Abstract
Purpose
NRG Oncology RTOG 0319 was the first cooperative group trial in the US to evaluate 3-dimensional conformal radiation therapy (3D-CRT) accelerated partial breast irradiation (APBI). This report updates secondary endpoints of toxicity and efficacy.
Methods
Patients (pts) with stage I/II invasive breast cancer (BrCa) (T≤3cm, N≤3 positive lymph nodes, negative margins) were eligible for 3D-CRT APBI - 38.5 Gy in 10 BID fractions. Pt characteristics and treatment details have previously been reported. Adverse Events (AEs) were graded with CTCAE v. 3.0. This analysis updates recurrences in the ipsilateral breast (IBR), contralateral breast (CBR), ipsilateral nodes (INR), and metastatic sites (DM), mastectomy rate (M), disease-free (DFS), mastectomy-free (MFS), and overall survival (OS).
Results
52 of 58 enrolled pts were eligible: median age 61 years (y), 94% stage I and 83% ER positive. Median follow up is 8y (min-max: 1.7–9.0). The 7y estimate of isolated IBR (no DM) is 5.9%. 7y estimates of all IBR, INR, M, and DM were 7.7%, 5.8%, 7.7%, and 7.7%, respectively. All 4 IBRs were invasive of which 3 had a component within the planning target volume. Patterns of failures were: 3 IBR, 1 INR, 2 DM, 1 INR+DM, and 1 IBR+INR+DM. 7y estimates of MFS, DFS, and OS are 71.2%, 71.2%, and 78.8%, respectively. 13 pts have died: 3 of BrCa, 10 of other causes. Four pts (7.7%) reported G3 treatment-related AE. No G3 pain, pulmonary, or cardiac toxicities were reported.
Conclusion
This phase I/II trial of 3D-CRT APBI continues to demonstrate durable tumor control and minimal G3 toxicity, comparable to other APBI techniques. Mature phase III results will determine the appropriateness and limitations of this non-invasive APBI technique.
Keywords: Breast Cancer, Partial Breast Irradiation, Clinical Trial, 3D Conformal Radiation Therapy
Introduction
NRG Oncology RTOG 0319 was the first prospective cooperative group trial in the US to evaluate 3D conformal radiation (3D-CRT) as a method of accelerated partial breast irradiation (APBI). The primary endpoint of this phase I/II trial was the reproducibility among multiple centers to perform 3D-CRT APBI according to protocol definitions and has previously been reported.1 The secondary end-points of cancer control and toxicity were reported in 20102 with a median follow up of 4.5 years. At that time, 4-year end-point estimates included ipsilateral breast recurrence (IBR) of 6%, ipsilateral nodal failure (INF) 2%, distant failure 8%, and disease-free survival 84%. An update of toxicity and patient self-assessment of breast appearance/satisfaction was reported in 20133 with a median follow-up of 5.3 years. Eighty-two percent of patients rated their cosmesis as good/excellent at 1 year, and 64% at 3 years. Thirty-one percent of patients were satis ed with the treatment at 3 years. The rate of any reported grade 3 (G3) toxicity was 5.8% and all were related to skin or musculoskeletal effects of treatment.
The adoption of external beam methods for APBI delivery has the potential to render APBI broadly available to nearly any radiation practice in comparison to brachytherapy APBI, which requires specialized equipment and expertise, conceivably limiting its access. However, there is less long term cancer outcome data for external beam APBI, as this technique emerged after brachytherapy APBI. This report serves to update the cancer outcomes and toxicity on NRG Oncology RTOG 0319, providing unique long term data on a group of patients treated prospectively with 3D-CRT APBI.
Methods and Materials
Patient eligibility
Details of patient eligibility and treatment specifications have been reported previously.1–3 Briefly, patients with American Joint Committee on Cancer stage I or II (T1N0, T1N1, T2N0, and T2N1) invasive ductal breast cancer, including not otherwise specified (NOS), medullary, papillary, colloid (mucinous), or tubular histologies with lesions ≤3 cm, and negative surgical margins were eligible. Patients were required to have uni-focal breast cancer without an extensive intra-ductal component. Patients with ≤3 positive axillary nodes were allowed. Negative margins (>2 mm) were required. Patients were ineligible if they had a history of malignancy within the previous 5 years (except for non-melanomatous skin cancer).
Treatment technique and imaging
Treatment planning and delivery were performed with the patient in the supine position. The clinical target volume (CTV) was defined by uniformly expanding the excision cavity volume by 10 to 15 mm; limited to 5 mm from the skin surface and lung–chest wall interface. Six surgical clips were used to help define the excision cavity volume. The CTV was uniformly expanded by 1 cm to define the planning target volume (PTV). The PTV was used to generate the beam aperture for treatment and modified to generate a PTV for evaluation (PTV_EVAL) for dose–volume histogram (DVH) constraints and analysis (limited to 5 mm from skin surface and at lung chest wall interface). Other required contoured structures included the ipsilateral and contralateral breast, thyroid gland, heart, and ipsilateral and contralateral lung. Field arrangements were at the physician's discretion. Credentialing was required for all institutions.
Radiation therapy was to begin within 8 weeks of surgery unless chemotherapy was given. If chemotherapy was given first, RT was recommended to start a minimum of 2 weeks after the last cycle of chemotherapy. A total of 38.5 Gy in 10 fractions were prescribed to the International Commission on Radiation Units and Measurements (ICRU) 50 reference point dose (usually isocenter). Two fractions per day, each 3.85 Gy, separated by at least 6 hours, were given in 5 consecutive week days (Monday-Friday). Dose calculations with tissue inhomogeneity correction were required. Portal films or portal images of each beam and an orthogonal pair (antero-posterior and lateral) were obtained for the first fraction. Subsequent films or images were obtained on fraction numbers 2, 5, and 9, including an orthogonal pair.
Dose–volume constraints and normal tissue tolerances
Dose–volume constraints established for the protocol have been published previously (1). These included limitations in dose to: (1) uninvolved breast tissue; (2) ipsilateral and contralateral lung; (3) contralateral breast; and (4) heart (different values for right- and left-sided lesions), and (5) thyroid. Quality assurance evaluations were established with an ideal plan having (1) the 95% isodose surface cover 100% of the PTV; and (2) the maximum dose to the PTV not to exceed the prescription dose by >10%.
Statistical considerations
An IBR is defined as biopsy proven invasive or non-invasive recurrence (except lobular carcinoma in situ) in the ipsilateral breast. IBRs were subdivided by occurring within the planning target volume, peripherally (in the skin of the treated breast), or at other locations of the breast. An INR consisted of any ipsilateral axillary, internal mammary, and/or supraclavicular recurrence; INRs with and without protocol-defined IBR were examined separately. IBR, INR, and contralateral breast recurrence, distant metastases (DM), and mastectomy failure rates were estimated using the cumulative incidence method4 in which death is a competing risk. The mastectomy rate included simple mastectomy or a modified radical mastectomy for any cause. For disease-free survival (DFS), a failure is any tumor recurrence (including ipsilateral breast recurrence, ipsilateral nodal recurrence), DM, CBR, or death. DFS and overall survival (OS) rates were estimated using the Kaplan-Meier method5. Adverse events (AEs) were graded with CTCAE version 3.0. All analyses were performed using SAS/STAT® software.
Results
NRG Oncology RTOG 0319 enrolled 58 patients between August 15, 2003 and April 30, 2004, of whom 52 were evaluable for follow up (three patients did not receive protocol therapy and three were ineligible). Median follow up for this analysis is 8 years (min-max: 1.7–9 years). As previously reported,2 the study population had very favorable patient and disease characteristics: 87% of patients were postmenopausal, 94% had T1 disease, 92% were axillary node negative, and 83% had estrogen receptor positive disease (Table 1). A total of 8 patients (15%) developed a cancer recurrence during the follow up period.
Table 1.
Pretreatment Characteristics for all Eligible Patients (n=52)
| Age (years) | |
| Median | 61 |
| Min - Max | 38 – 89 |
| Tumor dimension (cm) | |
| Median | 0.9 |
| Min - Max | 0.1 – 2.6 |
| <1 | 24 ( 46.2%) |
| 1≤ to < 2 | 20 ( 38.5%) |
| 2≤ to < 3 | 3 ( 5.8%) |
| Missing | 5 ( 9.6%) |
| Histology | |
| Invasive ductal | 45 ( 86.5%) |
| Colloid | 2 ( 3.8%) |
| Tubular | 5 ( 9.6%) |
| Stage | |
| I (T1, N0-1, M0) | 49 ( 94.2%) |
| II (T2, N0-1, M0) | 3 ( 5.8%) |
| Unknown | 4 ( 7.7%) |
| Menopausal status | |
| Premenopause | 7 ( 13.5%) |
| Postmenopause | 34 ( 65.4%) |
| Surgically menopausal | 11 ( 21.2%) |
| Final surgical margins | |
| Negative | 49 ( 94.2%) |
| Positive margin, negative at re-excision | 3 ( 5.8%) |
| Chemotherapy | |
| No | 43 ( 82.7%) |
| Yes, prior radiotherapy | 5 ( 9.6%) |
| Yes, after radiotherapy | 3 ( 5.8%) |
| Uknown | 1 ( 1.9%) |
| Hormonal therapy | |
| No | 37 ( 71.2%) |
| Yes | 15 ( 28.8%) |
| Axillary Nodes Positive | |
| No | 48 ( 92.3%) |
| Yes | 4 ( 7.7%) |
| ER Status | |
| Negative | 9 ( 17.3%) |
| Positive | 43 ( 82.7%) |
Ipsilateral Breast and Ipsilateral Nodal Recurrences
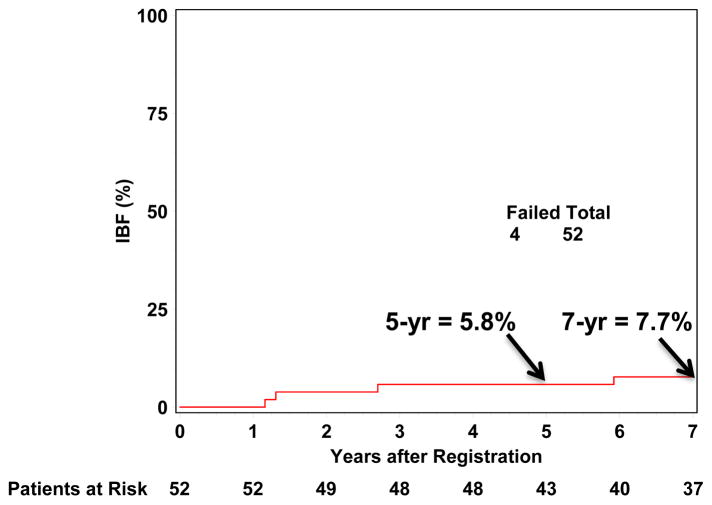
IBR occurred in four of 52 (7.7%) analyzed patients. Three of these patients had isolated ipsilateral breast recurrence and one had simultaneous breast and nodal recurrences. Of the four ipsilateral breast recurrences, two were within the prior APBI planning target volume (PTV) of which one had recurrence in both the breast tissue and skin, one was outside the APBI PTV, and one spanned both in and outside the PTV. All four IBRs occurred in postmenopausal women with stage I disease, of whom 3 were ER+, and 1 received chemotherapy. The cumulative incidence of IBR at 5 and 7-years were 5.8% (95% confidence interval (CI): 0.0%, 12.2%) and 7.7% (95% CI: 0.4%, 15.0%), respectively (Figure 1). Similarly, the cumulative incidence of undergoing a mastectomy at 5 and 7-years was 5.8% (95% CI: 0.0%, 12.2%) and 7.7% (95% CI: 0.4%, 15.0%), respectively. There were no ipsilateral or contralateral breast recurrences following regional or distant recurrence.
Figure 1.
Cumulative Ipsilateral Breast Recurrence
INR developed in 3 of 52 (5.8%) patients. Two patients had INR alone, and one had simultaneous nodal and breast recurrences, as described above. No nodal recurrences occurred in the small population of node positive patients (4/52), all of whom underwent axillary dissection. All 3 INRs occurred in postmenopausal women with stage I, ER+ tumors.
Overall, there were a total of six patients who developed loco-regional failure (LRF), defined as ipsilateral breast and/or nodal recurrence. All had stage I disease and were postmenopausal, 5/6 were ER+, and 1/6 received adjuvant chemotherapy. There was no clear pattern of patient, disease or treatment characteristics among the patients with LRF in this small cohort. Cumulative incidences of 5- and 7-year loco-regional failure (LRF) were 9.6% (95% CI: 1.5%, 17.7%) and 11.5% (95% CI: 2.8%, 20.3%). There were no contralateral breast cancers diagnosed during follow up.
Distant Metastases (DM) and Pattern of Failure
Four patients (7.7%) developed DM at any time during follow up, either as first recurrence (2 patients) or after LRF (2 patients). Of the eight patients (15.4%) who developed any cancer recurrence during the follow-up period, the site of first recurrence was ipsilateral breast in three (5.8%), ipsilateral nodes in two (3.8%), simultaneous ipsilateral breast and nodal recurrence in one (1.9%), and DM in two (3.8%). Of the 2 patients who developed DM after LRF, initial LRF consisted of INR in one and simultaneous ipsilateral breast/nodal recurrence the other. No patient with isolated IBR developed DM, and no patients with isolated DM developed subsequent LRF.
Survival
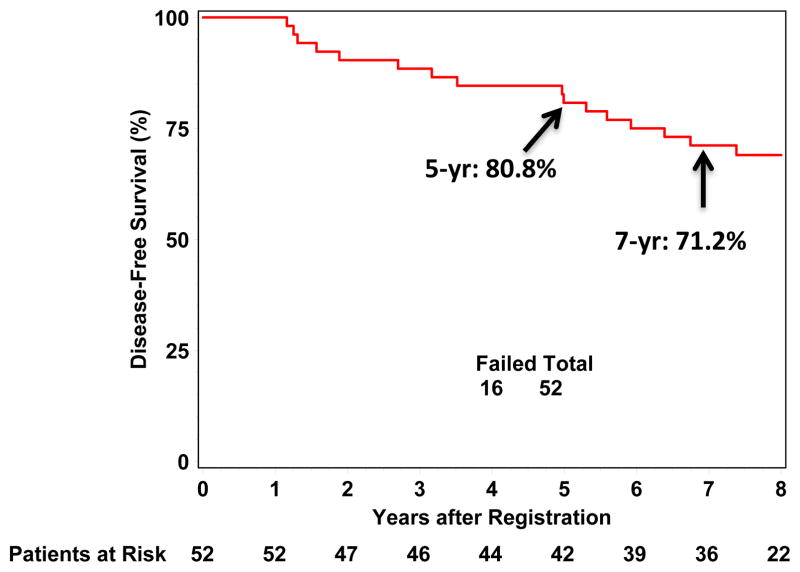
Of the 13 patients who died during the follow-up period, 10 died of causes other than breast cancer: eight were free of any disease recurrence at the time of death, and two had experienced disease recurrence but died of other causes. DFS is 80.8% (95% CI: 67.2%, 89.2%) at 5 years, and 71.2% (95% CI: 56.8%, 81.5%) at 7 years (Figure 2). Mastectomy-free survival at 7 years is 71.2% (95% CI: 56.8%, 81.5%). OS is 86.5% (95% CI: 73.8%, 93.3%) at 5 years and 78.8% (95% CI: 65.1%, 87.7%) at 7 years.
Figure 2.
Disease Free Survival
Toxicity
The worst reported adverse event(s) by any patient are presented in Table 2. Individual patients could report multiple toxicities. G3 treatment-related toxicities were reported by only four patients (7.7%). These toxicities consisted of radiation dermatitis (n=1), myositis (n=1), skin fibrosis (n=2), fibrosis- cosmesis (n=1), and telangiectasia (n=2). No G3 pain, pulmonary, or cardiac toxicities were reported. Most G3 toxicities were reported years after the start of treatment with a median reporting time of 3.9 years (min-max: 0.1–7.1 years).
Table 2.
Worst Reported Treatment-Related Adverse Event–Grade 3 in 4/52 Patients (7.7%)
| G3 Adverse Event* | n (%) | Months Since Start of Treatment (Mean) |
|---|---|---|
| Radiation Dermatitis | 1 (2%) | 1.2 |
| Myositis | 1 (2%) | 5.3 |
| Skin Fibrosis | 2 (4%) | 45.4 |
| Telangiectasia | 2 (4%) | 61.2 |
| Fibrosis - Cosmesis | 1 (2%) | 85.3 |
3 of 4 patients reported >1 adverse event
Discussion
This update presents the only long term cooperative group data of 3D-CRT APBI for the treatment of early stage invasive breast cancer in a small but mature data set. Trials with longer follow-up include the Hungary Trial6 (10.8 years), Boston Interstitial Trial7 (11.2 years), and RTOG 95-17 Trial8 (12.1 years), all of which investigated brachytherapy using a multi-catheter APBI technique. With a median follow up of 8 years, 3D-CRT APBI as delivered on this NRG Oncology RTOG trial demonstrates durable rates of ipsilateral breast tumor control. To put the results of this update in context, the 7-year cumulative incidence of ipsilateral breast recurrence reported here of 7.7% can be compared to IBR rates from other selected published APBI trials (Table 3). These trials vary widely in length of follow-up and sample size. The TARGIT9, American Society of Breast Surgeons (ASBS)10, NYU11, ELIOT12, Boston-3D-CRT13, Hungary6, Boston-Interstitial7 and RTOG 95-178 trials have follow-up of ranging from 2.4-12 years with sample sizes ranging from 50-1721 treated patients. Cumulative incidences of ipsilateral breast recurrence range widely, primarily associated with length of follow-up. The TARGIT9 trial, with a median follow up of only 2.4 years reported an IBR of 3.3% at 5 years. The Boston-Interstitial7 trial, with a much more mature median follow-up of 11.2 years, reported the highest IBR of 15% at 12 years. The two 3D-CRT APBI trials (Boston12 and NYU11) both included patients with more favorable tumor characteristics (stage I disease only) and report low recurrence rates (1–4.4%) with 5 year follow up. The cumulative incidence of iIBR reported here for NRG Oncology RTOG 0319 is within the range of other experiences, and lends confidence that 3D-CRT to 38.5 Gy in 10 fractions is an appropriate APBI option to be used alongside multi-catheter and balloon brachytherapy in the large randomized NSABP-B39/RTOG 0413 trial, now closed to accrual.
Table 3.
Ipsilateral Breast Recurrence in Selected APBI Trials
| Reference | Trial Technique | n | Follow-up | IBR rate |
|---|---|---|---|---|
| 9 | TARGIT Intrabeam kV IORT | 1721 | 2.4 yrs | 5 yr: 3.3% |
| 10 | ASBS Registry Trial Mammosite | 1449 | 5.3 yrs | 7 yr: 5.1% |
| 11 | NYU Prone 3D-CRT | 98 | 5.3 yrs | 5 yr: 1% |
| 12 | ELIOT Electron IORT | 651 | 5.8 yrs | 5 yr: 4.4% |
| 13 | Boston 3D-CRT | 98 | 5.9 yrs | 5 yr: 5% |
| RTOG 0319 3D-CRT | 52 | 8 yrs | 7 yr: 7.7% | |
| 6 | Hungary Multicatheter | 50 | 10.8 yrs | 12 yr: 9.3% |
| 7 | Boston Multicatheter | 48 | 11.2 yrs | 12 yr: 15% |
| 8 | RTOG 95-17 Multicatheter | 98 | 12.1 yrs | 10 yr 5.2% |
APBI – Accelerated Partial Breast Irradiation; IBR–Ipsilateral Breast Recurrence; IORT- Intraoperative Radiation Therapy; ASBS–American Society of Breast Surgeons; NYU–New York University; 3D-CRT–3 Dimensional Conformal Radiation Therapy
The worst reported treatment-related toxicity grade in this update was G3, reported on 4 patients (8%). This is a small increase from the 4-yr rate of 5.8% described in the last report3. Toxicity comparisons to other published APBI trials are difficult as most published APBI experiences use customized (non-validated) toxicity definitions. APBI trials which incorporated standardized toxicity criteria (Table 4) describe G3 toxicity rates of 1.4% at 3 years in RAPID14, 0–3% at 3.4 years in a preliminary report of NSABP-B39/RTOG 041315, and 8% for high dose rate and 21% for low dose rate multi-catheter brachytherapy at 7.6 years in RTOG 95-1716. With the longest follow-up, NRG Oncology RTOG 0319’s G3 toxicity is in line with these published trials. As most (5 of 7) G3 toxicities in 0319 were first reported years after treatment (3.6–7.1 years), long term follow-up of patients treated with this technique is important.
Table 4.
Comparison of Standardized Grade 3 Toxicities Among Prospective APBI Trials
| Trial | APBI Technique | n | Follow-up | Incidence G3 |
|---|---|---|---|---|
| RAPID14 NSABP B- | 3D-CRT | 1070 | 3 yrs | 1.4% |
| 39/RTOG 041315 | 3D-CRT | 1524 | 5.9 yrs | 0 – 3% |
| RTOG 951716 | Multi-Catheter | 100 | 7.6 yrs | 21% - LDR 8% - HDR |
| RTOG 0319 | 3D-CRT | 54 | 8 yrs | 7.7% |
APBI–Accelerated Partial Breast Irradiation; G–Grade; 3D-CRT–3 Dimensional Conformal Radiation Therapy; LDR–Low Dose-Rate; HDR–High Dose-Rate
A recent presentation of NSABP B-39/RTOG 0413,15 which closed in April 2013 with a total accrual of 4216 patients, described the early toxicity for the 1,391 patients who were randomized to APBI and treated with 3D-CRT. This early analysis demonstrated a G3 toxicity rate of ≤3% limited to fibrosis-cosmesis and fibrosis-deep connective tissue. While the median follow up for this large dataset is quite short (3.6 years), it further supports the observed low toxicity profile of 3D-CRT APBI in 0319 - a small but mature trial.
Conclusion
In summary, NRG Oncology RTOG 0319 was the first prospective cooperative group trial of APBI using 3D-CRT in the US. With a median follow-up of 8 years, NRG Oncology RTOG 0319 continues to demonstrate durable ipsilateral breast tumor control, in line with other published APBI trials. Similarly, the toxicity profile for this first cooperative group experience was low, also similar to other studies. Most G3 toxicities were reported many years after treatment, highlighting the importance of long term follow up.
After demonstrating feasibility in this trial, 3D-CRT APBI was incorporated into NSABP B-39/RTOG 0413, the randomized trial comparing whole breast irradiation to APBI. Mature results from this trial, in which approximately three-quarters of the patients who randomized to APBI were treated with 3D-CRT, will further define the efficacy, toxicity, and limitations of this technique.
Summary.
Updated follow up of patients treated with 3D-conformal accelerated partial breast irradiation on NRG Oncology RTOG 0319 demonstrates durable ipsilateral breast tumor control and minimal grade 3 toxicity at 7 years, comparable to other published prospective APBI trials. No G3 pain, pulmonary, or cardiac toxicities were reported. Mature phase III results from pending randomized trials utilizing this non-invasive treatment technique are awaited to fully assess its role in early stage breast cancer management.
Acknowledgments
This project was supported by RTOG grant U10 CA21661, and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflicts of interest: Dr. Wong reports grants and other from Elekta, during the conduct of the study. In addition, Dr. Wong has a patent ABC system for breath-hold with royalties paid to Elekta, and a patent Cone-beam CT for image guidance with royalties paid to Elekta.
Presented at the 55th annual meeting of the American Society of Therapeutic Radiation Oncology (ASTRO), September 2013 NRG Oncology RTOG 0319: Long term efficacy and toxicity
References
- 1.Vicini F, Winter K, Straube W, et al. A phase I/II trial to evaluate three-dimensional conformal radiation therapy confined to the region of the lumpectomy cavity for stage I/II breast carcinoma: Initial report of feasibility and reproducibility of Radiation Therapy Oncology Group (RTOG) Study 0319. Int J Radiat Oncol Bio Phys. 2005;63(5):1531–1537. doi: 10.1016/j.ijrobp.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Vicini F, Winter K, Wong J, Pass H, Rabinovitch R, Chafe S, Arthur D, Petersen I, White J, McCormick B. Initial Efficacy Results of RTOG 0319: Three-Dimensional Conformal Radiation Therapy (3D-CRT) Confined to the Region of the Lumpectomy Cavity for Stage I/ II Breast Carcinoma. Int J Radiat Oncol Bio Phys. 2010;77(4):1120–1127. doi: 10.1016/j.ijrobp.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chafe S, Moughan J, McCormick B, Wong J, Pass H, Rabinovitch R, Arthur DW, Petersen I, White J, Vicini F. Late Toxicity and Patient Self-Assessment of Breast Appearance/Satisfaction on RTOG 0319: A Phase 2 Trial of 3-Dimensional Conformal Radiation Therapy-Accelerated Partial Breast Irradiation Following Lumpectomy for Stages I and II Breast Cancer. Int J Radiat Oncol Bio Phys. 2013;86(5):854–859. doi: 10.1016/j.ijrobp.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Kalbfleish JD. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. pp. 167–169. [Google Scholar]
- 5.Kaplan EL, Meier P. Nonparameteric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 6.Polgar C, Major T, Fodor J, et al. Radiation Accelerated partial-breast irradiation using high dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:271–279. doi: 10.1016/j.radonc.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Hattangadi JA, Powell SN, MacDonald SM, et al. Accelerated partial breast irradiation with low-dose rate interstitial implant brachytherapy after wide local excision: 12-year outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):791–800. doi: 10.1016/j.ijrobp.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White JR, Winter KA, Kuske RR, et al. Long-term outcome from RTOG 9517: A phase I/II study of accelerated partial breast irradiation (APBI) with mulitcatheter brachytherapy (MCT) following lumpectomy for early-stage breast cancer. J Clin Oncol. 2012;30(suppl 27) abstr 147. [Google Scholar]
- 9.Vaidya J, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 6 year results for local control and overall survival from the TARGIT-A randomized trial. Lancet. 2014;383:603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 10.Shah C, Badiyan S, Wilkinson JB, et al. Treatment efficacy with accelerated partial breast irradiation (APBI): Final analysis of the American Society of Breast Surgeons MammoSite® Breast Brachytherapy Registry Trial. Ann Surg Oncol. 2013;20:3279–3285. doi: 10.1245/s10434-013-3158-4. [DOI] [PubMed] [Google Scholar]
- 11.Formenti SC, Hsu H, Fenton-Kerimian M, Roses D, Guth A, Jozsef G, Goldberg JD, Dewyngaert JK. Prone accelerated partial breast irradiation after breast-conserving surgery: five-year results of 100 patients. Int J Radiat Oncol Biol Phys. 2012 Nov 1;84(3):606–11. doi: 10.1016/j.ijrobp.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomized controlled equivalence trial. Lancet Oncol. 2013;14:1269–77. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 13.Pashtan IM, Recht A, Ancukiewicz M, Brachtel E, Abi-Raad RF, D'Alessandro HA, Levy A, Wo JY, Hirsch AE, Kachnic LA, Goldberg S, Specht M, Gadd M, Smith BL, Powell SN, Taghian AG. External beam accelerated partial-breast irradiation using 32 gy in 8 twice-daily fractions: 5-year results of a prospective study. Int J Radiat Oncol Biol Phys. 2012 Nov 1;84(3):e271–7. doi: 10.1016/j.ijrobp.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivotto IA, Whealn TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013 Nov 10;31(32):4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 15.Julia TB, Costantino JP, Vicini FA, White JR, Winter KA, Arthur DW, Kuske RR, Rabinovitch R, Curran WJ, Wolmark N. Early Toxicity Results with 3-D Conformal External Beam Therapy (CEBT) from the NSABP B-39/RTOG 0413 Accelerated Partial Breast Irradiation (APBI) Trial. Proceedings, 2011 ASCO Annual Meeting; abst 1011. [Google Scholar]
- 16.Rabinovitch R, Winter K, Kuske R, et al. RTOG 95-17, a Phase II trial to evaluate brachytherapy as the sole method of radiation therapy for Stage I and II breast carcinoma--year-5 toxicity and cosmesis. Brachytherapy. 2014;13(1):17–22. doi: 10.1016/j.brachy.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]