal sectioning for staining. Prepare 4 µm sections with a sliding microtome.
- Perform immunofluorescent staining on the sections after standard procedures of deparaffinization and rehydration using xylene and ethanol12.
- Block endogenous peroxidase activity by immersing the slide in a sufficient amount of 3% hydrogen peroxide solution for 3 min after Ag retrieval. Block slides for non-specific binding in 3% BSA in PBS at room temperature in a humidified chamber for 60 min.
- Stain sections with 200 µl of fluorochrome-conjugated TCRVβ5 antibody diluted 1:100 in the blocking solution. Incubate for 2 hr at room temperature in a 75 - 100% humidified chamber, and wash 5 times in 1x PBS for 5 min.
- Counterstain the slides for nuclear staining with an antifade reagent containing DAPI. Add approximately 300 μl of the diluted DAPI staining solution (300 nM in 1x PBS) to the coverslip, making certain that entire coverslip is covered. Store the slides in the dark at 4 °C until analysis under a fluorescent microscope (Figure 3).
- Arthritis suppression assay
- Measure the swelling of both knees of the mice before arthritis induction by using a dial-gauge caliper to establish a baseline.
- Follow steps (4.3 - 4.3.6) for arthritis induction and Treg transfer. Measure mouse knees with a dial-gauge caliper post-Treg transfer.
- Calculate the percent increase in the knee diameter: Percent Increase = (Knee diameter on day 1 - Knee diameter on day 0) / Knee diameter on day 0.
- Measurement of joint destruction and inflammatory cell infiltration in the knees by histology (Figure 4).
- On day 7 post-arthritis induction, euthanize the mice as described previously and excise the knees by surgical procedure. See step 4.7.1 - 4.7.2.
- Fix the knees in 4% formalin, decalcify in EDTA, and embed in paraffin. See step 4.7.3
- Remove both knees, fix in 10% formalin, and calcify in Formical-4. Embed the tissues in Paraffin, section at 4 µm, and stain with hematoxylin and eosin (H & E) or Safranin O staining (as in step 4.7.7) and observe under a microscope.
- Use H & E staining to evaluate bone erosion with a semi-quantitative scoring system (0: no erosions; 4: extended erosions and destruction of bone). Use Safranin O staining to evaluate the loss of proteoglycans and score with a semi-quantitative scoring system (0: no loss of proteoglycans; 3: complete loss of staining for proteoglycans).
- Use a semi-quantitative scoring system to score inflammatory cell infiltration on H & E stained slides (0: no cell infiltration; 4: abounded cell infiltration). Evaluate scores by examining both knees in a blinded manner.
5. Measurement of Bone Loss in the Knees with the High-resolution Micro-computed Tomography (micro-CT) System
On day 10 post-arthritis induction, anaesthetize mice with 2% isoflurane and prepare for imaging.
Position mice with knees facing upwards in the chamber.
Use a high-resolution micro-CT system to acquire in vivo imaging of the bone architecture around the knees of the mice.
Perform micro-CT scans with a 2.2 mm length, including mouse knee joints with the following parameters: 10.5 µm voxel size at 55 kv, 145 µA 200 msec integration time, 211 images.
Import micro-CT images and capture frontal plane images after further image processing (volume rendering and transformation) (Figure 5).
Representative Results
As shown here, on day 28, Ag-specific Tregs substantially expressed CD3 and Ag-specific TCR, two T cell markers. The CD3+TCRVβ5+ population expressed CD4. Most of the CD3+TCRVβ5+CD4+ cells also expressed CD25, CD127, and CTLA-4, which are typically expressed at elevated levels in naturally occurring Tregs (nTregs) and in T cells expressing FoxP3 ectopically. FoxP3 expression in iPSC-derived cells persisted even after long-term in vitro stimulation with the Notch ligand, as detected by intracellular staining analyzed by flow cytometry (Figure 1). In addition, the Ag-specific iPSC-Tregs produced suppressive cytokines, such as TGF-β and IL-10, when stimulated in vitro with Ag-pulsed splenocytes (Figure 2), indicating the iPSC-Tregs had potential suppressive activities. Fluorescence microscopy revealed that more FoxP3+ cells were present in the OVA-treated than the PBS-treated knees; there were no FoxP3+ cells existing in the knees in mice receiving the DsRed+ vector-transduced iPSCs. Many more CD4+FoxP3+ TCRVβ5+ cells presented in the knees of mice receiving iPSCs transduced with the MiDR-TCR-FoxP3 than the MiDR-FoxP3 (Figure 3). These observations suggest that Ag-specific iPSC-Tregs migrate to the AIA knee after the adoptive transfer into recipient mice. The transferred iPSC-derived cells substantially decreased the inflammatory knee swelling when OVA was present, but had no effect on the control knee that was only injected with mBSA in the murine model (Figure 4); reduced bone loss in the cell transfer knee visualized by the high-resolution micro-CT system (Figure 5).
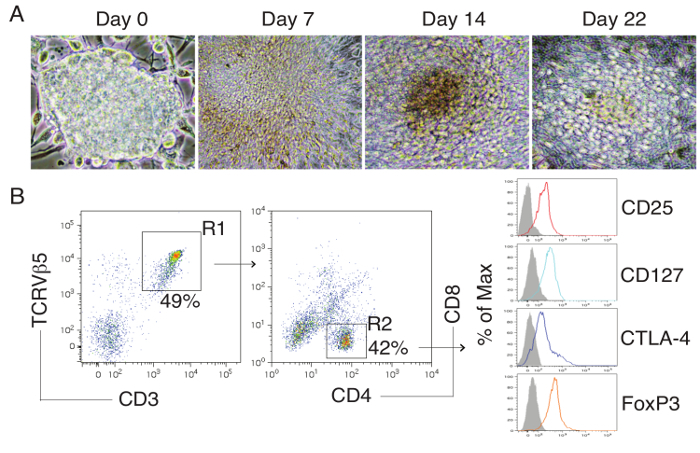 Figure 1:Differentiation of Ag-specific iPSC-Tregs. Murine iPSCs were transduced with a construct: MiDR-TCRα-2A-TCRβ-2A-FoxP3 containing genes of OVA-specific TCR and FoxP3. The gene-transduced cells (DsRed+) were co-cultured on OP9-DL1-DL4-I-Ab cells in the presence of mFlt3L and mIL-7. (A) Morphology of Tregs cell differentiation on day 0, 7, 14 and 22. (B) Flow cytometric analysis for the protein expression of iPSC-derived cells on day 28. CD3+ TCRVβ5+ cells were gated as indicated (R1) and analyzed for the expression of CD4 and CD8, with CD25, CD127, CTLA-4, and FoxP3 shown for cells gated as CD4+CD8- cells (R2) (dark lines; shaded areas indicate isotype controls). Please click here to view a larger version of this figure.
Figure 1:Differentiation of Ag-specific iPSC-Tregs. Murine iPSCs were transduced with a construct: MiDR-TCRα-2A-TCRβ-2A-FoxP3 containing genes of OVA-specific TCR and FoxP3. The gene-transduced cells (DsRed+) were co-cultured on OP9-DL1-DL4-I-Ab cells in the presence of mFlt3L and mIL-7. (A) Morphology of Tregs cell differentiation on day 0, 7, 14 and 22. (B) Flow cytometric analysis for the protein expression of iPSC-derived cells on day 28. CD3+ TCRVβ5+ cells were gated as indicated (R1) and analyzed for the expression of CD4 and CD8, with CD25, CD127, CTLA-4, and FoxP3 shown for cells gated as CD4+CD8- cells (R2) (dark lines; shaded areas indicate isotype controls). Please click here to view a larger version of this figure.
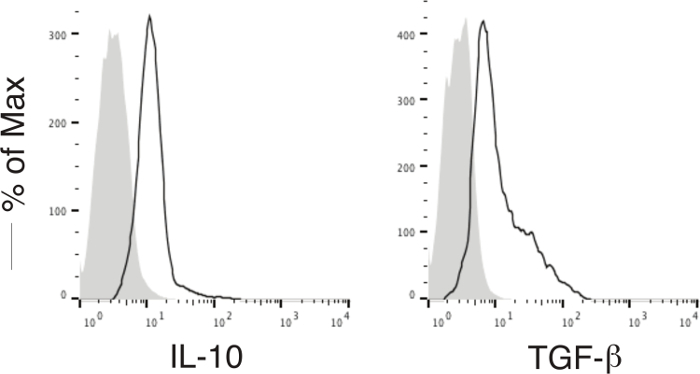 Figure 2:Functional Analyses ofIn Vitro Differentiated Ag-specific Tregs. Murine iPSC-Tregs were stimulated by splenocytes (APCs; Tregs/APCs = 1:4) and pulsed with OVA323-339 peptide. Intracellular cytokine production (TGF-β and IL-10) was analyzed by flow cytometry after gating on live CD4+ CD25+ cells (dark lines; shaded areas indicate isotype controls). Please click here to view a larger version of this figure.
Figure 2:Functional Analyses ofIn Vitro Differentiated Ag-specific Tregs. Murine iPSC-Tregs were stimulated by splenocytes (APCs; Tregs/APCs = 1:4) and pulsed with OVA323-339 peptide. Intracellular cytokine production (TGF-β and IL-10) was analyzed by flow cytometry after gating on live CD4+ CD25+ cells (dark lines; shaded areas indicate isotype controls). Please click here to view a larger version of this figure.
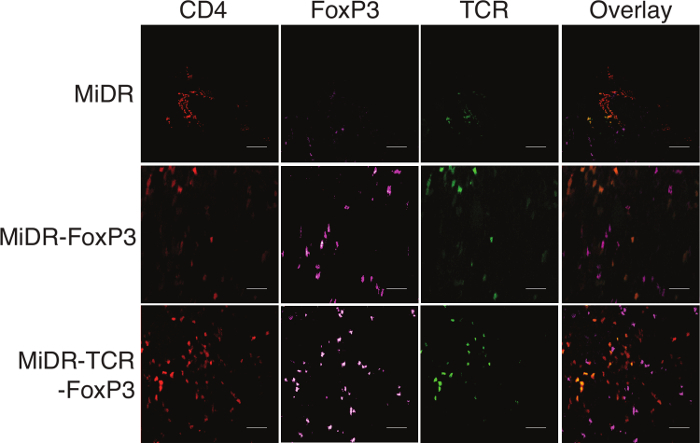 Figure 3:Ag-specific iPSC-Tregs Infiltrate into the Knee Joints. Ag-specific iPSC-Tregs were adoptively transferred into C57BL/6 mice. Shortly after arthritis induction (days 7 - 14), the knees were removed and stained for immunohistology (scale bars: 20 μm). Mice receiving OVA-specific iPSC-Tregs have large numbers of TCRVβ5+ cells in the knees. Please click here to view a larger version of this figure.
Figure 3:Ag-specific iPSC-Tregs Infiltrate into the Knee Joints. Ag-specific iPSC-Tregs were adoptively transferred into C57BL/6 mice. Shortly after arthritis induction (days 7 - 14), the knees were removed and stained for immunohistology (scale bars: 20 μm). Mice receiving OVA-specific iPSC-Tregs have large numbers of TCRVβ5+ cells in the knees. Please click here to view a larger version of this figure.
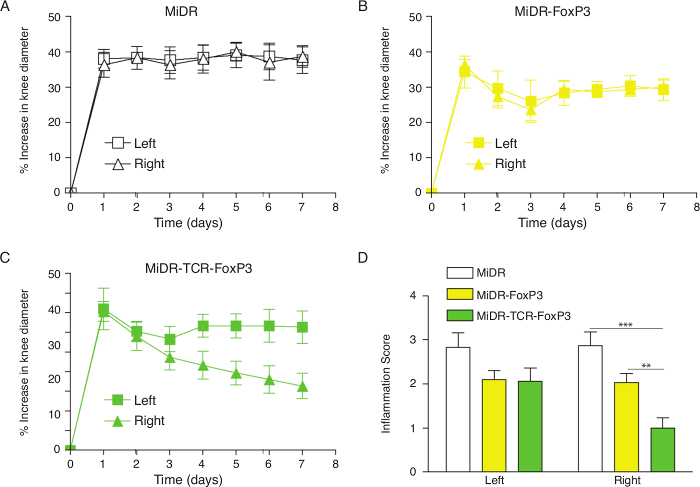 Figure 4:Adoptive Transfer of Ag-specific iPSC-Tregs Ameliorates AIA in Mice. Murine iPSCs were transduced with the retroviral construct MiDR, MiDR-FoxP3, or MiDR-TCR-FoxP3 and were co-cultured on the OP9-DL1/DL4/I-Ab cells. On day 7, the gene-transduced cells (3 × 106/mouse) were adoptively transferred into female C57BL/6 mice that were induced with AIA two weeks after the cell transfer. On the day following arthritis induction, the arthritis severity was monitored by measurement of the knee diameter. (A-C) Percent increase in knee diameter. Increase in knee diameter was calculated based on preinjection knee diameter for each mouse before injection on day 0. Arthritis score was evaluated by examining both knees in a blinded manner; each knee was assigned a score (0: no visible swelling or discoloration; 1: visible swelling with or without discoloration; 2: moderate swelling with discoloration; 3: severe swelling with discoloration). In each group, five mice were used, and data are representative of three independent experiments. Data are represented as the mean ± SD. (D) The mean scoring on day 7 for both knees from five mice. Data are represented as the mean ± SD from three independent experiments (** p< 0.01, *** p< 0.001, two-way ANOVA). Please click here to view a larger version of this figure.
Figure 4:Adoptive Transfer of Ag-specific iPSC-Tregs Ameliorates AIA in Mice. Murine iPSCs were transduced with the retroviral construct MiDR, MiDR-FoxP3, or MiDR-TCR-FoxP3 and were co-cultured on the OP9-DL1/DL4/I-Ab cells. On day 7, the gene-transduced cells (3 × 106/mouse) were adoptively transferred into female C57BL/6 mice that were induced with AIA two weeks after the cell transfer. On the day following arthritis induction, the arthritis severity was monitored by measurement of the knee diameter. (A-C) Percent increase in knee diameter. Increase in knee diameter was calculated based on preinjection knee diameter for each mouse before injection on day 0. Arthritis score was evaluated by examining both knees in a blinded manner; each knee was assigned a score (0: no visible swelling or discoloration; 1: visible swelling with or without discoloration; 2: moderate swelling with discoloration; 3: severe swelling with discoloration). In each group, five mice were used, and data are representative of three independent experiments. Data are represented as the mean ± SD. (D) The mean scoring on day 7 for both knees from five mice. Data are represented as the mean ± SD from three independent experiments (** p< 0.01, *** p< 0.001, two-way ANOVA). Please click here to view a larger version of this figure.
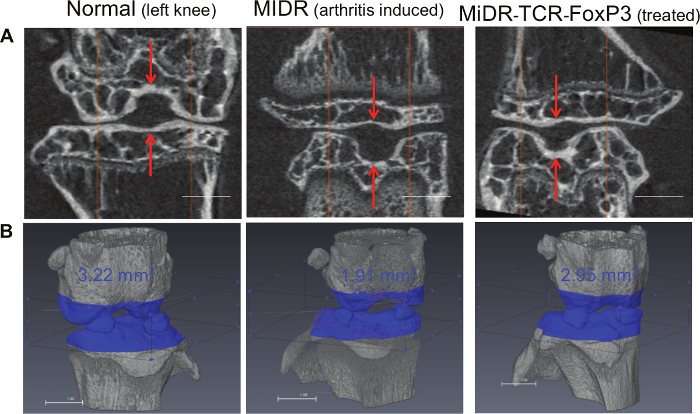 Figure 5:Ag-specific iPSC-Tregs Migrate to the OVA Injected Knee and Reduce the Bone Loss. iPSC-Tregs were adoptively transferred into C57BL/6 mice. Within 21 days post-arthritis induction, mice were anaesthetized and the bone architecture around the mouse knees was imaged by the micro-CT system. Mice receiving OT-II TCR/FoxP3 gene-transduced murine iPSC-Tregs exhibit significant reduction of bone loss as compared to control mice receiving vector-transduced iPSCs. (A) The scans were performed with a 2.2 mm length, and images were captured after further image processing (volume rendering and transformation; scale bars: 1 mm). (B) Bone volume around knee joint was evaluated using three-dimensional reconstruction of micro-CT images, and bone volume inside the volume of interest was calculated (scale bars: 1 mm). Please click here to view a larger version of this figure.
Figure 5:Ag-specific iPSC-Tregs Migrate to the OVA Injected Knee and Reduce the Bone Loss. iPSC-Tregs were adoptively transferred into C57BL/6 mice. Within 21 days post-arthritis induction, mice were anaesthetized and the bone architecture around the mouse knees was imaged by the micro-CT system. Mice receiving OT-II TCR/FoxP3 gene-transduced murine iPSC-Tregs exhibit significant reduction of bone loss as compared to control mice receiving vector-transduced iPSCs. (A) The scans were performed with a 2.2 mm length, and images were captured after further image processing (volume rendering and transformation; scale bars: 1 mm). (B) Bone volume around knee joint was evaluated using three-dimensional reconstruction of micro-CT images, and bone volume inside the volume of interest was calculated (scale bars: 1 mm). Please click here to view a larger version of this figure.
Discussion
In this protocol, a critical step is the in vitro differentiation of TCR/FoxP3 gene-transduced iPSCs. In vitro Notch signaling induces development towards the T cell lineage. To differentiate iPSCs into CD4+FoxP3+ Tregs, we used the OP9-DL1/DL4/I-Ab cells, which highly express MHC II (I-Ab) molecules. Most of the iPSCs differentiate into CD4+ cells. However, after the surface TCR expression, many differentiated pre-T cells lose the ability to differentiate and eventually die. As a result, the cell number of the iPSC-derived functional Tregs dramatically reduces after four weeks of in vitro differentiation. To avoid this, addition of IL-2 can improve cell survival at those time points. An alternative method to drive the maturation and survival of Ag-specific pre-Tregs is to transfer the pre-Tregs that have differentiated in vitro for a week into mice. These pre-Tregs can continue differentiating and maturing in vivo for another three weeks. Using this method, a functional Ag-specific Treg population can be generated from iPSCs, which are nTregs-like and have the ability to suppress autoimmune arthritis in the murine model.
To improve the efficacy of in vivo development of Ag-specific iPSC-Tregs, different compounds (e.g., retinoic acid, gelectin)13,14 or suppressive cytokines (e.g., TGF-β, IL-10) can be used after the adoptive transfer of the pre-Tregs. In addition to increasing the cell survival, this combined approach can maintain FoxP3 expression14,15 and enhance the quality of the Ag-specific iPSC-Tregs.
A potential problem that could arise for in vivo Treg development is overall immunosuppression, resulting in complications during infections or subsequent weight loss. A large number of Ag-specific Tregs developing in vivo may worsen infections. In this case, a suicide gene, the inducible caspase 9(iCasp9)15,16, can be incorporated into the TCR/FoxP3 vector. This approach allows the removal of the stem cell-derived Tregs by the injection of a bioinert small-molecule dimerizing agent (AP1903) to "shut off" the generation of stem cell-derived Tregs, which will overcome this potential issue.
A self-Ag is a typical protein recognized by the immune system of hosts suffering from an individual autoimmune disorder. This self-Ag is the target of the immune system, and the correlated T cells are not deleted. To generate self-Ag specific Tregs, the use of TCR transduction is a good choice. A self-Ag (e.g., heat shock protein) specific TCR can be transduced into mature CD4+ CD25+ Tregs from peripheral blood mononuclear cells (PBMCs), and this approach has been utilized in clinical trials. Alternatively, as described in this protocol, using the gene transduction of self-Ag specific TCR with FoxP3 and stimulation with Notch signaling, stem cell-derived Tregs can be self-Ag specific Tregs.
Cell-based therapies for autoimmune diseases utilizing engineered Tregs may be useful17. Although TCR transduction in T cells has proved to be safe, feasible, and applicable in clinical trials, there are still major safety concerns due to the autoimmunity caused by cross-reactivity with healthy tissues18. Furthermore, using current methods, genetically modified Tregs usually have a short-term persistence in vivo19. Alternatively, a promising source for developing large numbers of monoclonal Ag-specific Tregs is stem cells. Embryonic stem cells (ESCs) have the best pluripotency and self-renewal, but it is not feasible to obtain them from patients. Although easily isolated from the peripheral blood, hematopoietic stem cells (HSCs) are multi-potent stem cells and can be expanded in cell culture similarly to ESCs20. Present iPSC technology has advanced to a more efficient generation of PSC from patients' somatic cells by transduction of different transcription factors. A number of methodological improvements have been developed in recent years to generate iPSCs by maximally reducing potential hazards such as immunogenicity and tumorigenicity. Compared to ESCs, iPSCs have identical pluripotency and self-renewal. As a result, iPSC technology can provide an advantage in developing patient- and/or disease-specific PSCs. The use of iPSCs to develop Ag-specific Tregs may advance the field of cell-based therapies for autoimmune diseases10,11.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This project was funded, in part, under grants from the National Institutes of Health (R01AI121180, R21AI109239 and K18CA151798), the American Diabetes Association (1-16-IBS-281), and the Pennsylvania Department of Health (Tobacco Settlement Funds) to J.S.
References
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Ferraro A, et al. Interindividual variation in human T regulatory cells. Proc Natl Acad Sci U S A. 2014;111:E1111–E1120. doi: 10.1073/pnas.1401343111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herwijnen MJ, et al. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. 2012;109:14134–14139. doi: 10.1073/pnas.1206803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GP, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S A. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TM, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- Lei F, Haque R, Weiler L, Vrana KE, Song J. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260:1–5. doi: 10.1016/j.cellimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Lei F, Haque R, Xiong X, Song J. Directed differentiation of induced pluripotent stem cells towards T lymphocytes. J Vis Exp. 2012. p. e3986. [DOI] [PMC free article] [PubMed]
- Lei F, et al. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71:4742–4747. doi: 10.1158/0008-5472.CAN-11-0359. [DOI] [PubMed] [Google Scholar]
- Haque R, et al. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189:1228–1236. doi: 10.4049/jimmunol.1200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi V, Chandy KG. Immunohistochemistry: paraffin sections using the Vectastain ABC kit from vector labs. J Vis Exp. 2007. [DOI] [PMC free article] [PubMed]
- Lu L, et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A. 2014;111:E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41:270–282. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos CA, et al. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010;28:1107–1115. doi: 10.1002/stem.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Lei F, Xiong X, Wu Y, Song J. FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development. Arthritis Res Ther. 2010;12:R66. doi: 10.1186/ar2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenen MM, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, et al. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg HA, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]


