Abstract
Sarcoidosis is a chronic and systemic disorder characterized by the formation of non-caseating granulomas. Very few cases of isolated gastrointestinal sarcoidosis have been reported, and even fewer, if any, report gastrointestinal sarcoidosis within multiple gastrointestinal sites concomitantly. We present a 42-year-old white man with chronic diarrhea and abdominal pain for more than 3 years. Mucosal biopsies revealed non-caseating microgranulomas in the stomach, throughout the small intestine, colon, and rectum. Prednisone therapy was initiated with a rapid improvement in symptoms and complete resolution of diarrhea within 3 weeks.
Introduction
Sarcoidosis is a disorder that affects multiple organ systems and is characterized by non-caseating granulomas. Although pulmonary involvement is most common, many patients have multi-organ system involvement.1 Extrapulmonary organ involvement is seen in less than 10% of patients. Gastrointestinal (GI) sarcoidosis is a rare disease entity that can be found isolated or as part of a systemic disease. The stomach is the most frequently affected hollow organ.2,3 GI sarcoidosis presents subclinically, with patients reporting symptoms only 0.1–0.9% of the time.4 The incidence of intestinal involvement is unknown, although it is estimated at less than 1% and there are only a few reported cases.1,5 In such cases, the small intestine was noted as the least common site of GI involvement. The clinical diagnosis of colonic sarcoidosis is difficult, as symptoms are uncommon and the endoscopic findings are nonspecific.5
Case Report
A 42-year-old white man presented with abdominal pain and a 1.5-year history of loose, watery stools occurring 3 times per day. He had a past medical history of gastroesophageal reflux disease and recent diagnosis of hereditary hemochromatosis demonstrated by grade 3 iron overload with mild portal fibrosis on liver biopsy, and a homozygous mutation of C282Y on genetic testing. He denied any rectal bleeding, fever, or weight loss. Physical exam was normal. Infectious work-up including ova and parasites, culture, fecal leukocytes, and Clostridium difficile nucleic acid amplification were negative. Histoplasma antigen, anti-transglutaminase, and anti-endomysial antibodies were also negative.
Colonoscopy revealed erythematous mucosa in the transverse colon and rectum, as well as congestion in the terminal ileum (Figure 1). Esophagogastroduodenoscopy (EGD) revealed diffuse gastric erythema with erosions and duodenitis (Figure 2). Biopsies of the stomach and duodenum demonstrated non-caseating microgranulomas, as did the terminal ileum, colon, and rectum (Figure 3). Acid-fast bacilli and fungi stains were negative. No Helicobacter pylori organisms were identified. Computed tomography (CT) of the abdomen and pelvis showed hepatosplenomegaly, although recent liver biopsy did not show granulomas. Chest CT was unremarkable. Angiotensin-converting enzyme (ACE) was elevated at 77 U/L, and rapid plasma reagin and Tropheryma whipplei testing were negative.
Figure 1.
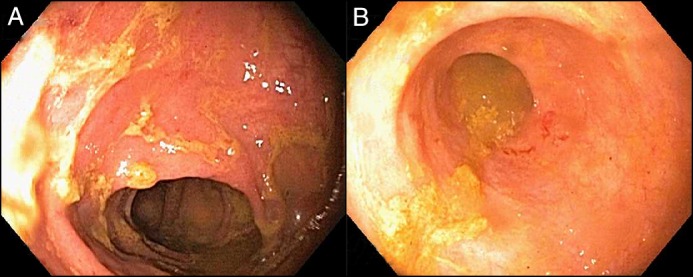
Colonoscopy showing (A) moderately erythematous mucosa in the sigmoid, transverse, ascending colon, and cecum and (B) congested mucosa in the terminal ileum.
Figure 2.
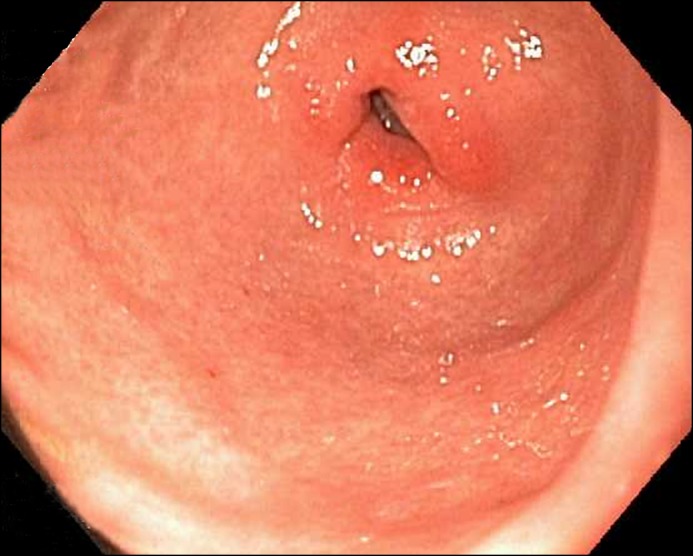
EGD showing moderately erythematous mucosa at the pylorus.
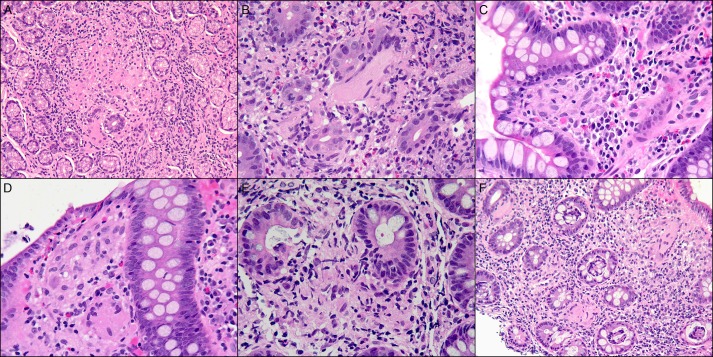
Figure 3.
Small non-caseating granulomas in the (A) stomach, (B) duodenum, (C) terminal ileum, (D) right colon, (E) left colon, and (F) rectum.
With a high suspicion for sarcoidosis, the patient was referred to rheumatology. He was lost to follow-up and returned 2 years later with abdominal pain and diarrhea that had progressively worsened up to 15 episodes daily. Repeat EGD and colonoscopy revealed moderate to marked chronic colitis with mild activity in the right and left colon. Biopsies from the stomach, terminal ileum, and right and left colon again revealed non-caseating granulomas. Stains for Whipple’s disease and Mycobacterium were negative. Prednisone 40 mg daily was started for GI sarcoidosis, and within 3 weeks the patient reported his abdominal pain and diarrhea had resolved.
Discussion
Sarcoidosis is a multi-organ systemic disease most commonly involving the lungs. The index of clinical suspicion for isolated sarcoidosis in the GI tract, in the setting of an unremarkable chest CT and no respiratory symptoms, is extremely low. Gastric sarcoidosis may result in ulcers, gastritis, or overall erythematous and friable mucosa on endoscopy.6 There are two disease patterns reported in the literature based on previous cases: gastric ulcer formation infiltrated with sarcoid granulomas, and diffuse infiltration with granulomas resulting in fibrosis with a reduced lumen size.6 The sigmoid colon is the most common location of colonic sarcoidosis involvement. Endoscopic findings may include friable mucosa that mimics colitis, erosions, fold thickening, focal nodularity, and elevated lesions that may appear as tumors.5 Lesions resembling polyps, carcinomas, and diverticuli have also been reported.
Because sarcoidosis of the colon is rare and may mimic many other diseases, it is important to rule out other differential causes, especially those which cause granulomatous disease (such as tuberculosis, syphilis, inflammatory bowel disease, Whipple’s disease), fungal infections (such as Histoplasma), and H. pylori.1,4,5 Gastric sarcoidosis, specifically, can mimic peptic ulcer disease, Ménétrier’s disease, hypertrophic gastritis, Mycobacterium infection, syphilis, histoplasmosis, cancer, lymphoma, and Langerhans cell histiocytosis.7
Non-caseating granulomas that contain multinucleate giant cells in the absence of crypt abscesses on histology are the gold standard diagnostic findings.5 In addition to histological findings, rapid improvement with corticosteroid therapy and elevated ACE levels are supportive of the diagnosis.
ACE levels are elevated in 60% of patients with sarcoidosis.8 Sarcoidosis of the terminal ileum and colon can resemble inflammatory bowel disease, but serum ACE levels in Crohn’s disease are not significantly elevated.8,9 Patients with ulcerative colitis can have elevated ACE levels, but levels seen in sarcoidosis are significantly higher.9 One study reported ACE levels are lower in patients with ulcerative colitis and Crohn’s disease than healthy controls when ACE genotypes are accounted for.10 A rare association between sarcoidosis and Crohn’s disease has been reported, and additional targeted therapy against tumor necrosis factor-α and integrins has been associated with the induction of sarcoidosis when used to treat inflammatory bowel disease.11-13
The decision to treat GI sarcoidosis is based upon disease severity and activity.6 Asymptomatic patients do not need to be treated.7 Corticosteroids are the initial choice of treatment in sarcoidosis; however, their role in GI sarcoidosis is unclear.3 Other agents may be necessary in patients who do not respond to steroids. The recommended dose is 30–40 mg prednisone daily, gradually tapering to a maintenance dose of 10–15 mg over 6 months. Response to therapy is monitored clinically, radiographically, and with trending serum ACE levels.3
To the best of our knowledge, this is the only case in the English literature where GI symptoms were the presenting symptom of sarcoidosis in the absence of other organ system involvement, and the only case reported with histological evidence of sarcoidosis throughout the entire GI tract (stomach, small intestine, and colon). Endoscopic and histological findings, along with the rapid response to oral corticosteroid therapy, highly support the diagnosis of sarcoidosis in this patient.
Disclosures
Author contributions: L. Stemboroski wrote and revised the manuscript. B. Gaye and C. Monteiro analyzed the data. R. Makary analyzed the data and critically revised the manuscript. E. Eid designed and critically revised the manuscript, and is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Vahid B, Spodik M, Braun KN, Ghazi LJ, Esmaili A. Sarcoidosis of gastrointestinal tract: A rare disease. Dig Dis Sci. 2007; 52(12):3316–20. [DOI] [PubMed] [Google Scholar]
- 2.Giovinale M, Fonnesu C, Soriano A, et al. . Atypical sarcoidosis: Case reports and review of the literature. Eur Rev Med Pharmacol Sci. 2009; 13(Suppl 1):37–44. [PubMed] [Google Scholar]
- 3.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008; 103(12):3184–92; quiz 3193.doi: 10.1111/j.1572-0241.2008.02202.x [doi]. [DOI] [PubMed] [Google Scholar]
- 4.Erra P, Crusco S, Nugnes L, et al. . Colonic sarcoidosis: Unusual onset of a systemic disease. World J Gastroenterol. 2015; 21(11):3380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daldoul S, Triki W, El Jeri K, Zaouche A. Unusual presentation of a colonic sarcoidosis. Case Rep Med. 2012; 2012:169760.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman M, Ali MA, Borum ML. Gastric sarcoidosis: A case report and review of the literature. South Med J. 2007; 100(3):301–3.doi: 10.1097/SMJ.0b013e318030ed94 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Tokala H, Polsani K, Kalavakunta JK. Gastric sarcoidosis: A rare clinical presentation. Case Rep Gastrointest Med. 2013; 2013:260704.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmadi M, Ahmad DS, Odum B, Diaz-Arias A, Hammad H. Sarcoidosis: An extremely rare cause of granulomatous enterocolitis. J Gastrointestin Liver Dis. 2012; 21(4):423–5. [PubMed] [Google Scholar]
- 9.Letizia C, D'Ambrosio C, Agostini D, et al. . Serum angiotensin-converting enzyme activity in crohn's disease and ulcerative colitis. Ital J Gastroenterol. 1993; 25(1):23–5. [PubMed] [Google Scholar]
- 10.Matsuda T, Suzuki J, Furuya K, Masutani M, Kawakami Y. Serum angiotensin I-converting enzyme is reduced in Crohn's disease and ulcerative colitis irrespective of genotype. Am J Gastroenterol. 2001; 96(9):2705–10. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Kaneta K, Honma M, et al. . Sarcoidosis during infliximab therapy for Crohn's disease. J Dermatol. 2010; 37(5):471–4. [DOI] [PubMed] [Google Scholar]
- 12.Parisinos CA, Lees CW, Wallace WA, Satsangi J. Sarcoidosis complicating treatment with natalizumab for Crohn's disease. Thorax. 2011; 66(12):1109–10. [DOI] [PubMed] [Google Scholar]
- 13.Fok KC, Ng WW, Henderson CJ, Connor SJ. Cutaneous sarcoidosis in a patient with ulcerative colitis on infliximab. J Crohns Colitis. 2012; 6(6):708–12. [DOI] [PubMed] [Google Scholar]