Abstract
Dcw1p and Dfg5p in Saccharomyces cerevisiae are homologous proteins that were previously shown to be involved in cell wall biogenesis and to be essential for growth. Dcw1p was found to be a glycosylphosphatidylinositol-anchored membrane protein. To investigate the roles of these proteins in cell wall biogenesis and cell growth, we constructed mutant alleles of DCW1 by random mutagenesis, introduced them into a Δdcw1 Δdfg5 background, and isolated a temperature-sensitive mutant, DC61 (dcw1-3 Δdfg5). When DC61 cells were incubated at 37°C, most cells had small buds, with areas less than 20% of those of the mother cells. This result indicates that DC61 cells arrest growth with small buds at 37°C. At 37°C, fewer DC61 cells had 1N DNA content and most of them still had a single nucleus located apart from the bud neck. In addition, in DC61 cells incubated at 37°C, bipolar spindles were not formed. These results indicate that DC61 cells, when incubated at 37°C, are cell cycle arrested after DNA replication and prior to the separation of spindle pole bodies. The small buds of DC61 accumulated chitin in the bud cortex, and some of them were lysed, which indicates that they had aberrant cell walls. A temperature-sensitive dfg5 mutant, DF66 (Δdcw1 dfg5-29), showed similar phenotypes. DCW1 and DFG5 mRNA levels peaked in the G1 and S phases, respectively. These results indicate that Dcw1p and Dfg5p are involved in bud formation through their involvement in biogenesis of the bud cell wall.
Fungal cells including yeast cells have sturdy shells, i.e., cell walls, to endure osmotic stress in their environment. Without the cell wall, they easily burst and die. The cell wall also must be plastic so that it can expand outside the lipid bilayer during growth (6). Maintaining both plasticity and rigidity during bud cell wall construction during the cell cycle is a complex process that involves many factors. Saccharomyces cerevisiae is a good model for studying this process. In S. cerevisiae, when cells reach a critical size in late G1, they simultaneously start budding. In the very first stage of budding, when the shape of the bud is nearly spherical, the newly synthesized mannoproteins and glucan are uniformly incorporated over the whole surface of the emerging bud (10). During the S phase, DNA replication occurs and the polarized tip growth becomes predominant as the bud becomes larger (5, 7). When the size of the bud is approximately one-third the size of the mother cell, which coincides with the completion of the S phase, separation of spindle pole bodies (SPBs) occurs and bipolar spindles are formed (3). When the size of the bud is two-thirds the size of the mother cell, the maturation phase of the bud growth starts and the cell wall components are again incorporated uniformly over the whole bud surface (5, 7, 10). One of the gene products involved in the construction of the cell wall of the growing bud is Fks1p, which functions in the biosynthesis of β-1,3-glucan (9), the major constituent of the cell wall. Still, much is unknown about the construction of other constituents of the bud cell wall.
We previously showed that Dcw1p and Dfg5p are required for cell growth and cell wall biogenesis and that Dcw1p is a glycosylphosphatidylinositol-anchored membrane protein (16). Subsequently, another group found that Dfg5p of Candida albicans is required for hypha formation at alkaline pH and is found in the cell membrane and cell wall extract fractions (31). To investigate the roles of Dcw1p and Dfg5p, a double disruptant of dcw1 and dfg5 was generated. The double disruptant was transformed with a plasmid containing a functional DFG5 gene under the control of the GAL1 promoter, so that its expression could be shut off by transferring the cells to a glucose-containing medium. When DFG5 expression was repressed, the cells were round and large, and Cwp1p, a major cell wall protein (28), was secreted into the medium. In the absence of DFG5 expression, chitin was delocalized and the amount of chitin was increased. However, even after DFG5 expression is turned off, the cells are considered to continue to have Dfg5p activity until all of the residual Dfg5p is degraded, a process which appears to take about 18 h. In order to see the phenotype of the double disruptant of DCW1 and DFG5, in which the enzymatic activities of Dcw1p and Dfg5p are rapidly destroyed, i.e., to obtain further insight into the enzymatic activities of Dcw1p and Dfg5p, we constructed mutant alleles of DCW1 and DFG5 whose gene products function normally at 25°C but immediately lose most of their activity at 37°C. The mutant containing the dcw1 allele was defective in bud growth, the cell cycle was arrested after DNA replication and prior to the separation of SPBs, and the buds had aberrant cell walls, when incubated at 37°C. The mutant containing the dfg5 allele showed similar phenotypes. These results indicated that Dcw1p and Dfg5p are involved in bud formation through their involvement in biogenesis of the bud cell wall.
MATERIALS AND METHODS
Yeast strains, culture conditions, and synchronization procedure.
The yeast strains used in this work are summarized in Table 1. The yeast strains were grown at 25 or 37°C in YPAD medium, which contained 1% yeast extract, 2% Bacto Peptone, 0.01% adenine sulfate, and 2% glucose with or without 1 M sorbitol. For selection of 5-fluoroorotic acid (5-FOA)-resistant clones, we used medium containing 0.67% Bacto Yeast Nitrogen Base, 0.08% complete supplement mixture without uracil (Bio 101), 2% glucose, 50 μg of uracil/ml, 0.01% adenine sulfate, 0.1% 5-FOA, and 2% agar. Escherichia coli strain JM109 was used for the preparation of plasmid DNA. E. coli strains were grown at 37°C in Luria-Bertani broth containing 100 μg of ampicillin/ml for the selection of transformants. The cell cycle was synchronized as previously described (18) with slight modifications. After wild-type cells were grown to an optical density at 660 nm (OD660) of 0.3 in YPAD, hydroxyurea was added to a final concentration of 0.15 M. The cells were incubated at 30°C for 2 h, washed with chilled water three times, and incubated in YPAD at 30°C for the indicated times.
TABLE 1.
Strains used in this studya
| Strain | Relevant genotype | Reference |
|---|---|---|
| 56FCF | Δdcw1::HIS3 Δdfg5::HIS3 pYC2-DFG5 | 16 |
| DC61 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-dcw1-3 | This study |
| DC62 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-dcw1-7 | This study |
| DC63 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-DCW1 | This study |
| DF64 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-dfg5-13 | This study |
| DF65 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-dfg5-17 | This study |
| DF66 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-dfg5-29 | This study |
| DF67 | Δdcw1::HIS3 Δdfg5::HIS3 pRS405-DFG5 | This study |
| DC68 | pRS416Y (DCW1) in DC61 | This study |
| DF69 | pRS416Y (DCW1) in DF66 | This study |
| DC70 | SPC42-GFP in DC61 | 1 |
All strains have a YPH499 (MATa ura3 lys2 ade2 trp1 his3 leu2) background (30).
PCR mutagenesis and plasmid construction.
Random mutations in the DCW1 and DFG5 genes were generated with a Gene Morph PCR mutagenesis kit (Stratagene). A DCW1 or DFG5 fragment was generated by mutagenic PCR with primers BamDCW1-1 and BamDCW1-2 or BamDFG5-1 and BamDFG5-2 (Table 2), cut with BamHI, and ligated into BamHI-cut pRS415 (pRS415-dcw1 or -dfg5). The plasmids were amplified in E. coli, purified with Wizard Midiprep (Promega), and transformed into strain 56FCF (Δdcw1 Δdfg5 pYC2-DFG5) (16). Clones which formed colonies at 25°C but did not form colonies at 37°C on a glucose-containing medium were selected and plated on 5-FOA medium to counterselect for pYC2-DFG5 (16), which originally kept 56FCF alive. The plasmids were extracted from the selected clones and amplified in E. coli. The amplified plasmids were cut with BamHI and ligated into BamHI-cut pRS405 (pRS405-dcw1 or -dfg5). The plasmids were transformed into 56FCF and plated on 5-FOA medium to counterselect for pYC2-DFG5. Construction of pRS416Y was described in a previous report (16). (Plasmid pRS416 contains wild-type DCW1.) A plasmid harboring SPC42-GFP (1), which encodes green fluorescent protein (GFP)-tagged Spc42p, a component of SPB (8), was a kind gift from John V. Kilmartin.
TABLE 2.
Primers used in this study
| Name | Nucleotide sequence (5′-3′) |
|---|---|
| BamDCW1-1 | CCCGGATCCTTGAACTTAAGATGATCTGGT |
| BamDCW1-2 | CCCGGATCCCTTTTCTAGAGTCGTTCAAA |
| BamDFG5-1 | CCCGGATCCATGCGAAAGATGGTTGGATAA |
| BamDFG5-2 | CCCGGATCCGGCTACCCGTAATCAAGTT |
| DCW1Probe-1 | TGGTGGTGGTTTGAGGTGGCA |
| DCW1Probe-2 | GAGCCAGCCAACAGCAAACCT |
| DFG5Probe-1 | GATTGCGGCGAGGTTGGGCAG |
| DFG5Probe-2 | GATTGCGGCGAGGTTGGGCAG |
| CLN2-1 | GCTGAACCAAGACCCCGTATG |
| CLN2-2 | GAAATTAAAGATGAGGCACTG |
| ACT1-1 | AGGTTGCTGCTTTGGTTATT |
| ACT1-2 | TTAGAAACACTTGTGGTGAA |
Fluorescence-activated cell sorting (FACS) analysis.
Cells were grown in YPAD to a density of 5 × 106 to 10 × 106 cells/ml at 25°C and incubated at 37°C for 3 h. Culture samples corresponding to 107 cells were washed in 1 ml of 0.2 M Tris-HCl (pH 7.5), fixed in 1 ml of 0.2 M Tris-HCl (pH 7.5) containing 70% ethanol, washed in 1 ml of 0.2 M Tris-HCl (pH 7.5), dissolved in 1 ml of 0.2 M Tris-HCl (pH 7.5) containing 0.25 mg of RNase A (Wako)/ml, incubated at 50°C for 1 h, mixed with 20 μl of 50-mg/ml proteinase K (Wako), incubated at 50°C for 1 h, pelleted by centrifugation at 10,000 × g for 2 min, dissolved in 1 ml of 0.2 M Tris-HCl (pH 7.5) containing 16 μg of propidium iodide/ml, incubated at room temperature for 30 min in a dark room, sonicated, and analyzed by flow cytometry (Coulter; Epics Elite ESP) (13).
Cell staining and microscopy.
Cells were grown in YPAD to a density of 5 × 106 to 10 × 106 cells/ml at 25°C; incubated at 37°C for 3 h; and stained with 4′,6-diamidino-2-phenylindole (DAPI), Calcofluor white (25), or rhodamine-phalloidin as previously described (2). An indirect immunofluorescence assay was done as previously described (16) using monoclonal antitubulin antibody (Chemicon International) as the first antibody (1:200 dilution in phosphate-buffered saline [PBS]-bovine serum albumin). Microscopic observations were done with a Nikon Eclipse E800 fluorescence microscope equipped with a charge-coupled device camera (ORCA-ER; Hamamatsu). Bud areas were measured with the computer program AQUA LITE (Hamamatsu).
Viability assay (4).
Cells were grown as described above, collected, sonicated, and resuspended in distilled water. Five milliliters of 100 mM Tris-HCl (pH 9.5)-100 mM NaCl-5 mM MgCl2 was mixed with 33 μl of nitroblue tetrazolium (50 mg/ml; Promega) in 70% dimethyl formamide and 16.5 μl of 5-bromo-4-chloro-3-indolylphosphate (50 mg/ml; Promega) in dimethyl formamide to form solution A. Five hundred microliters of solution A was mixed with 5 ml of solution B (0.05 M Gly-HCl [pH 9.7]). The mixture was added to the cell suspension (1:1) and observed with the microscope.
Northern analysis.
Total RNA was isolated, transferred to Hybond XL (Amersham Pharmacia Biotech), and probed with a DNA fragment that was PCR amplified with primers DCW1Probe-1 and -2, DFG5Probe-1 and -2, and ACT1-1 and -2 or an 0.86-kb XhoI-HindIII product of the fragment which was PCR amplified with primers CLN2-1 and CLN2-2.
Statistical analysis.
The significance of differences was determined by using the t test with adjustment for unequal variances (Welch test).
RESULTS
Isolation of DCW1 and DFG5 mutants defective in growth at 37°C.
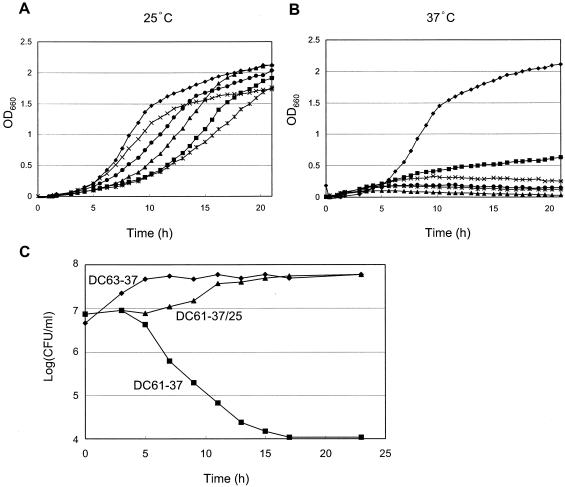
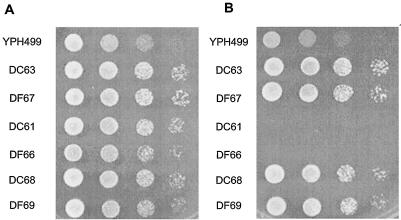
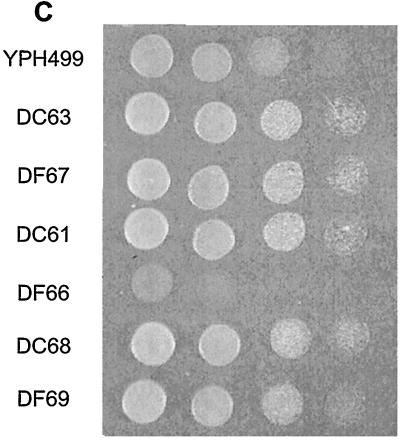
To obtain a mutant in which the enzymatic function shared by Dcw1p and Dfg5p is normal at 25°C but is rapidly destroyed at 37°C, we attempted to generate a mutant that contains a mutagenized dcw1 gene and a disrupted dfg5 gene, or a disrupted dcw1 gene and a mutagenized dfg5 gene. To do this, we constructed mutant alleles of DCW1 or DFG5 by mutagenic PCR. The PCR-amplified fragment was ligated into a plasmid, the plasmid was introduced into a Δdcw1 Δdfg5 background (56FCF) (16), and 50 colonies were obtained from both transformations. From the obtained transformants, we selected five clones that grew well at 25°C but did not grow at 37°C (Fig. 1A and B). Sequence analysis revealed that the open reading frames (ORFs) of DCW1 and DFG5 of the five selected mutants contained three to six point mutations, all of which were predicted to lead to amino acid substitutions in Dcw1p or Dfg5p (Table 3). Site-directed mutagenesis of DCW1 and DFG5 confirmed that none of the single amino acid substitutions of the mutants contributes to the temperature-sensitive defects of Dcw1p and Dfg5p (data not shown). This suggests that more than one mutation is involved in the temperature-sensitive phenotype, as was found in other reported mutants (17, 23, 27). Of these five mutants, the mutants that contained alleles of dcw1-3 and dfg5-29 (designated DC61 and DF66, respectively) were selected for further examination because their growth was clearly shut off at the nonpermissive temperature (37°C) (Fig. 1A and B). We confirmed that they formed colonies at 25°C but not at 37°C (Fig. 2A and B). The temperature sensitivity of these two mutants was complemented by transforming a plasmid containing wild-type DCW1. Both DC61 and DF66 formed colonies at 37°C in YPAD agar containing 1 M sorbitol, although DF66 grew rather slowly (Fig. 2C). This result indicates that DC61 and DF66 are unable to form colonies at 37°C because of a cell wall defect. DC61 cells incubated at 37°C for 3 h could grow again at 25°C (Fig. 1C).
FIG. 1.
DCW1 and DFG5 point mutants display temperature-sensitive growth. (A and B) Cells were grown to saturation in 5 ml of YPAD. An aliquot of the culture (0.2 ml) was used to inoculate 5 ml of YPAD, and the new culture was incubated at 25°C (A) or 37°C (B). Closed diamonds, wild type; closed squares, DF64; closed triangles, DF65; multiplication signs, DF66; asterisks, DC61; closed circles, DC62. Growth was scored with an automatic absorbance recorder (Biophotorecorder; Advantec Toyo). (C) Growth of DC63 and DC61 cells under different temperature regimens. Growth is expressed as the number of CFU per milliliter. All cells were initially incubated at 25°C to an OD660 of 0.5. DC63-37, DC63 (wild-type) cells incubated at 37°C; DC61-37, DC61 cells incubated at 37°C; DC61-37/25, DC61 cells incubated at 37°C for 3 h and then transferred to 25°C.
TABLE 3.
Estimated amino acid substitutions of Dcw1p or Dfg5p in the alleles of dcw1 and dfg5
| Allele | Estimated amino acid substitutions |
|---|---|
| dfg5-13 | T244I, T358S, G452R |
| dfg5-17 | L11I, M152V, Q154L, N273K, G276S, A414V |
| dfg5-29 | S33R, L101I, N138K, P333S, G415S, G451C |
| dcw1-3 | R17T, V235I, G367D |
| dcw1-7 | A68V, T267M, T364I |
FIG. 2.
Temperature sensitivity of DC61 and DF66 is rescued by osmotic support. Mid-log-phase cells were diluted to an OD660 of 0.5, and 5 μl of this suspension and three subsequent 10-fold serial dilutions were spotted onto YPAD agar (A and B) or YPAD agar containing 1 M sorbitol (C) (left to right). Growth was scored after 2 days at 25°C (A) or 37°C (B and C).
DC61 and DF66 cells arrest growth with small buds at 37°C.
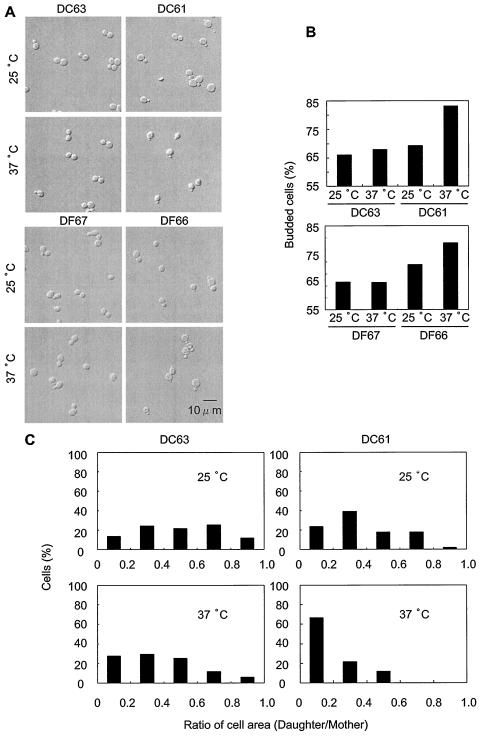
To determine the optimal time for microscopic observations, we measured the viability of these cells as estimated by CFU per milliliter. DC61 cells remained viable for 3 h after upshift to 37°C although they showed increasing loss of viability after 5 h at 37°C (Fig. 1C). DF66 showed a similar viability transition (data not shown). From these data, we selected an incubation time of 3 h at 37°C for further investigation, because, at this time, the DC61 cells were still viable and the effect of depletion of the enzymatic activity of Dcw1p and Dfg5p on living cells could be clearly observed. At this time, DC61 and DF66 cells incubated at 37°C had significantly more buds than did the ones incubated at 25°C (Fig. 3A and B) (P < 0.05). Under these conditions, most of the cells had only a single bud. At 25°C, the areas of most of the buds were between 20 and 80% of the area of the mother cells (Fig. 3C, upper right panel). However, at 37°C, the areas of most of the buds of DC61 cells were less than 20% of the area of the mother cells (Fig. 3C, lower right panel). For the wild type (DC63), the bud area distributions were not significantly different between 25 and 37°C (Fig. 3C, left panels). Measurement of the bud areas of DF66 gave similar results (data not shown). These results indicate that, in cells depleted of Dcw1p or Dfg5p, bud emergence is normal but bud growth is defective.
FIG. 3.
DC61 and DF66 cells arrest growth with small buds when incubated at 37°C. The cells were grown to an OD660 of 0.5 to 1.0 in YPAD medium at 25°C and incubated at 37°C for 3 h. (A) Morphology of DC63, DC61, DF66, and DF67 incubated at 25 or 37°C for 3 h. (B) Ratio of cells with buds to total cells. (C) Distribution of bud area, expressed as a percentage of mother cell area, for DC63 and DC61 cells incubated at 25 and 37°C for 3 h.
DC61 cells are arrested after DNA replication and prior to the separation of SPBs and show actin delocalization.
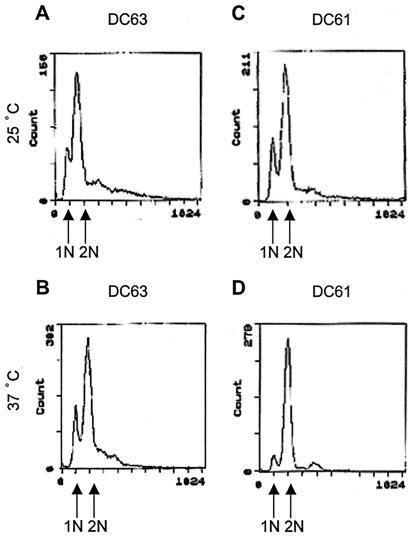
The preceding results suggested that, when DC61 cells are incubated at 37°C, their cell cycle is arrested. This was confirmed by a FACS analysis, which showed that the ratio of DC61 cells with 1N DNA content to cells with 2N DNA content was lower in cells incubated at 37°C (Fig. 4D) than in cells incubated at 25°C (Fig. 4C). This was not the case with DC63 (Fig. 4A and B). This result indicates that the cell cycle progression of DC61 is arrested after DNA replication when incubated at 37°C.
FIG. 4.
DNA content of DC63 and DC61. DC63 and DC61 cells were grown to an OD660 of 0.5 to 1.0 in YPAD medium at 25°C, and the cells were incubated at 37°C for 3 h. The cells were fixed, stained with propidium iodide, and subjected to FACS analysis. (A) DC63, 25°C; (B) DC63, 37°C; (C) DC61, 25°C; (D) DC61, 37°C.
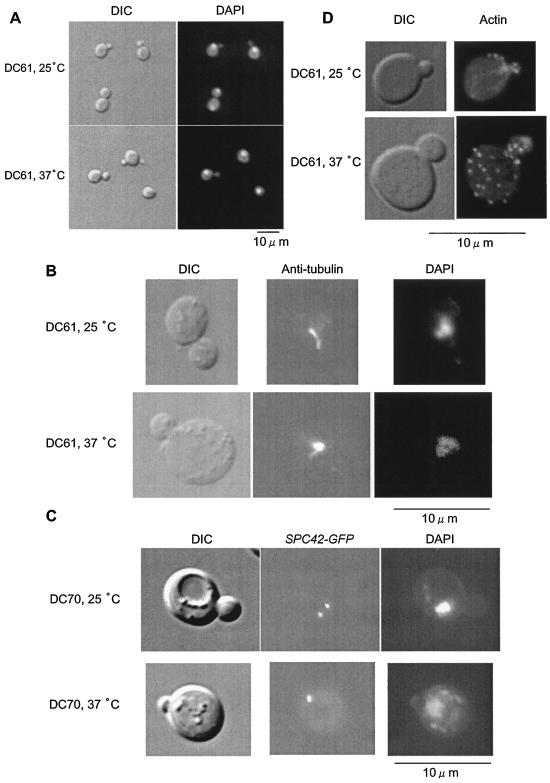
We examined the cell cycle of DC61 cells in more detail. The nucleus of many DC61 cells incubated at 25°C was close to the bud neck, while the nucleus of almost all DC61 cells incubated at 37°C was not localized near the bud neck (Fig. 5A). The percentage of DC61 cells with an elongated distribution of tubulin was 35.8% at 25°C but only 9.8% at 37°C (Fig. 5B). This suggested that, in DC61 cells incubated at 37°C for 3 h, bipolar spindles are not formed. This was confirmed by examining the state of SPBs by using GFP-tagged Spc42p (1), which is a component of SPB (8). The percentage of DC61 cells with double dots was 44.6% at 25°C but only 11.6% at 37°C (Fig. 5C). These results clearly indicate that, when incubated at 37°C for 3 h, DC61 cells are arrested prior to SPB separation. In addition, actin patches were delocalized in DC61 cells incubated at 37°C for 3 h (Fig. 5D), showing that the polarity of the cells is lost.
FIG. 5.
DC61 cells are arrested prior to SPB separation and show actin delocalization when incubated at 37°C. DC61 and DC70 cells expressing SPC42-GFP cells were grown to an OD660 of 0.5 to 1.0 in YPAD medium at 25°C and incubated at 37°C for 3 h. (A) Nuclear morphology of DC61 cells incubated at 25 or 37°C as shown by DAPI staining. (B) Microtubule morphologies of DC61 cells incubated at 25 or 37°C visualized by staining with antitubulin antibody. (C) SPB separation in DC70 cells incubated at 25 or 37°C. DAPI and tubulin or Spc42p were visualized at different excitation wavelengths. (D) Actin staining of DC61 cells incubated at 25 or 37°C. DIC, differential interference contrast.
Small buds of DC61 accumulate chitin in the bud cortex and are lysed at 37°C.
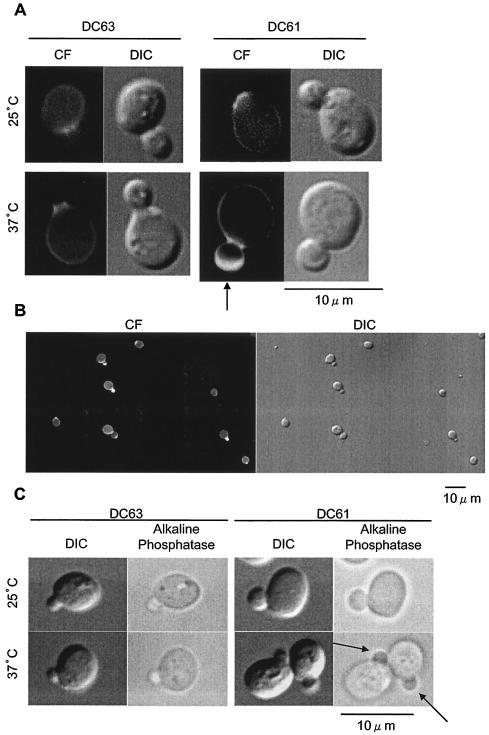
To verify that the small buds of DC61 incubated at 37°C have aberrant cell walls, we examined the distribution of chitin in the buds. Calcofluor white, a chitin stain, stained only the bud neck in the DC61 cells incubated at 25°C and wild-type DC63 cells (Fig. 6A and B). However, in the DC61 cells incubated at 37°C, it stained the bud cortex, especially the tips of the small buds. These results indicate that chitin is deposited in the bud cortex, especially in the tips of the small buds of DC61 cells incubated at 37°C. We conclude that the abnormal distribution of chitin is due to a cell wall defect. In addition, alkaline phosphatase activity, which is normally confined to the vacuole, was detected in some of the small buds of DC61 incubated at 37°C, indicating that alkaline phosphatase leaked from the vacuole as a result of a defect of the cell wall of these buds (Fig. 6C). These results show that the small buds of DC61 incubated at 37°C have aberrant cell walls.
FIG. 6.
Small buds of DC61 incubated at 37°C have aberrant cell walls. (A and B) Chitin localization after Calcofluor staining. (A) DC63 and DC61 cells were incubated at 25°C to an OD660 of 0.5 to 1.0 in YPAD medium, and cells were incubated at 37°C for 3 h, stained with Calcofluor white (CF), and observed under a fluorescence microscope. The arrow indicates the localization of chitin in DC61 cells incubated at 37°C. DIC, differential interference contrast. (B) The same view of DC61 incubated at 37°C with different magnification. (C) DC63 and DC61 were prepared as described above, alkaline phosphatase substrate was added, and cells were observed under the microscope. Arrows indicate the localization of alkaline phosphatase activity in DC61 cells incubated at 37°C.
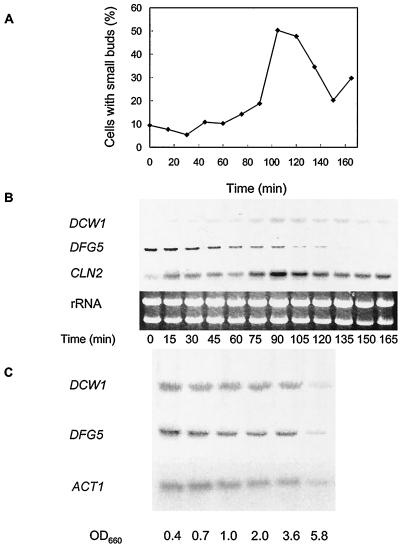
Accumulations of DCW1 and DFG5 mRNAs are cell cycle and growth phase regulated.
The preceding results indicate that Dcw1p and Dfg5p have important roles in cell cycle progression. Therefore, we examined whether mRNA levels of these genes are cell cycle regulated. The mRNA levels of DCW1 and DFG5 peaked at the G1 and S phases, respectively (Fig. 7B). These results clearly show that the mRNA levels of DCW1 and DFG5 are regulated according to the cell cycle. We next investigated how the mRNA levels of DCW1 and DFG5 vary among the different growth phases. Genes involved in cell wall synthesis are differentially transcribed at different growth phase. Among the genes involved in cell wall biosynthesis, those required for growth and proliferation are transcribed in the exponential phase (35), while those required for protection against the environment and for long-term survival are transcribed in the stationary phase (29, 34). We found that DCW1 and DFG5 mRNAs, like ACT1 mRNA, were abundant in the exponential phase (Fig. 7B). ACT1 transcription is elevated during periods of growth (19). This shows that DCW1 and DFG5 are involved in exponential growth, which is consistent with the fact that Dcw1p and Dfg5p are required for normal bud formation.
FIG. 7.
Cell cycle- and growth-phase-dependent regulation of DCW1 and DFG5 mRNAs. (A and B) Cell cycle-dependent regulation of DCW1 and DFG5 mRNAs. Wild-type cells were released from hydroxyurea-induced S arrest and incubated in YPAD, and samples were taken at the times indicated. (A) Budding index. Only the percentage of cells with small buds is shown. (B) Northern analysis. RNA was prepared and used for Northern analysis of DCW1 (upper panel), DFG5 (middle panel), and CLN2 (lower panel). (C) Growth-phase-dependent regulation of DCW1 and DFG5 mRNAs. Wild-type cells were grown to an OD660 of 0.4, 0.7, 1.0, 2.0, 3.6, and 5.8 in YPAD at 30°C. RNA was prepared and used for Northern analysis of DCW1 (upper panel), DFG5 (middle panel), and ACT1 (lower panel).
DISCUSSION
Although it has been indicated that Dcw1p and Dfg5p are involved in cell wall biogenesis (16, 33), the concrete step in which these proteins are involved was not known. In this report, we showed that cells with temperature-sensitive alleles of dcw1 (DC61) and dfg5 (DF66) at the nonpermissive temperature exhibited three remarkable phenotypes: (i) cessation of bud growth as shown by small buds, (ii) cell cycle arrest after DNA replication and prior to SPB separation, and (iii) defective bud cell walls as shown by chitin accumulation and by alkaline phosphatase leakage. These phenotypes indicate that Dcw1p and Dfg5p are required for formation of the cell wall in growing buds. These results are consistent with the finding that mRNA levels of DCW1 and DFG5 are abundant at the G1 and S phases, respectively, and in exponentially growing cells. Since the cell wall is a large complex structure comprised of many constituents including glucan, chitin, and proteins, many gene products are considered to be involved in bud cell wall formation. We believe that Dcw1p and Dfg5p are two of these gene products.
We previously showed that the promoter shutoff of DFG5 in the background of the double disruptant of dcw1 and dfg5 causes a large and round cell morphology, delocalization of chitin, and an increase in the amount of chitin. On the other hand, the dcw1ts (DC61) cells form small buds and cease growing. The difference in the phenotypes in these two strains can be explained by the rate at which the enzymatic function of Dcw1p or Dfg5p disappears. In promoter shutoff cells, depletion of the enzymatic function of Dfg5p depends on the turnover of Dfg5p, because only transcription of DFG5 ceases when cells are transferred to a medium containing glucose. In contrast, in the dcw1ts cells, the enzymatic activity of Dcw1p is thought to be rapidly destroyed when the incubation temperature is increased. Therefore, the effect of the loss of activity of Dcw1p should be clearly observed in dcw1ts cells. The dcw1ts and dfg5ts (DF66) cells could grow at the nonpermissive temperature under osmotic support (i.e., in YPAD agar containing 1 M sorbitol). This is also in contrast to the promoter shutoff cells, which could not grow even under osmotic support. A possible explanation for this phenotype is that the enzymatic activities of Dcw1p or Dfg5p in the DC61 and DF66 cells did not completely disappear at the nonpermissive temperature, whereas the transcription of DFG5 completely disappeared after shutoff of the promoter.
The mRNA levels of DCW1 and DFG5 peaked at the G1 and S phases, respectively (Fig. 7B). The genes involved in the G1-S transition possess SCB (Swi4p-dependent cell cycle box) or MCB (MluI cell cycle box) elements upstream of the ORFs (15). The G1-S transition events include bud growth, cell wall biosynthesis, and DNA replication (12, 14, 20, 24). One of these genes is FKS1, which has SCB and MCB elements. FKS1 mRNA accumulates at the G1 phase, and Fks1p is involved in the construction of the bud cell wall (22, 26). DCW1 also has two SCB elements and two MCB elements upstream of its ORF, and DFG5 has one SCB element upstream of its ORF (Table 4). These findings support our hypothesis that Dcw1p and Dfg5p are involved in bud growth, which is a G1-S transition event. Because the temperature-sensitive mutants of dcw1 and dfg5 show similar phenotypes, they are considered to have similar roles in bud growth. However, Dcw1p is thought to exert its function during initial bud growth late in the G1 phase, while Dfg5 is thought to exert its function when the buds become larger in the S phase.
TABLE 4.
SCB and MCB elements upstream of DCW1 and DFG5
| Gene | Position | Sequence | Orientation | Element | Consensusa |
|---|---|---|---|---|---|
| DCW1 | −313 | TACGAAA | Reverse | SCB | CACGAAA |
| DCW1 | −126 | TACGAAA | Forward | SCB | CACGAAA |
| DCW1 | −704 | ACGCGT | Forward | MCB | ACGCGN |
| DCW1 | −719 | ACGCGA | Forward | MCB | ACGCGN |
| DFG5 | −230 | CCCGAAA | Forward | SCB | CACGAAA |
The consensus sequence of the SCB element is 5′-CACGAAA, 5′-NACGAAA, or 5′-CNCGAAA (24). N indicates any nucleotide.
Suzuki et al. described a new cell cycle checkpoint that ensures coupling of cell wall synthesis and mitosis (32). This checkpoint monitors the progress of cell wall synthesis and causes a cell cycle arrest after DNA replication and prior to SPB separation in response to the defect of cell wall synthesis. This checkpoint was found in an fks1ts mutant in which an enzyme that catalyzes β-1,3-glucan biosynthesis (9) is deficient at the nonpermissive temperature. The cell cycle arrest of this fks1ts mutant is strikingly similar to that of dcw1ts cells in this report, suggesting that dcw1ts cells are cell cycle arrested by this cell wall integrity checkpoint. In addition to the cell cycle arrest, fks1ts cells share several other characteristics with dcw1ts cells, including cell lysis, chitin distribution in the bud tip, and arrest with a small bud (11). Because Dcw1p is localized mainly in the membrane and partly in the cell wall (16), Dcw1p and Dfg5p might be involved in construction of bud cell wall components like Fks1p. Because Dcw1p and Dfg5p are homologous to bacterial glycanases (16, 21), they might participate in remodeling of newly synthesized cell wall components. However, the exact enzymatic functions of Dcw1p and Dfg5p are still unknown. Closer examination of the cell walls of the dcw1ts or dfg5ts cells should clarify their functions.
Acknowledgments
We thank Masaki Mizunuma for teaching us the procedure for cell cycle synchronization, John V. Kilmartin for providing the plasmid harboring SPC42-GFP, Hiroko Ikeda for FACS analysis, and Mihoko Tominaga for DNA sequencing.
REFERENCES
- 1.Adams, I. R., and J. V. Kilmartin. 1999. Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae. J. Cell Biol. 145:809-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedetti, H., S. Raths, F. Crausaz, and H. Riezman. 1994. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell 5:1023-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers, B., and L. Goetsch. 1975. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J. Bacteriol. 124:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabib, E., and A. Duran. 1975. Simple and sensitive procedure for screening yeast mutants that lyse at nonpermissive temperatures. J. Bacteriol. 124:1604-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabib, E., R. Roberts, and B. Bowers. 1982. Synthesis of the yeast cell wall and its regulation. Annu. Rev. Biochem. 51:763-793. [DOI] [PubMed] [Google Scholar]
- 6.Cabib, E., D. H. Roh, M. Schmidt, L. B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679-19682. [DOI] [PubMed] [Google Scholar]
- 7.De Nobel, J. G., F. M. Klis, A. Ram, H. Van Unen, J. Priem, T. Munnik, and H. Van Den Ende. 1991. Cyclic variations in the permeability of the cell wall of Saccharomyces cerevisiae. Yeast 7:589-598. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson, A. D., and J. V. Kilmartin. 1996. Spc42p: a phosphorylated component of the S. cerevisiae spindle pole body (SPB) with an essential function during SPB duplication. J. Cell Biol. 132:887-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, M. el-Sherbeini, J. A. Clemas, S. Mandala, B. R. Frommer, and M. B. Kurtz. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farkas, V., J. Kovarik, A. Kosinova, and S. Bauer. 1974. Autoradiographic study of mannan incorporation into the growing cell walls of Saccharomyces cerevisiae. J. Bacteriol. 117:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Rodriguez, L. J., J. A. Trilla, C. Castro, M. H. Valdivieso, A. Duran, and C. Roncero. 2000. Characterization of the chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett. 478:84-88. [DOI] [PubMed] [Google Scholar]
- 12.Horak, C. E., N. M. Luscombe, J. Qian, P. Bertone, S. Piccirrillo, M. Gerstein, and M. Snyder. 2002. Complex transcriptional circuitry at the G1/S transition in Saccharomyces cerevisiae. Genes Dev. 16:3017-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howlett, N. G., and S. V. Avery. 1999. Flow cytometric investigation of heterogeneous copper-sensitivity in asynchronously grown Saccharomyces cerevisiae. FEMS Microbiol. Lett. 176:379-386. [DOI] [PubMed] [Google Scholar]
- 14.Igual, J. C., A. L. Johnson, and L. H. Johnston. 1996. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15:5001-5013. [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer, V. R., C. E. Horak, C. S. Scafe, D. Botstein, M. Snyder, and P. O. Brown. 2001. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409:533-538. [DOI] [PubMed] [Google Scholar]
- 16.Kitagaki, H., H. Wu, H. Shimoi, and K. Ito. 2002. Two homologous genes, DCW1 (YKL046c) and DFG5, are essential for cell growth and encode glycosylphosphatidylinositol (GPI)-anchored membrane proteins required for cell wall biogenesis in Saccharomyces cerevisiae. Mol. Microbiol. 46:1011-1022. [DOI] [PubMed] [Google Scholar]
- 17.Lerner, C. G., P. S. Gulati, and M. Inouye. 1995. Cold-sensitive conditional mutations in Era, an essential Escherichia coli GTPase, isolated by localized random polymerase chain reaction mutagenesis. FEMS Microbiol. Lett. 126:291-298. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman, H. B., and K. M. Hopkins. 2004. Methods to induce cell cycle checkpoints. Methods Mol. Biol. 241:3-10. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig, J. R., II, J. J. Foy, S. G. Elliott, and C. S. McLaughlin. 1982. Synthesis of specific identified, phosphorylated, heat shock, and heat stroke proteins through the cell cycle of Saccharomyces cerevisiae. Mol. Cell. Biol. 2:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama, Y., and T. Nakajima. 2000. The aman6 gene encoding a yeast mannan backbone degrading 1,6-α-d-mannanase in Bacillus circulans: cloning, sequence analysis, and expression. Biosci. Biotechnol. Biochem. 64:2018-2020. [DOI] [PubMed] [Google Scholar]
- 22.Mazur, P., N. Morin, W. Baginsky, M. el-Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihara, Y., T. Utagawa, H. Yamada, and Y. Asano. 2000. Phosphorylation of nucleosides by the mutated acid phosphatase from Morganella morganii. Appl. Environ. Microbiol. 66:2811-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogas, J., B. J. Andrews, and I. Herskowitz. 1991. Transcriptional activation of CLN1, CLN2, and a putative new G1 cyclin (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell 66:1015-1026. [DOI] [PubMed] [Google Scholar]
- 25.Pringle, J. R., R. A. Preston, A. E. Adams, T. Stearns, D. G. Drubin, B. K. Haarer, and E. W. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357-435. [DOI] [PubMed] [Google Scholar]
- 26.Ram, A. F., S. S. Brekelmans, L. J. Oehlen, and F. M. Klis. 1995. Identification of two cell cycle regulated genes affecting the β-1,3-glucan content of cell walls in Saccharomyces cerevisiae. FEBS Lett. 358:165-170. [DOI] [PubMed] [Google Scholar]
- 27.Sekiya-Kawasaki, M., M. Abe, A. Saka, D. Watanabe, K. Kono, M. Minemura-Asakawa, S. Ishihara, T. Watanabe, and Y. Ohya. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoi, H., Y. Iimura, and T. Obata. 1995. Molecular cloning of CWP1: a gene encoding a Saccharomyces cerevisiae cell wall protein solubilized with Rarobacter faecitabidus protease I. J. Biochem. (Tokyo) 118:302-311. [DOI] [PubMed] [Google Scholar]
- 29.Shimoi, H., H. Kitagaki, H. Ohmori, Y. Iimura, and K. Ito. 1998. Sed1p is a major cell wall protein of Saccharomyces cerevisiae in the stationary phase and is involved in lytic enzyme resistance. J. Bacteriol. 180:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spreghini, E., D. A. Davis, R. Subaran, M. Kim, and A. P. Mitchell. 2003. Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot. Cell 2:746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki, M., R. Igarashi, M. Sekiya, T. Utsugi, S. Morishita, M. Yukawa, and Y. Ohya. 2004. Dynactin complex is involved in a novel checkpoint to monitor cell wall synthesis in Saccharomyces cerevisiae. Nat. Cell Biol. 6:861-871. [DOI] [PubMed] [Google Scholar]
- 33.Tomishige, N., Y. Noda, H. Adachi, H. Shimoi, A. Takatsuki, and K. Yoda. 2003. Mutations that are synthetically lethal with a gas1Δ allele cause defects in the cell wall of Saccharomyces cerevisiae. Mol. Genet. Genomics 269:562-573. [DOI] [PubMed] [Google Scholar]
- 34.Werner-Washburne, M., E. Braun, G. C. Johnston, and R. A. Singer. 1993. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 57:383-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]