Abstract
Background
Understanding the relationship between alcohol abuse, a common and theoretically modifiable condition, and the most common cause of death in the world, cardiovascular disease, may inform potential prevention strategies.
Objective
To investigate the associations between alcohol abuse and atrial fibrillation (AF), myocardial infarction (MI), and congestive heart failure (CHF).
Methods
Using the Healthcare Cost and Utilization Project database, we performed a longitudinal analysis of California residents ≥21 years old who received ambulatory surgery, emergency, or inpatient medical care in California between 2005 and 2009. We determined the risk of an alcohol abuse diagnosis on incident AF, MI, and CHF. Patient characteristics modifying the associations and population attributable risks were determined.
Results
Among 14,727,591 patients, 268,084 (1.8%) had alcohol abuse. After multivariable adjustment, alcohol abuse was associated with an increased risk of incident AF (HR 2.14, 95% CI 2.08–2.19, P<0.0001), MI (HR 1.45, 95% CI 1.40–1.51, P <0.0001), and CHF (HR 2.34, 95% CI 2.29–2.39, P<0.0001). In interaction analyses, individuals without conventional risk factors for cardiovascular disease exhibited a disproportionately enhanced risk of each outcome. The population attributable risk of alcohol abuse on each outcome was of similar magnitude to other well-recognized modifiable risk factors.
Conclusions
Alcohol abuse increased the risk of AF, MI, and CHF to a similar degree as other well-established risk factors. Those without traditional cardiovascular risk factors are disproportionately prone to these cardiac diseases in the setting of alcohol abuse. Thus, efforts to mitigate alcohol abuse might result in meaningful reductions of cardiovascular disease.
Keywords: Atrial fibrillation, myocardial infarction, congestive heart failure, alcohol abuse, epidemiology
Despite advances in prevention and treatments, cardiovascular disease continues to be the most prevalent threat to health and survival in the United States, comprising >25% of all deaths (1,2). More than 500,000 Americans suffered a first-time myocardial infarction (MI) in 2015, there are >870,000 new congestive heart failure (CHF) diagnoses annually, and atrial fibrillation (AF), the most common cardiac arrhythmia in the United States, affects >6 million Americans and imposes a high risk of embolic stroke (3). Furthermore, with an aging population and enhanced detection techniques, prevalent MI and incident CHF and AF are on the rise, with a combined projected annual financial burden approaching $400 billion by 2030 (3–5). As such, defining and understanding modifiable risk factors for cardiovascular disease is of particular importance, and targeting modifiable risk factors common to AF, MI, and CHF could have a particularly broad impact.
Alcohol is the most commonly consumed drug in the United States (6). Several studies suggest that moderate levels of alcohol consumption may help prevent incident MI and CHF (7–15). Conversely, even low to moderate levels of alcohol consumption have been shown to increase the incidence of AF (16–18). Nonetheless, the lay press often highlights the potential health benefits of alcohol consumption (19,20), and the research that promulgates that it is heart healthy to drink more alcohol (21).
Alcohol abuse is present in 10–15 million Americans (6). While particular harms of alcohol abuse are appropriately frequently highlighted, including increased risk of domestic violence, suicide, accidents, cirrhosis, and some cancers (22), to some extent the cardiovascular literature may justify the moderate consumption of alcohol (23). The cardiovascular effects of alcohol abuse are not well characterized in population-based research. Indeed, this is generally difficult to study as the great majority of epidemiological studies rely on self-reported alcohol consumption, an ascertainment method known to be particularly inaccurate in individuals who drink heavily (24,25).
With evidence supporting cardiovascular protection and harm as a result of alcohol consumption, the question arises as to whether particular subgroups of patients might be more negatively impacted by alcohol consumption in regards to cardiovascular risk. No study has rigorously examined the interactions between alcohol use and known cardiovascular risk factors, likely because such interaction analyses requires substantial statistical power and therefore a particularly large number of subjects. We therefore leveraged data from every ambulatory surgical, emergency department, and inpatient encounter in California from 2005 through 2009 to evaluate the association between alcohol abuse and AF, MI, and CHF. We then identified subgroups most affected by these associations and determined the relative population-level burdens of AF, MI, and CHF that can be attributed to alcohol abuse.
Methods
All California residents ≥21 years old who received care in a California ambulatory surgery unit, emergency department, or inpatient hospital unit between January 1, 2005 and December 31, 2009 were identified using the Healthcare Cost and Utilization Project (HCUP) California State Ambulatory Surgery Databases, Emergency Department Databases, and State Inpatient Databases (26). The individual HCUP databases specific to healthcare setting and calendar year were merged using an encrypted unique patient identifier to capture repeated visits for a given patient. Participants entered the cohort at first healthcare encounter and were censored upon incident diagnosis of the given outcome of interest (AF, MI, and CHF in separate analyses), at the time of inpatient death, or, in the absence of either, were administratively censored at the end of follow-up (December 31, 2009). Patients with residence outside of California or with missing visit date information were excluded, as well as patients with prevalent AF, MI, or CHF in the respective analyses (defined as carrying the diagnosis at the first recorded hospital encounter).
We recorded demographic data, including age, gender, race, and income at each healthcare encounter. Race and Hispanic ethnicity are reported separately in HCUP, and race was coded as either white or other for the vast majority of individuals with Hispanic ethnicity. Therefore, those with Hispanic ethnicity were treated as a distinct group that superseded the coded race. Income level was categorized by quartiles using the median household income for the patient’s ZIP code; observations with missing data had the value carried forward from the most recent encounter, and those patients without any income data over the study period were excluded. Up to 25 International Classification of Diseases–9th Edition (ICD-9) codes and 21 Current Procedural Terminology (CPT) codes were provided for each encounter. The specific codes used for alcohol abuse, covariates, and each outcome variable are described in Online Table 1.
Because postoperative AF after cardiothoracic surgery may have a different underlying mechanism than AF occurring outside of this acute surgical setting, AF was not recorded if a patient had undergone cardiothoracic surgery during the same hospitalization or within the previous 30 days (27). Such patients remained under observation and could be diagnosed with AF outside of this blanking period. As systolic heart failure is mechanistically different from heart failure with preserved ejection fraction, systolic dysfunction was the primary CHF outcome. A sensitivity analysis utilizing heart failure without systolic dysfunction alone was also performed. MI diagnoses were restricted to acute MI, and an a priori subgroup analysis was performed restricting the endpoint to ST-elevation MI (STEMI) only. Across each analysis, medical comorbidities postulated to confound or mediate the association between alcohol abuse coding and AF, MI, or CHF, respectively, were also recorded using ICD-9 and CPT codes. Dichotomous medical comorbidity variables were accumulated at each healthcare encounter and carried forward over time.
Statistical Analysis
Continuous variables with a normal distribution are presented as mean ± standard deviation. The cumulative incidences of AF, MI, and CHF were estimated, treating death as a competing risk in each. Because results treating deaths as a competing risk did not substantially differ from the standard estimates, Cox models were used to assess the independent effects of alcohol abuse, and to estimate cumulative incidence in both groups under a proportional hazards assumption. Cox proportional hazards models were used to investigate associations with incident events both before and after controlling for mediators and confounders. In these models, demographic characteristics and medical comorbidities were treated as time dependent covariates. All analyses were controlled for potential confounders identified a priori that were available in the dataset. The proportional hazards assumption was assessed using Kaplan–Meier versus predicted survival plots and log-minus-log survival plots and was met for each outcome.
Effect modification of the relationship between alcohol abuse and the outcomes was assessed by testing for interactions with demographic characteristics and established cardiovascular risk factors. To enhance interpretation of these results, race was dichotomized as white versus non-white, and age was dichotomized as age < or ≥ to 60 years. Each interaction term was analyzed separately and adjusted for all other covariates. All interaction terms and potential confounders in each analysis were identified a priori from those available in the administrative dataset. Differences in absolute 3-year risks were calculated for each outcome, stratified by cardiovascular risk factors and presence or absence of alcohol abuse.
The population attributable risk for each outcome was calculated for all covariates in each model. Population attributable risk was estimated by calculating the ratio of the total excess risk associated with the exposure of interest to the total observed risk. Each measured comorbid exposure was compared to those in the cohort without that respective comorbidity. Confidence intervals for population attributable risk estimates were obtained using nonlinear combinations of estimators.
To assess the validity of the alcohol abuse codes, we examined the relationship between those codes and the incidence of the first diagnosis (in a separate encounter occurring after the first identification of an alcohol abuse code) of esophageal varices and hepatic cirrhosis using Cox proportional hazard models.
Analyses were performed using Stata 14 (StataCorp, College Station, Texas) and SAS 9.4 (SAS Institute Inc., Cary, North Carolina). A 2-tailed P<0.05 was considered statistically significant. Certification to use de-identified HCUP data was obtained from the University of California, San Francisco Committee on Human Research.
Results
Of the 20,390,778 patients receiving care in California ambulatory surgery centers, emergency departments, and inpatient wards, 14,727,591 patients were included in analyses examining incident AF, MI, and CHF (Online Figure 1). During the study period, 268,084 patients (1.8% of patients, with 6.3 events/1,000 person-years, 95% CI 6.3–6.4) were coded with an alcohol abuse diagnosis. Patient characteristics at baseline are provided in Table 1. The incidences of AF, MI, and CHF in patients with and without alcohol abuse are shown in Table 2. The incidences of AF, MI, and CHF by patient subgroups are shown in Online Table 2.
Table 1.
Patient Characteristics by Outcome Analysis, Stratified by Presence of Alcohol Abuse.
| Atrial Fibrillation n = 14 378 483 |
Myocardial Infarction n = 14 286 472 |
Congestive Heart Failure n = 14 043 590 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| No Alcohol Abuse n = 14 118 785 |
Alcohol Abuse n = 259 688 |
No Alcohol Abuse n = 14 028 715 |
Alcohol Abuse n = 257 757 |
No Alcohol Abuse n = 13 794 083 |
Alcohol Abuse n = 249 507 |
|
| No. Medical Encounters, (per patient) | 44 673 579 (3.2) |
2 342 998 (9.0) |
45 715 014 (3.3) |
2 383 827 (9.2) |
44 321 427 (3.2) |
2 298 681 (9.2) |
|
| ||||||
| Age - yr. ± SD | 49.1 ± 18.5 | 49.4 ± 14.2 | 49.0 ± 18.5 | 49.3 ± 14.2 | 48.6 ± 18.3 | 49.0 ± 14.1 |
|
| ||||||
| Male sex | 5 871 304 (42.5) |
175 634 (69.0) |
5 814 268 (42.3) |
174 054 (68.9) |
5 701 438 (42.2) |
167 655 (68.6) |
|
| ||||||
| Race | ||||||
| White | 7 643 090 (54.1) |
149 206 (57.5) |
7 827 602 (54.5) |
153 828 (57.9) |
7 665 362 (54.4) |
148 295 (57.9) |
|
| ||||||
| Black | 1 029 331 (7.3) |
20 908 (8.1) |
1 035 538 (7.2) |
21 134 (8.0) |
1 008 516 (7.2) |
19 926 (7.8) |
|
| ||||||
| Hispanic | 3 534 696 (25.0) |
65 518 (25.2) |
3 554 723 (24.8) |
66 363 (25.0) |
3 513 290 (24.9) |
64 192 (25.1) |
|
| ||||||
| Asian/Pacific Islander | 1 192 147 (8.4) |
5 884 (2.3) |
1 207 360 (8.4) |
6 012 (2.3) |
1 188 485 (8.4) |
5 726 (2.2) |
|
| ||||||
| Native American | 34 151 (0.2) |
2 037 (0.8) |
34 446 (0.2) |
2 056 (0.8) |
33 896 (0.2) |
1 995 (0.8) |
|
| ||||||
| Other | 685,337 (4.9) |
16,132 (6.2) |
692,220 (4.8) |
16,350 (6.2) |
681,702 (4.8) |
15,839 (6.2) |
| Income Quartile | ||||||
| 1 Lowest | 3 360 654 (23.8) |
75 038 (28.9) |
3 339 355 (23.8) |
74 505 (28.9) |
3 273 977 (23.7) |
71 887 (28.2) |
|
| ||||||
| 2 | 3 520 100 (25.0) |
68 732 (26.5) |
3 497 753 (24.9) |
68 249 (26.5) |
3 436 910 (24.9) |
66 037 (26.5) |
|
| ||||||
| 3 | 3 620 695 (25.6) |
63 734 (24.5) |
3 596 512 (25.6) |
63 217 (24.5) |
3 537 080 (25.6) |
61 222 (24.5) |
|
| ||||||
| 4 Highest | 3 617 336 (25.6) |
52 184 (20.1) |
3 595 095 (25.6) |
51 786 (20.1) |
3 546 116 (25.7) |
50 361 (20.2) |
|
| ||||||
| HTN | 2 463 603 (17.5) |
61 864 (23.8) |
2 403 689 (17.1) |
60 614 (23.5) |
2 244 850 (16.3) |
55 683 (22.3) |
|
| ||||||
| DM | 1 189 756 (8.4) |
32 126 (12.4) |
1 157 306 (8.3) |
31 542 (12.2) |
1 063 343 (7.7) |
28 807 (11.6) |
|
| ||||||
| CAD | 604 139 (4.3) |
11 715 (4.5) |
538 602 (3.8) |
10 507 (4.1) |
445 679 (3.2) |
8 161 (3.3) |
|
| ||||||
| CKD | 178 883 (1.3) |
4 083 (1.6) |
170 020 (1.2) |
3 941 (1.5) |
134 695 (1.0) |
3 074 (1.2) |
|
| ||||||
| VHD | 134 595 (1.0) |
2 691 (1.0) |
126 091 (0.9) |
2 514 (1.0) |
95 148 (0.7) |
1 609 (0.6) |
|
| ||||||
| DL | 1 002 639 (7.1) |
17 902 (6.9) |
955 148 (6.8) |
17 099 (6.6) |
886 336 (6.4) |
15 359 (6.2) |
|
| ||||||
| Smoking | 595 787 (4.2) |
48 833 (18.8) |
579 178 (4.1) |
48 013 (18.6) |
560 075 (4.1) |
45 442 (18.2) |
|
| ||||||
| Obese | 431 950 (3.1) |
8 431 (3.3) |
421 487 (3.0) |
8 231 (3.2) |
393 784 (2.9) |
7 279 (2.9) |
|
| ||||||
| OSA | 104 979 (0.7) |
2 287 (0.9) |
102 755 (0.7) |
2 236 (0.9) |
91 745 (0.7) |
1 777 (0.7) |
Baseline characteristics are for patients included in the incident myocardial infarction, congestive heart failure, and atrial fibrillation analyses; individuals with prevalent MI, CHF, and AF have been excluded from each analysis, respectively. CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DL, dyslipidemia; DM, diabetes; OSA, obstructive sleep apnea; SD, standard deviation; Smoking, current cigarette smoking; VHD, valvular heart disease.
Dichotomous variables (using Chi2 Test) and continuous variables (using Student’s t-test) were compared within each analysis – P<0.0001 for all comparisons, except in CHF analysis: CAD, P=0.264, VHD, P=0.007; Obese, P=0.063; OSA, P=0.004
Values are numbers (percentages) unless otherwise indicated.
Table 2.
Risk of Atrial Fibrillation, Myocardial Infarction, and Congestive Heart Failure with Alcohol Abuse.
| Atrial Fibrillation |
Myocardial Infarction |
Congestive Heart Failure |
|
|---|---|---|---|
| Incidence Rate in events per 1,000 person-years (95% CI) | |||
| Overall Population | 7.4 (7.4–7.4) | 3.1 (3.0–3.1) | 10.0 (10.0–10.0) |
| Alcohol Abuse | 8.5 (8.3–8.7) | 4.6 (4.6–4.7) | 14.0 (13.7–14.2) |
| No Alcohol Abuse | 7.4 (7.4–7.4) | 3.1 (3.1–3.1) | 9.8 (9.8–9.9) |
CI, confidence interval; HR, hazard ratio
Each model is adjusted for age, sex, race, hypertension, diabetes, coronary artery disease (for the AF and CHF outcomes), congestive heart failure (for the AF and MI outcomes), chronic kidney disease, valvular heart disease (for the AF and CHF outcomes), dyslipidemia, obesity, obstructive sleep apnea, cigarette smoking, and income.
Validation Analyses
In an analysis intended to validate alcohol abuse coding in our model, presence of alcohol abuse coding resulted in an approximately 130-fold adjusted hazard of varices or cirrhosis (HR 132.6, 95% CI 130.7–134.4, P<0.0001) (Online Table 3 and Online Figure 2).
Alcohol and Atrial Fibrillation
After excluding patients with prevalent AF, 14,378,483 patients were included in the AF analysis, exhibiting 358,887 incident AF events (2.5%, 7.4 events/1,000 person-years, 95% CI 7.4–7.4). Alcohol abuse predicted a greater risk of incident AF (HR 1.93, 95% CI 1.88–1.98, P <0.0001). After multivariable adjustment for age, gender, race, and presence of hypertension, diabetes, coronary disease, congestive heart failure, chronic kidney disease, valvular heart disease, dyslipidemia, current smoking, obesity, obstructive sleep apnea, and income, those with alcohol abuse had a greater than two fold higher risk of AF (HR 2.14, 95% CI 2.08–2.19, P <0.0001) (Figures 1 and 2. Unadjusted and sex and age adjusted only incidence curves are shown in Online Figures 3–5). The increased hazard of AF due to alcohol abuse was higher than the majority of well-established AF risk factors. The specific hazard ratios for each outcome are shown in Online Table 4.
Figure 1. Cumulative Probability of Atrial Fibrillation, Myocardial Infarction, and Congestive Heart Failure by Presence or Absence of Alcohol Abuse.
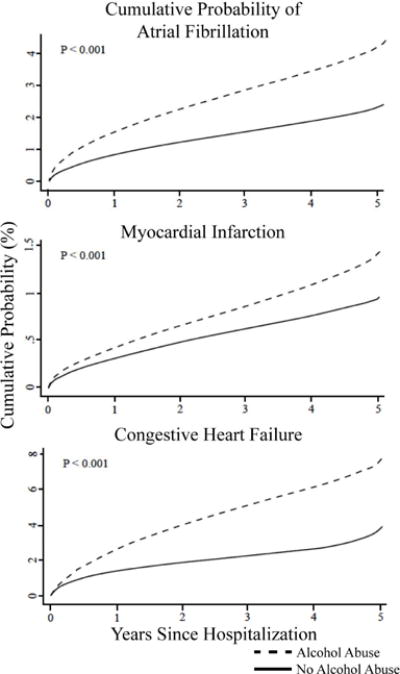
These curves were generated under a proportional hazards assumption. Each model is adjusted for age, sex, race, hypertension, diabetes, coronary artery disease (for the AF and CHF outcomes), congestive heart failure (for the AF and MI outcomes), chronic kidney disease, valvular heart disease (for the AF and CHF outcomes), dyslipidemia, obesity, obstructive sleep apnea, cigarette smoking, and income.
Figure 2. Association of alcohol abuse and known risk factors for Atrial Fibrillation, Myocardial Infarction, and Congestive Heart Failure.
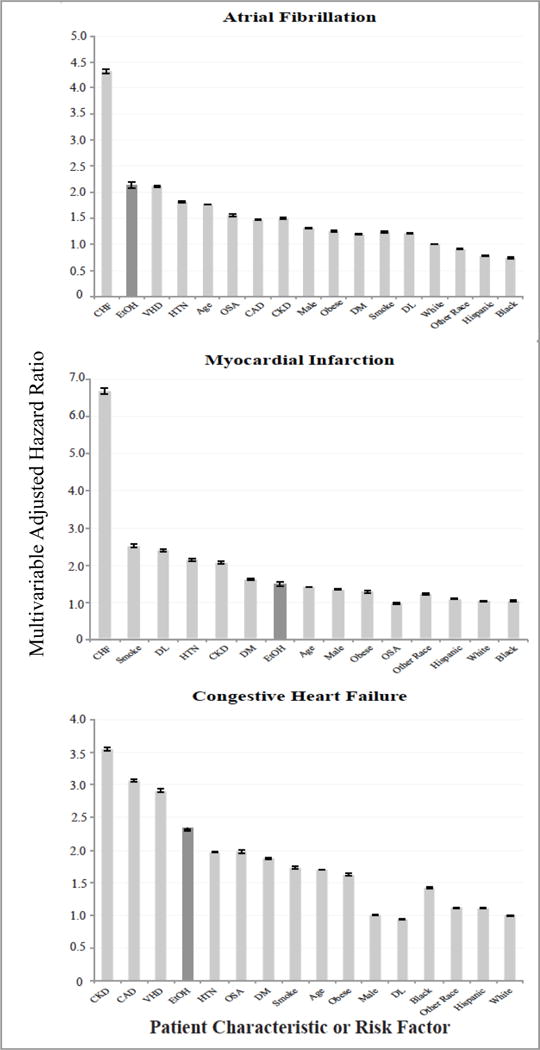
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DL, dyslipidemia; DM, diabetes; OSA, obstructive sleep apnea; Smoking, current cigarette smoking; VHD, valvular heart disease. White race is the referent for other racial hazard ratios. Other Race denotes patients self-identified as Asian, Pacific Islander, Native American, or Other. Patient characteristics ordered by strength of association for each given outcome, followed by race. Error bars denote 95% confidence intervals.
With the exception of male sex and smoking, the relative increased risk of AF associated with alcohol abuse was significantly greater in the absence of established AF risk factors (Figure 3). While the enhanced risk in the setting of alcohol abuse was substantial in every group (Central Illustration), the lower baseline risk in patients without risk factors accounted for the significant interactions favoring a stronger relative effect in those patients.
Figure 3. Association between Alcohol Abuse and Atrial Fibrillation, Myocardial Infarction, or Congestive Heart Failure, Stratified by Patient Characteristics.
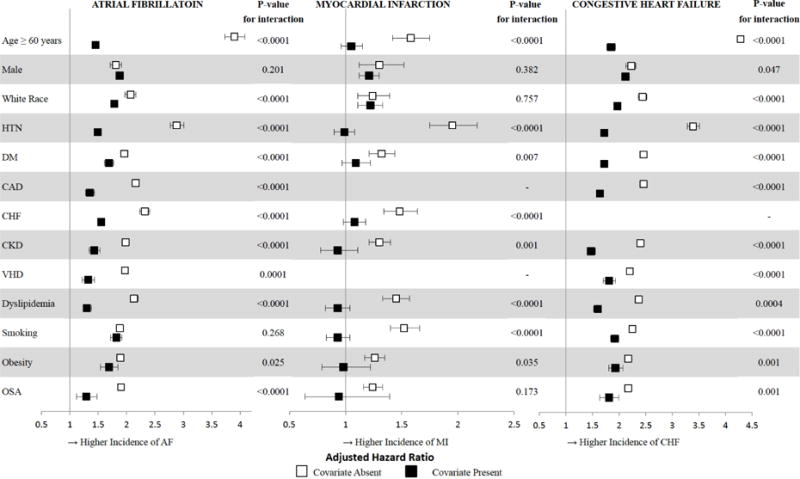
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DL, dyslipidemia; DM, diabetes; OSA, obstructive sleep apnea; Smoking, current cigarette smoking; VHD, valvular heart disease. Age dichotomized to Age < 60 versus ≥ 60, and race dichotomized to White Race versus Non-White Race for analysis. Error bars denote 95% confidence intervals.
Central Illustration. Alcohol Abuse and Cardiac Disease: Risk, Patient Characteristics, and Population Attributable Risk.
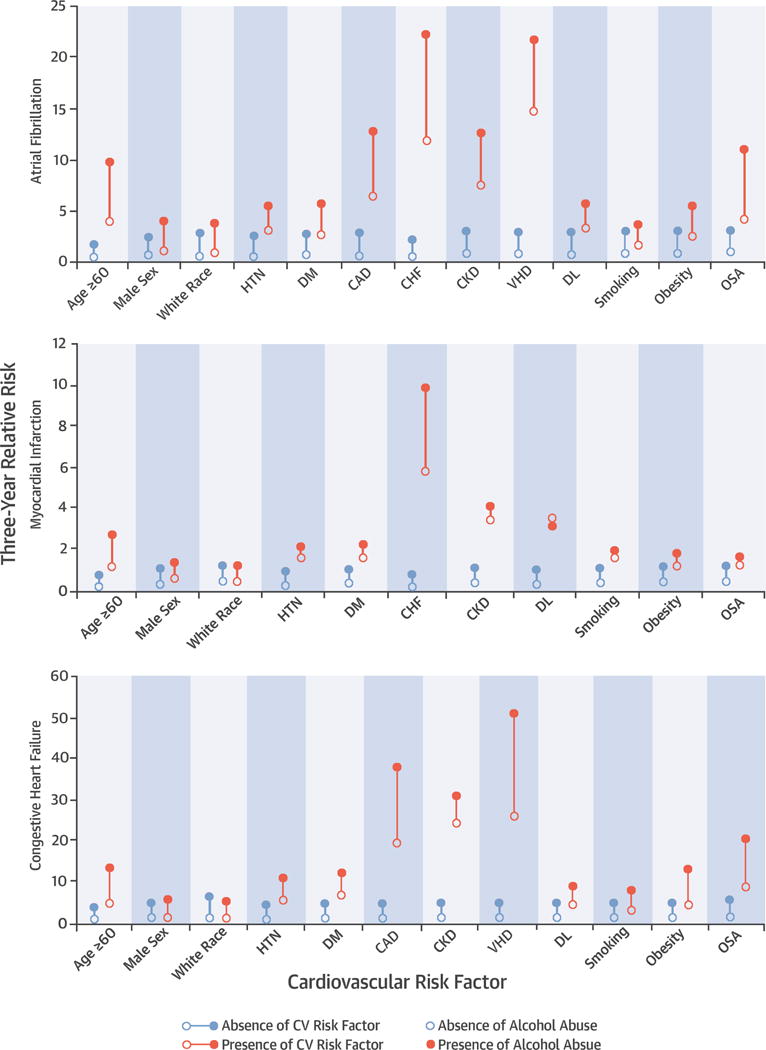
Absolute Risk of Atrial Fibrillation, Myocardial Infarction, or Congestive Heart Failure, Stratified by Presence or Absence of Risk Factors and Presence or Absence of Alcohol Abuse. CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DL, dyslipidemia; DM, diabetes; OSA, obstructive sleep apnea; Smoking, current cigarette smoking; VHD, valvular heart disease. Age dichotomized to Age < 60 versus ≥ 60, and race dichotomized to White Race versus Non-White Race for analysis.
Alcohol and Myocardial Infarction
Among 14,286,427 patients, incident MI was diagnosed in 157,254 individuals (1.1%, 3.1 events/1,000 person-years, 95% CI 3.0–3.1). Presence of alcohol abuse increased the risk of acute MI both before (unadjusted HR 2.03, 95% CI 1.95–2.11, P<0.0001) and after adjustment for age, gender race, hypertension, diabetes, congestive heart failure, chronic kidney disease, dyslipidemia, current smoking, obesity, obstructive sleep apnea, and income, exhibited a relative risk similar to multiple well-established risk factors (adjusted HR 1.45, 95% CI 1.4–1.51, P<0.0001) (Figures 1 and 2). This increase in risk associated with alcohol was of a magnitude similar to that of diabetes and obesity. When restricting these analyses to only STEMI as the outcome (N = 48,467 events, 0.3% of patients for an incidence rate of 1.1 [95% CI 1.1–1.1] per 1,000 person/years), there remained a two-fold increased in risk in unadjusted analyses (HR 2.04, 95% CI 1.90–2.18, P <0.0001); this was attenuated, but still revealed a 30% increased risk after inclusion in the same adjusted model as used for all acute MIs (HR 1.30, 95% CI 1.21–1.39, P <0.0001).
Although the relative risk of alcohol abuse on MI was of lower magnitude compared to AF, a similar pattern was observed in interaction analyses by various subgroups: the relative risk was most prominent in the absence of risk factors (Figure 3), driven primarily by the particularly low baseline risk when conventional risk factors and alcohol abuse were both absent (Central Illustration).
Alcohol and Congestive Heart Failure
After exclusions, 14,043,590 patients remained in our model for the CHF outcome, revealing 411,983 incident CHF events (2.9%, 10.0 events/1,000 person-years, 95% CI 10.0–10.0). Alcohol abuse imposed an unadjusted hazard ratio of 2.23 (95% CI 2.19–2.28, P <0.0001) in predicting incident CHF; the risk remained similar after adjusting for potential confounders (HR 2.34, 95% CI 2.29–2.39, P <0.0001) (Figure 1), exhibiting a similar relative hazard as well-established predictors such as hypertension and diabetes (Figure 2). In a sensitivity analysis, 858,604 patients exhibited the outcome of heart failure in the absence of systolic dysfunction. Alcohol abuse was associated with a three-fold higher risk of heart failure with preserved systolic function (adjusted HR 3.2, 95% CI 1.86–5.51). The results of the interaction analyses using this outcome were also similar to those obtained using the primary outcome of heart failure with systolic dysfunction, again demonstrating that younger age and the absence of hypertension especially enhanced the relative risk in the setting of alcohol abuse.
After stratification by risk factors for CHF, it was again those patients without a given risk factor that tended to be disproportionately affected by alcohol abuse (Figures 3 and 4).
Figure 4. Population attributable risk of alcohol abuse and other modifiable risk factors for Atrial Fibrillation, Myocardial Infarction, and Congestive Heart Failure.
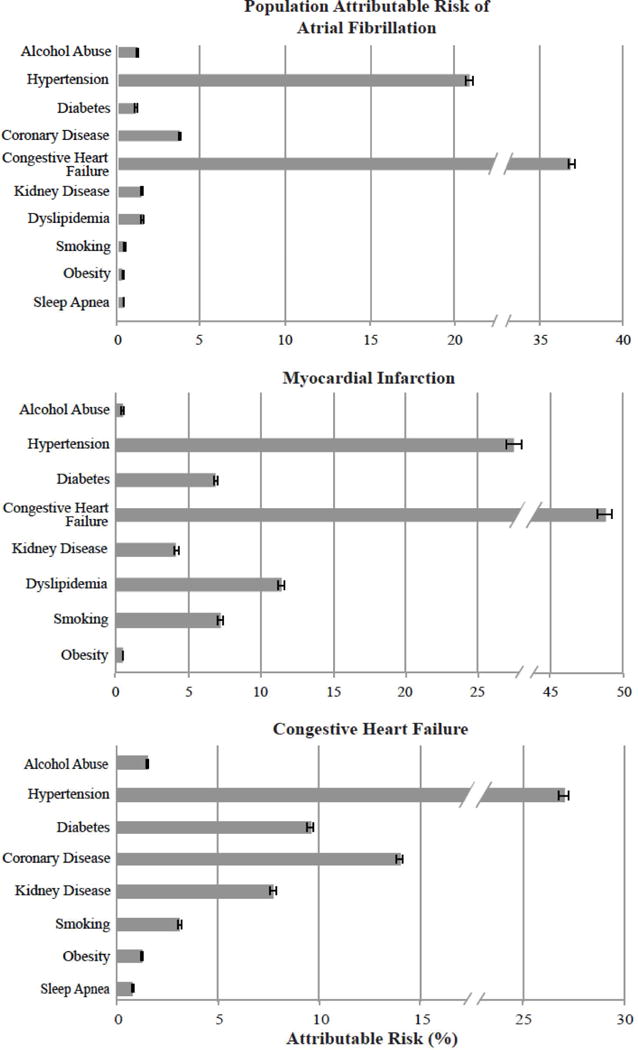
Patient characteristics with negative hazard ratios in the Cox proportional hazard adjusted model were excluded from the figure: obstructive sleep apnea (from MI), dyslipidemia (from CHF).
Although hypertension and CHF were by far the most important contributors to the population attributable risk for each outcome, the population attributable risk for alcohol abuse on each outcome was similar in magnitude to the majority of well-established and at least theoretically modifiable risk factors (Figure 4).
Discussion
Leveraging state-wide ambulatory surgery visits, emergency room encounters, and hospitalizations in California over five consecutive years, we found that alcohol abuse was an important predictor of AF, MI, and CHF, exhibiting both relative hazards and population attributable risks similar to the majority of established modifiable risk factors in each case. Although alcohol abuse substantially heightened the risk in all subgroups, the relative increase in risk was observed most prominently among those without established risk factors.
There is much research into the role of alcohol, the nation’s most consumed drug (28), and its effect on cardiovascular disease, the principle threat to health in the United States (29). Even mild to moderate levels of alcohol consumption have been associated with increased risk of AF, yet this level of exposure has been demonstrated observational studies to decrease the risk of MI and CHF (10,11,13,14,30,31). The impact of the most extreme form of alcohol consumption, alcohol abuse, on these cardiovascular endpoints across the population has previously remained unknown.
A recent study examining access to alcohol as an instrumental variable among counties with various alcohol sales laws in Texas revealed a consistent association between greater alcohol access and AF and inconsistent results regarding MI and HF (32). While the paper utilized novel ecological methods to demonstrate important health effects related to alcohol exposure, a major limitation of the study was the inability to examine variable degrees of that exposure. Indeed, the impact of the most extreme form of alcohol consumption, alcohol abuse, on these cardiovascular endpoints across the population has previously remained unknown.
Given the association of AF with cardiovascular complications and death (27,33) and few interventions to this point that modify that risk (34,35), prevention of AF is paramount. While the data in favor of an association between alcohol consumption and AF are conflicting (14,34,35), the majority of studies suggest that chronic alcohol consumption increases the risk for incident AF (18). The great majority of these studies have relied on participant self-report and none was able to examine interactions by various participant characteristics. Among common risk factors for AF, our data demonstrate that only CHF is a stronger risk factor than alcohol abuse. There was a disproportionate effect of alcohol abuse in predicting AF in patients who lacked other known risk factors for AF, suggesting that alcohol abuse may be a particularly important cause of lone AF. Interaction analyses revealed that younger patients and those without hypertension exhibited a particularly disproportionately heightened relative risk of AF. This demonstrates that the mechanisms of AF in the context of alcohol abuse do not rely on atrial changes that occur with age or hypertension, perhaps suggesting an electrical rather than a structural effect. Conversely, alcohol abuse did not appear to confer increased risk associated with male sex or smoking, perhaps suggesting that these share common mechanistic pathways that promote AF.
Despite the preponderance of observational literature favoring a protective effect of alcohol on MI risk (11–15), our data demonstrate the reverse – an increased risk of MI (whether including all acute MIs or only STEMIs) both before and after multivariable adjustment. Taken along with prior literature on gradations of alcohol consumption and MI risk, these data on alcohol abuse therefore suggest that the impact of alcohol consumption on incident MI may mirror the “U-shaped curve” found with alcohol consumption and overall mortality (36–38) and are consistent with the observation that acute binges of alcohol increase the risk of MI (39). Of interest, a recent analysis suggests that the apparent protective effect of light to moderate drinking on mortality maybe confounded by physical activity and perceived health status (40). Therefore, our findings may simply better elucidate the harmful effects of alcohol related to MI by examining alcohol in excess as the predictor. Although the magnitude of increased risk for MI was lower than for AF (30% versus more than a two-fold increased risk), again alcohol abuse appears to confer both an increased relative risk and population attributable risk similar to well-established modifiable risk factors. In no circumstance did the absence of a cardiovascular comorbidity display a protective effect against the increased MI risk among those with alcohol abuse. Alcohol abuse had a negligible impact in increasing the relative risk among those with chronic kidney disease, dyslipidemia, smoking, and obesity, perhaps related to alcohol’s favorable effects on lipid profiles (41–47). Importantly, in each case, although the relative risk of alcohol abuse was not different in those with and without these risk factors, the absolute risk was increased in every case, demonstrating the important impact in these populations. In patients without these diseases, the ill effects of alcohol are especially severe regarding MI risk. Clearly, it is not appropriate to extrapolate from evidence regarding moderate drinking that “more is better” in terms of MI risk, arguing for the importance of moderation even in regards to an outcome where alcohol has demonstrated some benefit.
While alcoholic cardiomyopathy is known to occur in the setting of alcohol abuse (48,49), the population-level effects of alcohol abuse on incident CHF remain unclear. Indeed, several large studies have demonstrated that moderate alcohol consumption may have a protective effect against CHF (7–9). Our data show a more than two-fold increased risk of CHF with alcohol abuse in the general population – an even stronger predictive association than many other established risk factors, including hypertension. Interestingly, we were unable to identify any meaningful differences in our sensitivity analyses restricting the outcome to heart failure in the presence or absence of systolic dysfunction, suggesting volume overload due to excessive alcohol may occur due to mechanisms more complicated than simply weakening the ventricles. Stratification by patient characteristics for incident CHF yielded similar results to that of AF and MI: healthier patients tended to exhibit a disproportionately greater relative risk of CHF in the setting of alcohol abuse. Interestingly, just as with AF, the most diametric interactions were observed when stratifying by age and by presence of hypertension, again implicating some distinct mechanistic pathway regarding alcohol-induced myocardial toxicity versus age-related or hypertensive cardiomyopathy.
On a population level, the risk of AF, MI, and CHF that could be attributed to alcohol was on a par with multiple other established risk factors. Extrapolating from our data to the estimated prevalence of each outcome, a theoretical complete eradication of alcohol abuse would result in over 73,000 fewer AF cases, 34,000 fewer MIs, and 91,000 fewer patients with CHF in the US alone (3).
This study had several important limitations. HCUP relies on physician coding; however, coding for alcohol abuse, AF, MI, and CHF have been shown to be highly specific with variable sensitivity (50–54). Importantly, limited sensitivity would be expected only to decrease power and would not be expected to result in false positives. Research using these methods and particularly the HCUP database in this regard is nonetheless a powerful tool and an accepted approach for large population studies (26,55,56). Some potential confounders are not measured by ICD-9 codes, such as specific diets or levels of activity; however, we adjusted for demographic information, smoking, and obesity, which may parallel and even direct reflect many of these unmeasured factors (57,58). Smoking in particular may be an important confounder underlying the observation that alcohol abuse was associated with a higher risk of MI. However, the prevalence of smoking ascertained from HCUP of 8% is of a similar magnitude to the estimate of 13% provided by the Center for Disease Control and Prevention in California over that same time period (59); importantly, approximately 18% of those who abused alcohol were coded as smokers in HCUP versus approximately 4% of patient who did not abuse alcohol. Given the effects observed for smoking on MI and assuming under-ascertainment of smoking (if present) was non-differential (which we believe to be conservative as smoking was more likely more often coded among those also coded with alcohol abuse), we calculated that 82% of those with alcohol abuse would have had to also smoke cigarettes to explain the increased hazard of MI observed. While we cannot exclude this possibility, we do not believe such a high prevalence of smoking in California over this time period, particularly given the CDC-based estimates, is likely. In addition, use of physician coding eliminates the important bias of patient self-report that had been prevalent in studies on this topic to this point. We further validated our primary predictor (alcohol abuse) by demonstrating exceedingly strong associations with esophageal varices and cirrhosis; that well-established risk factors predicted AF, MI, and CHF as expected also serves to validate our covariates and outcomes. Our endpoints fail to capture any outpatient encounters, which has particular relevance to the potentially insensitive diagnoses of alcohol abuse and AF. However, by capturing patients treated in ambulatory procedural units, emergency departments, and inpatient settings, we likely studied the sicker cohort of patients with alcohol abuse and these cardiovascular outcomes and certainly captured those responsible for the majority of healthcare utilization. As with any observational study, we cannot exclude residual confounding as an explanation for our results. However, we adjusted for conventionally recognized confounders in as exhaustive a fashion as possible and as appropriate. Finally, it is important to emphasize that these data cannot prove causality. In addition, while the population attributable risk calculations must be interpreted with this caution, we believe such estimates provide substantial value in modeling potential public health impacts given a common and theoretically modifiable condition such as alcohol abuse.
Finally, by relying on physician coding of alcohol abuse, the proportion of alcohol abusers identified in our study is approximately one third of national estimates (6), and we were not able to comment on the quantity of alcohol. While this limits our ability to determine specific amounts that may be harmful, it also very accurately and specifically identifies patients wherein alcohol abuse was not only recognized but also clinically documented by a healthcare professional. Therefore, while treating or preventing alcohol abuse is no doubt challenging, in our study the predictor is already recognized by at least one healthcare professional, suggesting that these are the patients already accessible to possible intervention within the current healthcare system. That some patients who suffer from alcohol abuse were not identified as such in our study has the likely effect of biasing the results toward the null, suggesting that the association between alcohol abuse and cardiac disease may be even more marked than measured in the current study.
Conclusions
Alcohol abuse increases the risk of incident AF, MI, and CHF, exhibiting magnitudes of risk similar to other well-established risk factors. Although nearly all subgroups exhibited increased risk in the setting of alcohol abuse, those without a given risk factor for each outcome were disproportionately prone to enhanced cardiovascular risk. The risk of AF, MI, and CHF that can be attributed to alcohol abuse is large, suggesting that efforts to mitigate this addictive disease might result in substantial reductions of cardiac disease. Taken together, these data demonstrate that alcohol in excess should not be considered cardio-protective, but rather cardio-toxic, contributing to heightened risk for all three major, yet distinct, cardiac adverse outcomes.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Alcohol abuse increases the risk of atrial fibrillation, myocardial infarction (MI), and heart failure to an extent similar to that of other strong risk factors, and affects healthier individuals disproportionately. Protective effects of alcohol against MI are outweighed at heavy levels of consumption by its adverse effects.
Translational Outlook
More research is needed to understand the mechanisms by which alcohol influences the risk of cardiac disease in specific populations.
Acknowledgments
Sources of Funding: Research reported in this manuscript was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA022222 (G.M.M.) and 1F32HL129759-01 (I.R.W.).
ABBREVIATIONS
- AF
atrial fibrillation
- MI
myocardial infarction
- CHF
congestive heart failure
- HCUP
Healthcare Cost and Utilization Project
- ICD-9
International Classification of Diseases–9th Edition
- CPT
Current Procedural Terminology
- STEMI
ST elevation myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationships with Industry: None to report
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. World Health Organization - Non Communicable Diseases. Country Profiles. 2014 [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–9. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 6.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 7.Abramson JL, Williams SA, Krumholz HM, Vaccarino V. Moderate alcohol consumption and risk of heart failure among older persons. JAMA. 2001;285:1971–7. doi: 10.1001/jama.285.15.1971. [DOI] [PubMed] [Google Scholar]
- 8.Bryson CL, Mukamal KJ, Mittleman MA, et al. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol. 2006;48:305–11. doi: 10.1016/j.jacc.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 9.Djousse L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians’ Health Study I. Circulation. 2007;115:34–9. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- 10.Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Current atherosclerosis reports. 2008;10:117–20. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo CA, Jr, Stampfer MJ, Glynn RJ, et al. Moderate alcohol consumption and risk for angina pectoris or myocardial infarction in U.S. male physicians. Ann intern med. 1997;126:372–5. doi: 10.7326/0003-4819-126-5-199703010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiologic reviews. 1993;15:328–51. doi: 10.1093/oxfordjournals.epirev.a036124. [DOI] [PubMed] [Google Scholar]
- 13.Yano K, Rhoads GG, Kagan A. Coffee, alcohol and risk of coronary heart disease among Japanese men living in Hawaii. N Eng J Med. 1977;297:405–9. doi: 10.1056/NEJM197708252970801. [DOI] [PubMed] [Google Scholar]
- 14.Mukamal KJ, Chung H, Jenny NS, et al. Alcohol consumption and risk of coronary heart disease in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:30–7. doi: 10.1111/j.1532-5415.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 15.Tavani A, Bertuzzi M, Negri E, Sorbara L, La Vecchia C. Alcohol, smoking, coffee and risk of non-fatal acute myocardial infarction in Italy. Eur J Epidemiol. 2001;17:1131–7. doi: 10.1023/a:1021276932160. [DOI] [PubMed] [Google Scholar]
- 16.Conen D, Tedrow UB, Cook NR, et al. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300:2489–96. doi: 10.1001/jama.2008.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodama S, Saito K, Tanaka S, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427–36. doi: 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- 18.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281–9. doi: 10.1016/j.jacc.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Bakalar N. Study Links Alcohol to Lower Risk of Coronaries. The New York Times. 2007 Jan 9; [Google Scholar]
- 20.Mann D. In: Moderate Alcohol Drinking May Boost Heart Health. Martin LJ, editor. Feb 24, 2011. [Google Scholar]
- 21.Whitman IR, Pletcher MJ, Vittinghoff E, et al. Perceptions, Information Sources, and Behavior Regarding Alcohol and Heart Health. Am J Cardiol. 2015;116:642–6. doi: 10.1016/j.amjcard.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gmel G, Rehm J. Harmful alcohol use. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2003;27:52–62. [PMC free article] [PubMed] [Google Scholar]
- 23.United States. Department of Health and Human Services., United States. Department of Agriculture., United States. Dietary Guidelines Advisory Committee. Dietary guidelines for Americans, 2010. 7th. Washington, D.C.: G.P.O.; 2010. [Google Scholar]
- 24.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 25.Heeb JL, Gmel G. Measuring alcohol consumption: a comparison of graduated frequency, quantity frequency, and weekly recall diary methods in a general population survey. Addictive behaviors. 2005;30:403–13. doi: 10.1016/j.addbeh.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–7. doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- 27.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 28.Administration SAaMHS. National Institute of Health: Nationwide Trends. 2015: Annual National Survey on Drug Use and Health [Google Scholar]
- 29.Centers for Disease Control and Prevention: Leading Causes of Death. 2014: Number of Deaths for Leading Causes of Death [Google Scholar]
- 30.Solomon CG, Hu FB, Stampfer MJ, et al. Moderate alcohol consumption and risk of coronary heart disease among women with type 2 diabetes mellitus. Circulation. 2000;102:494–9. doi: 10.1161/01.cir.102.5.494. [DOI] [PubMed] [Google Scholar]
- 31.Suh I, Shaten BJ, Cutler JA, Kuller LH. Alcohol use and mortality from coronary heart disease: the role of high-density lipoprotein cholesterol. The Multiple Risk Factor Intervention Trial Research Group. Ann Intern Med. 1992;116:881–7. doi: 10.7326/0003-4819-116-11-881. [DOI] [PubMed] [Google Scholar]
- 32.Dukes JW, Dewland TA, Vittinghoff E, et al. Access to alcohol and heart disease among patients in hospital: observational cohort study using differences in alcohol sales laws. BMJ. 2016;353:i2714. doi: 10.1136/bmj.i2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–7. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 35.Mukamal KJ, Psaty BM, Rautaharju PM, et al. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J. 2007;153:260–6. doi: 10.1016/j.ahj.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Klatsky AL, Armstrong MA, Friedman GD. Alcohol and mortality. Annals of internal medicine. 1992;117:646–54. doi: 10.7326/0003-4819-117-8-646. [DOI] [PubMed] [Google Scholar]
- 37.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Ann Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg RJ, Burchfiel CM, Reed DM, Wergowske G, Chiu D. A prospective study of the health effects of alcohol consumption in middle-aged and elderly men. The Honolulu Heart Program. Circulation. 1994;89:651–9. doi: 10.1161/01.cir.89.2.651. [DOI] [PubMed] [Google Scholar]
- 39.Mostofsky E, Chahal HS, Mukamal KJ, et al. Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation. 2016;133:979–87. doi: 10.1161/CIRCULATIONAHA.115.019743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muscari A, Bianchi G, Conte C, et al. No Direct Survival Effect of Light to Moderate Alcohol Drinking in Community-Dwelling Older Adults. J Am Geriatr Soc. 2015;63:2526–2533. doi: 10.1111/jgs.13837. [DOI] [PubMed] [Google Scholar]
- 41.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–23. [PubMed] [Google Scholar]
- 42.Gaziano JM, Buring JE, Breslow JL, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Eng J Med. 1993;329:1829–34. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 43.Hines LM, Stampfer MJ, Ma J, et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Eng J Med. 2001;344:549–55. doi: 10.1056/NEJM200102223440802. [DOI] [PubMed] [Google Scholar]
- 44.Paunio M, Heinonen OP, Virtamo J, et al. HDL cholesterol and mortality in Finnish men with special reference to alcohol intake. Circulation. 1994;90:2909–18. doi: 10.1161/01.cir.90.6.2909. [DOI] [PubMed] [Google Scholar]
- 45.Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–7. doi: 10.1136/bmj.316.7137.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 47.Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 48.Regan TJ. Alcohol and the cardiovascular system. JAMA. 1990;264:377–81. [PubMed] [Google Scholar]
- 49.Urbano-Marquez A, Estruch R, Navarro-Lopez F, Grau JM, Mont L, Rubin E. The effects of alcoholism on skeletal and cardiac muscle. N Engl J Med. 1989;320:409–15. doi: 10.1056/NEJM198902163200701. [DOI] [PubMed] [Google Scholar]
- 50.Kim HM, Smith EG, Stano CM, et al. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use. BMC Health Serv Res. 2012;12:18. doi: 10.1186/1472-6963-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PloS one. 2014;9:e92286. doi: 10.1371/journal.pone.0092286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):129–40. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Glynn RJ, Dreyer NA, et al. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20:700–8. doi: 10.1002/pds.2146. [DOI] [PubMed] [Google Scholar]
- 54.Jensen PN, Johnson K, Floyd J, et al. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141–7. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 56.Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312:616–22. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–66. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turrell G, Kavanagh AM. Socio-economic pathways to diet: modelling the association between socio-economic position and food purchasing behaviour. Public health nutrition. 2006;9:375–83. doi: 10.1079/phn2006850. [DOI] [PubMed] [Google Scholar]
- 59.California Adult Smoking Prevalence, 1984 – 2009. California Department of Public Health, California Tobacco Control Program; Mar, 2010. http://www.cdph.ca.gov. [Google Scholar]
- 60.Higgins ST, Kurti AN, Redner R, et al. Co-occurring risk factors for current cigarette smoking in a U.S. nationally representative sample. Preventive medicine. 2016 doi: 10.1016/j.ypmed.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


