Abstract
Bone healing models are essential to the development of new therapeutic strategies for clinical fracture treatment. Furthermore, mouse models are becoming more commonly used in trauma research. They offer a large number of mutant strains and antibodies for the analysis of the molecular mechanisms behind the highly differentiated process of bone healing. To control the biomechanical environment, standardized and well-characterized osteosynthesis techniques are mandatory in mice. Here, we report on the design and use of an intramedullary nail to stabilize open femur osteotomies in mice. The nail, made of medical-grade stainless steel, provides high axial and rotational stiffness. The implant further allows the creation of defined, constant osteotomy gap sizes from 0.00 mm to 2.00 mm. Intramedullary locking nail stabilization of femur osteotomies with gap sizes of 0.00 mm and 0.25 mm result in adequate bone healing through endochondral and intramembranous ossification. Stabilization of femur osteotomies with a gap size of 2.00 mm results in atrophic non-union. Thus, the intramedullary locking nail can be used in healing and non-healing models. A further advantage of the use of the nail compared to other open bone healing models is the possibility to adequately fix bone substitutes and scaffolds in order to study the process of osseous integration. A disadvantage of the use of the intramedullary nail is the more invasive surgical procedure, inherent to all open procedures compared to closed models. A further disadvantage may be the induction of some damage to the intramedullary cavity, inherent to all intramedullary stabilization techniques compared to extramedullary stabilization procedures.
Keywords: Medicine, Issue 117, bone healing, animal model, mice, intramedullary locking nail, bone defect healing, non-union, biomechanics, bone substitutes
Introduction
The biology of bone healing may be studied in vitro using cell and spheroid cultures, but it also requires in vivo approaches using animal studies. While large-animal experiments still play an important role in preclinical testing, early stage testing of products or hypotheses has changed during the last 10 years and is nowadays often conducted in small animal models1. This switch was performed for several reasons. Production and maintenance of mice and rats are cheaper compared to pigs and sheep. In addition, small animals have shorter reproduction times and shorter normal healing periods, both of which facilitate the performance of large series of chronic experiments. Finally, the availability of gene-targeted animals and specific antibodies allows for the analysis of molecular mechanisms in bone healing. However, while the previously used osteosynthesis techniques in the larger animal models could be translated with minimal variation from similar procedures used in human or veterinary clinical patient care, the development and application of osteosynthesis techniques in the small-sized rats and mice turned out to be challenging.
It is well known that the biomechanical environment significantly influences the bone healing process2. As known from fracture healing in humans, differences in fracture stabilization result in distinct modes of healing, including intramembranous ossification after rigid fixation and endochondral ossification after less rigid fixation with micromovements. Complete axial or rotational instability may delay the healing process or may result in non-unions3. Accordingly, we feel that it is necessary to develop sophisticated implant systems and osteosynthesis techniques in mice and rats. In this way, the biomechanical conditions can be standardized appropriately, guaranteeing valid results when analyzing the healing process.
Although a considerable number of highly sophisticated murine stabilization techniques have been introduced during the last few years, the most commonly used technique is still the simple intramedullary pin. The major disadvantage of this technique, however, is the lack of rotational and axial stability4. To improve rotational and axial stability, an intramedullary screw was introduced to stabilize femur fractures in mice5. However, the screw fixation cannot be used to analyze bone-defective healing due to the need for contact and compression between the bone fragments in order to maintain rotational stability.
The intramedullary locking nail offers higher axial and rotational stability compared to the simple pin and the intramedullary screw4. A highly reproducible femur osteotomy, possible because of the guide for the Gigli saw and the ability to create defined gap sizes, allows for the analysis of both normal bone healing and bone-defective healing6. Due to the insertion of interlocking pins, the intramedullary locking nail guarantees a constant gap size during the entire healing process, even while bearing full weight. Here, we report on the design and application of the intramedullary locking nail, as well as on its advantages and disadvantages in experimental studies on normal and delayed bone healing.
Protocol
All procedures were IACUC-approved and followed institutional guidelines (Landesamt für Verbraucherschutz, Zentralstelle Amtstierärztlicher Dienst, Saarbrücken, Germany). Analgesia and infection prevention should be in agreement with the respective guidelines of the country and institution where the experiments are to be performed.
1. Preparation of Implants and Surgical Instruments
Select a scalpel blade (size 15), small preparation scissors, fine forceps, dressing forceps, small pincers, 24 gauge (G) and 27 G needles, a non-resorbable 5-0 suture, and a needle holder from the microsurgical instrument box.
Unpack the intramedullary nail, the interlocking pins, the special aiming device, the Gigli saw, the template for the Gigli saw, the centering drill bit (1 mm diameter), the drill bit (0.3 mm diameter), and the hand drill (Figure 2; see List of Materials). NOTE: The intramedullary nail (0.8 mm diameter, 15.7 mm length) is an intramedullary locking nail made of medical-grade stainless steel for retrograde implantation into the femur. The nail has a proximal thread (4 mm length) and two holes for the insertion of the interlocking pins (0.3 mm diameter) to achieve axial and rotational stability (Figure 1).
Expose the implants and all surgical instruments to a disinfecting solution (e.g., 96% alcohol) for 5 min or sterilize them (steam sterilization, 130 °C, 25 min). After disinfection or sterilization, place the instruments on a sterile operation cloth. Position the sterile operation cloth directly adjacent to the small animal operation table.
2. Animals, Anesthesia, and Analgesia
Choose the strain, age, and sex of the mice as necessary for the study and the question to be addressed. NOTE: For this study 12- to 14-week-old male CD-1 mice were used. For nail implantation, the ideal body weight of the animals is 25 - 35 g.
Anesthetize the mice with an intraperitoneal injection of 15 mg/kg xylazine and 75 mg/kg ketamine. Confirm the anesthetization by toe pinch. Apply eye lubricant to protect the animals' eyes from drying during anesthesia. After induction of anesthesia, place the mouse under a heat lamp to keep the body temperature constant.
Apply tramadol-hydrochloride in the drinking water (2.5 mg/100 ml) for analgesia from day 1 before the surgery until day 3 after the surgery.
3. Surgical Procedure and Nail Implantation
Before surgery, shave the entire right hind leg and apply a depilatory cream. After 5 min, remove the cream and clean the leg with water. Expose the implants and all surgical instruments to a disinfecting solution (e.g., 96% alcohol) or sterilize them (steam sterilization,130 °C, 25 min).
Under aseptic conditions, place the mouse in the supine position on the small animal operation table. Bend the right knee to allow for an anterior approach to the condyles of the knee. Perform a 5 mm medial parapatellar incision at the right knee using the scalpel blade.
Lift the patellar ligament with the fine forceps and mobilize the ligament carefully with the scalpel blade. Then, shift the patella laterally with the scalpel blade to expose the intercondylar notch of the femur.
- Open the intercondylar notch by drilling until the intramedullary cavity is reached.
- Start drilling with a 45º offset to the femur axis using the 1 mm centering drill bit. Slowly change the direction of the drill bit during drilling until it parallels the bone axis of the femur. Stop drilling if the intramedullary cavity is reached.
After opening the bone at the intercondylar notch, insert the 24 G needle into the intramedullary cavity over the whole length of the femur. Ream the intramedullary cavity of the femur manually through rotary motions of the 24 G needle. Remove the 24 G needle and insert the thinner 27 G needle into the intramedullary cavity. Push the needle forward to perforate the cortical bone of the femur proximally at the greater trochanter.
Remove the 27 G needle from the femur. Using the hand drill, implant the intramedullary nail through the intercondylar notch under continuous rotation and axial pressure until the distal end of the nail reaches the level of the condyles. NOTE: The distal end of the nail can be identified with a small mark.
Place the mouse in the left lateral position. Perform a longitudinal skin incision using the scalpel blade along the diaphyseal part of the lateral femur from the knee joint to the hip joint in order to surgically expose the midshaft of the femur.
- Using small preparation scissors, split the fascia and spread the muscles in the direction of the femur axis from the lateral side. Spread the muscles until the diaphyseal part of the femur is exposed. Preserve the sciatic nerve.
- Prepare the whole circumference of the femur by undermining the bone with the dressing forceps. Then, retract the muscles by spreading the dressing forceps and expose the femur.
Mount the aiming device to the distal end of the nail. Advance the device until it attaches to the adapter flange of the nail and turn the aiming device in anterolateral position to the femur.
- Interlock the nail with a proximal and a distal interlocking pin.
- Start with the proximal interlocking pin.
- Insert the centering drill bit (1 mm diameter) into the hand drill. Countersink the bone at the proximal interlocking hole position. NOTE: By countersinking, a small cavity is created in the facing cortical bone without drilling through the bone. This cavity allows for improved centering and guiding of the thinner drill bit (0.3 mm diameter), used later.
- Insert the drill bit (0.3 mm diameter) into the hand drill. Using the aiming device, drill the hole through both the facing and the averted cortical bone (bicortical). Insert the first interlocking pin through the aiming device. The interlocking pin drive shaft shears off as soon as the interlocking torque is achieved.
- Repeat this procedure for the distal interlocking pin.
- Perform the diaphyseal osteotomy.
- Attach the saw guide to the aiming device on the lateral side between the two interlocking pins. Then, saw the bone with the Gigli saw under continuous irrigation with saline. After the osteotomy is completed, cut the saw at one end, close to the bone. Remove the saw carefully to avoid causing damage to the soft tissue.
Remove the aiming device and, with the small pincers, clip off the remaining shaft of the intramedullary nail at the marked line.
Close the muscle layers at the lateral site of the femur and perform the skin closure with single sutures. At the anterior site of the knee, reposition the patella and fix the patella tendon to the muscles with one single suture. Use single sutures to close this wound as well.
Keep the animals under the heat lamp until they recover from anesthesia. Do not leave the animals unattended until they have regained sufficient consciousness to maintain ventral recumbency. Return the animals to single cages in the animal facility.
Monitor the animals carefully every day. Maintain postoperative analgesia during the first three days. Continue analgesia if, on day 4 after surgery, the animals still show evidence of pain, as indicated by vocalization, restlessness, lack of mobility, failure to groom, abnormal posture, and lack of normal interest in surroundings. Terminate analgesia when the animals are pain free.
Representative Results
The overall time for the surgical procedure was about 30 min from skin incision to wound closure. Using the surgical implants provided, surgery can be performed without a stereo-microscope. Postoperatively, the animals were monitored daily. Post-operative analgesia was terminated after 3 days because none of the animals showed evidence of pain (vocalization, restlessness, lack of mobility, failure to groom, abnormal posture, or lack of normal interest in surroundings) after this time period. The animals showed normal weight-bearing within 2 days after surgery. Wound infection or secondary fractures were not observed during the entire observation period.
The most important complication that can occur is the incorrect implantation of the locking nail, with the protrusion of the nail level with the condyles of the knee joint (Figure 3 A). This mainly occurs due to incorrect handling of the aiming device or due to the use of an animal with a too-small femur, particularly in mice with body weights below 20 g. Another complication is the dislocation of an interlocking pin (Figure 3 B). This complication can be avoided by radiographic confirmation of correct implant placement during or immediately after surgery. This issue is mainly caused by incomplete insertion of the pin. Finally, bone harvest at the end of the experiment was impeded a few times because it was difficult to remove the interlocking pins. This was due to bony bridging around the pin position.
Radiological analyses after 5 weeks confirmed complete healing of the 0.25 mm osteotomy gap. At this time point, the periosteal callus was almost completely remodeled (Figure 4 A). In contrast, in femora stabilized with a 2.00 mm gap, the osteotomy was not healed. The femora reliably showed an atrophic non-union formation. This was also confirmed after 10 weeks of bone healing (Figure 4 B).
After stabilization with a 0.25 mm osteotomy gap, histological analyses revealed a typical pattern of secondary fracture healing with callus formation, including intramembranous and endochondral ossification. After 5 weeks, the osteotomy was completely bridged with osseous tissue. At this time point, woven bone was already remodeled into lamellar bone (Figure 5 A). In contrast, the femora stabilized with 2.00 mm osteotomy gaps showed atrophic non-union after 10 weeks of observation. This was associated with a high amount of fibrous tissue within the osteotomy gap. None of the osteotomies showed signs of bone healing or bridging when analyzed histologically (Figure 5 B).
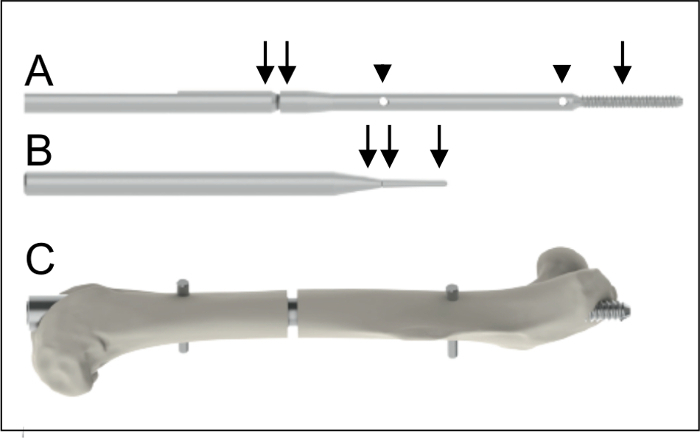 Figure 1: Implants. A. Intramedullary nail (0.8 mm diameter, 15.7 mm length) with a proximal thread (arrow, 4 mm length) and two holes (arrow heads) for the insertion of the interlocking pins. The nail is connected to a shaft (double arrow) to facilitate application. B. Interlocking pin (0.3 mm diameter, arrow) to achieve rotational and axial stability. The interlocking pin is also connected to a shaft (double arrow) to facilitate application. C. Intramedullary nail after implantation into a mouse femur. Please click here to view a larger version of this figure.
Figure 1: Implants. A. Intramedullary nail (0.8 mm diameter, 15.7 mm length) with a proximal thread (arrow, 4 mm length) and two holes (arrow heads) for the insertion of the interlocking pins. The nail is connected to a shaft (double arrow) to facilitate application. B. Interlocking pin (0.3 mm diameter, arrow) to achieve rotational and axial stability. The interlocking pin is also connected to a shaft (double arrow) to facilitate application. C. Intramedullary nail after implantation into a mouse femur. Please click here to view a larger version of this figure.
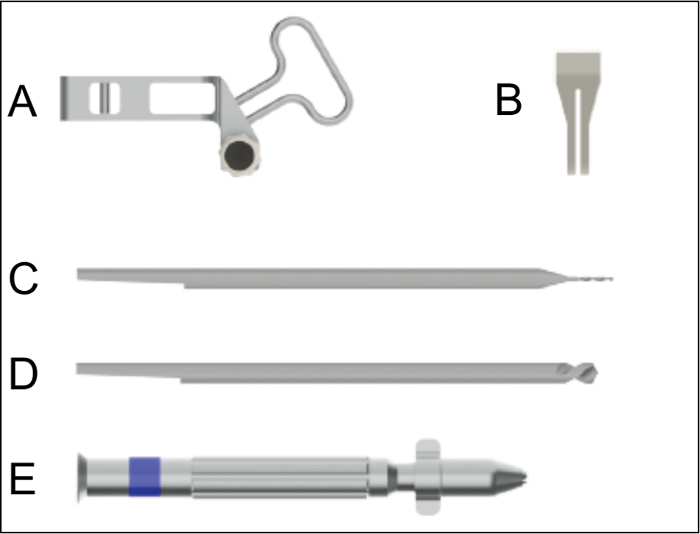 Figure 2: Surgical instruments for nail implantation. A. Aiming device for the insertion of the nail. B. Saw guide to be used for the creation of the osteotomy with a gap size of 0.25 mm. C. Drill bit for drilling the hole for the interlocking pins. D. Centering drill bit for countersinking of the interlocking pin holes. E. Hand drill used for the insertion of the nail, the countersinking, the hole drilling, and the insertion of the interlocking pins. Please click here to view a larger version of this figure.
Figure 2: Surgical instruments for nail implantation. A. Aiming device for the insertion of the nail. B. Saw guide to be used for the creation of the osteotomy with a gap size of 0.25 mm. C. Drill bit for drilling the hole for the interlocking pins. D. Centering drill bit for countersinking of the interlocking pin holes. E. Hand drill used for the insertion of the nail, the countersinking, the hole drilling, and the insertion of the interlocking pins. Please click here to view a larger version of this figure.
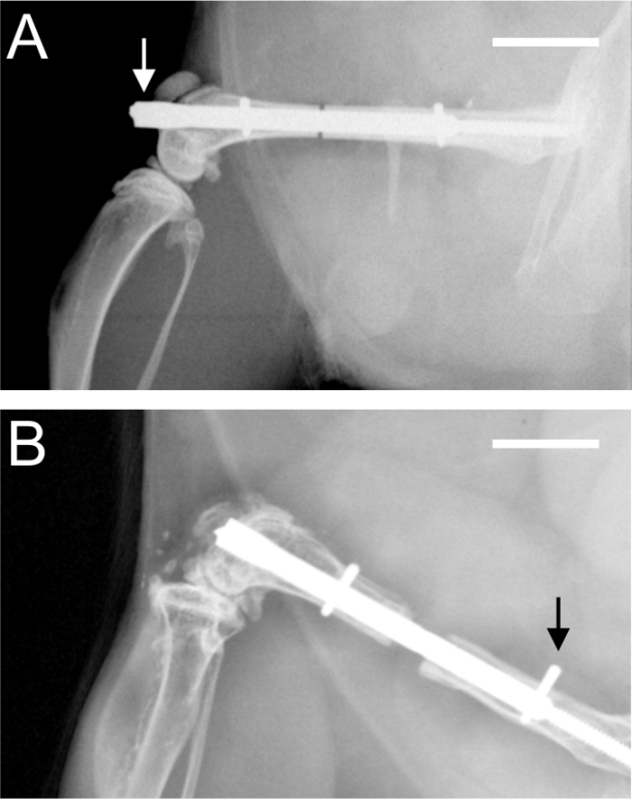 Figure 3:Postoperative radiographs. A. Radiograph demonstrating a protrusion (arrow) of the nail into the knee joint at the level of the condyles. B. Radiograph demonstrating an incomplete insertion of the proximal interlocking pin (arrow). Scale bars represent 4 mm.
Figure 3:Postoperative radiographs. A. Radiograph demonstrating a protrusion (arrow) of the nail into the knee joint at the level of the condyles. B. Radiograph demonstrating an incomplete insertion of the proximal interlocking pin (arrow). Scale bars represent 4 mm.
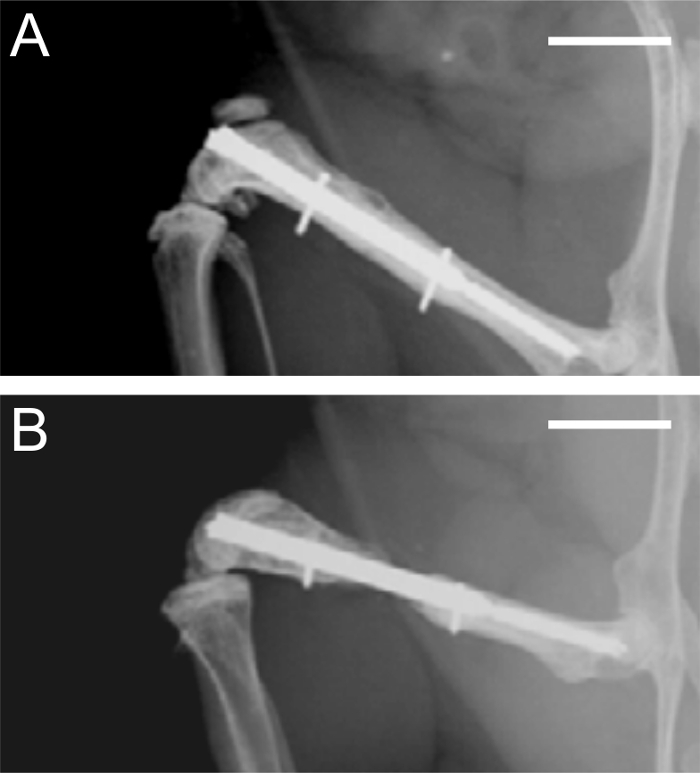 Figure 4: Radiographs after 5 and 10 weeks of bone healing. A. Radiographic analysis of a femur stabilized with a 0.25 mm osteotomy gap after 5 weeks, demonstrating adequate bone healing. B. Radiographic analysis of a femur stabilized with a 2.00 mm osteotomy gap after 10 weeks, demonstrating atrophic non-union. Scale bars represent 4 mm.
Figure 4: Radiographs after 5 and 10 weeks of bone healing. A. Radiographic analysis of a femur stabilized with a 0.25 mm osteotomy gap after 5 weeks, demonstrating adequate bone healing. B. Radiographic analysis of a femur stabilized with a 2.00 mm osteotomy gap after 10 weeks, demonstrating atrophic non-union. Scale bars represent 4 mm.
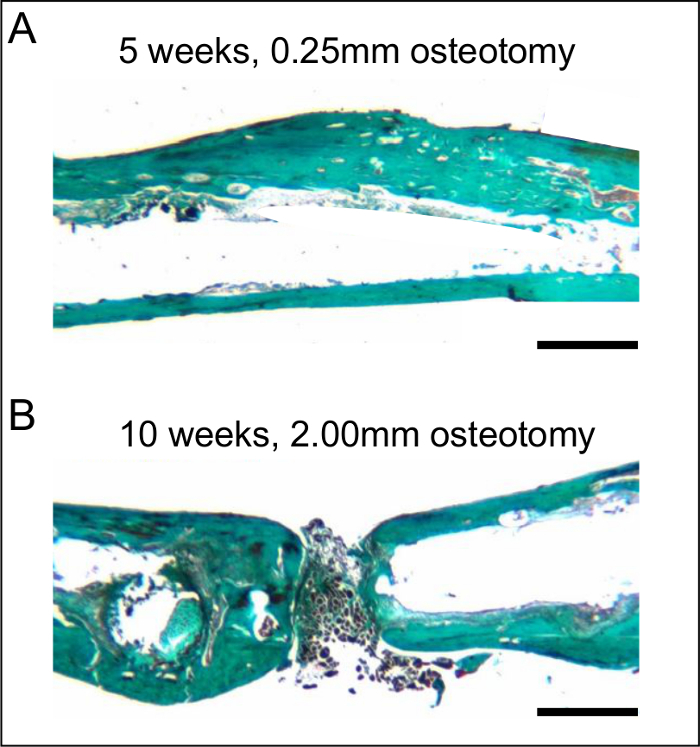 Figure 5:Histological sections after 5 and 10 weeks of bone healing. A. Histological analysis of a femur stabilized with a 0.25 mm osteotomy gap after 5 weeks, demonstrating adequate bone healing. Note the almost-complete remodeling with lamellar bone. B. Histological analysis of a femur stabilized with a 2.00 mm osteotomy gap after 10 weeks, demonstrating atrophic non-union. Note the fibrous tissue in the osteotomy gap. The histological sections were stained according to the trichrome method. Scale bars represent 800 µm.
Figure 5:Histological sections after 5 and 10 weeks of bone healing. A. Histological analysis of a femur stabilized with a 0.25 mm osteotomy gap after 5 weeks, demonstrating adequate bone healing. Note the almost-complete remodeling with lamellar bone. B. Histological analysis of a femur stabilized with a 2.00 mm osteotomy gap after 10 weeks, demonstrating atrophic non-union. Note the fibrous tissue in the osteotomy gap. The histological sections were stained according to the trichrome method. Scale bars represent 800 µm.
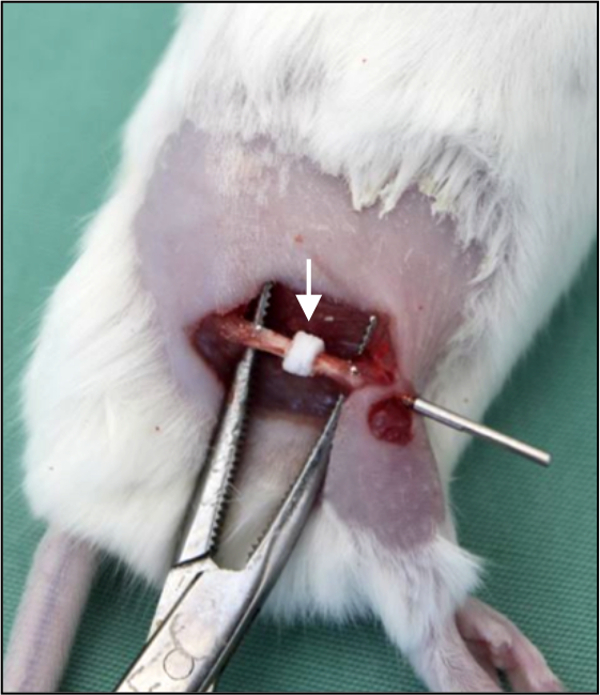 Figure 6: Bone substitute implantation.In vivo photograph demonstrating a segmental bone defect in the right femur of a mouse. The defect is filled by a bone substitute (arrow). The bone substitute is implanted over the nail, providing adequate positioning and fixation.
Figure 6: Bone substitute implantation.In vivo photograph demonstrating a segmental bone defect in the right femur of a mouse. The defect is filled by a bone substitute (arrow). The bone substitute is implanted over the nail, providing adequate positioning and fixation.
Discussion
The most critical steps of the surgical technique are the correct positioning of the nail, the aiming device, and the pins. The nail has to be inserted completely to the marked indent at the distal end of the nail, because a protrusion of the nail into the knee joint at the level of the condyles can restrict the movement of the knee (Figure 3 A). Therefore, the size of the femur and, accordingly, the body weight of the animals, must be considered. The surgeon should also pay special attention to the final position of the aiming device attaching the adapter flange of the nail. This guarantees that the osteotomy is always in an identical midshaft position. Finally, the interlocking pins have to be inserted completely bicortically to avoid pin dislocation during the observation period (Figure 3 B). Therefore, the pins should be inserted into the femur using rotational movements and continuous axial load. The pin drive shaft shears off as soon as the interlocking torque is achieved. The final position of the nail and the pins must be confirmed through radiography before the animals are included into the study protocol.
At the end of the experiment, the most critical step during the bone harvest is the removal of the interlocking pins from the nail. Quite frequently, the ends of the pins are covered by newly formed bony tissue. In fact, this bony tissue has to be resected until the pins can be removed. This must be performed very carefully, because any damage to the femur can influence the biomechanical properties of the healed bone. Sometimes, the pins can be more easily removed from the dorsal side of the femur. The nail itself can be removed without any difficulties using a simple needle holder.
The instruments and implants for this surgical procedure are highly specific, so modifications to the procedure cannot be made. While the procedure may develop complications, in our hands, they are rare (i.e., below 2%). For example, the patellar ligament, which is shifted laterally during the procedure, may rupture. This requires suturing of the ligament after nail implantation. During the insertion of the 24 G needle and the reaming of the femur, the condyles may burst. During pin insertion and osteotomy with the Gigli saw, the femoral bone may break in the midshaft region. There is no possibility of troubleshooting for these complications, so these animals cannot be used for a standardized experiment.
One limitation of the technique is that different sizes of animals and, thus, femora, require different sizes of implants. A further limitation on the use of the implant is that in vivo micro CT analyses of the osteotomy during the healing process are almost impossible due to the implant material (medical-grade stainless steel), which affects the image quality.
In vivo bone healing studies may be performed using open or closed models. In most studies, the healing of the femur or the tibia is analyzed. This holds true for the techniques and models developed for mice during the last decade. These include open7-10 models as well as closed5,11,12 approaches, which may provide a rigid or a less rigid fixation. Of interest, previous studies in mice used a simple intramedullary pin. Although the healing of fractures stabilized with such a simple pin was associated with huge callus formation, the technique bore a considerable number of disadvantages. These included pin dislocation and a heterogeneous healing response due to the failure of axial and rotational stability. Although these disadvantages are known to influence experimental results, recent studies, which intend to analyze the mechanisms of bone healing, still use murine models in which the fracture is stabilized only with a pin13 or is even left unstabilized14. Thus, because the mechanics during healing markedly influence the process of healing15, we feel that stable osteosynthesis techniques, comparable to those used in clinical practice, should also be used in mice.
The cost of the intramedullary locking nail is substantially higher when compared to that of the simple intramedullary pin. However, the pin bears the risk of dislocation and does not provide axial and rotational stability. This may affect the quality of the results and requires a greater number of animals for the study. In contrast, the intramedullary locking nail allows for stabilization of osteotomies and bone defects with a high degree of standardization, resulting in reduced variability of the results. This leads to a decrease in the necessary number of animals.
To overcome axial and rotational instability of the commonly used pin, several implants have been introduced during the last few years. These include the intramedullary screw, which induces fracture compression by a particularly modified distal head and proximal thread5.
The surgical technique necessary to implant the screw is simple and less invasive than that of the intramedullary nail. However, the screw shows lower rotational stiffness compared to the nail4. Furthermore, it cannot be used as a model of defective healing because the axial stability is achieved through axial compression of the bone fragments across the bone fracture.
The internal locking plate, which can be used for rigid fixation, results in bone healing, which is dominated by intramembranous ossification9. Because this type of healing is associated with little callus formation, this model may not be preferred in experiments that require larger amounts of callus tissue for biochemical and molecular analyses. Of interest, the internal locking plate can also be designed for a more flexible fixation technique16. Using this flexible plate, bone healing is dominated by endochondral ossification and thus results in larger amounts of callus tissue. However, callus formation is heterogeneous, occurring predominantly at the site opposite plate placement. The locking plate also allows for the stabilization of bone defects. However, the gap size is limited and does not result in a reliable non-union formation6.
The external fixator for mice offers a well-defined alternative to the nail for analyzing bone defect healing. The major advantage of the external fixator compared to the nail introduced here is the possibility to verify the implant stiffness in vivo during bone healing17. However, pin infections and alterations of normal physical activity due to the externally applied fixation components must also be considered.
Of special interest, neither the internal locking plate nor the external fixator allow standardized fixation of distinct bone substitutes and tissue-engineering constructs, which may be analyzed in bone defect healing. When using these two techniques, the bone substitutes or tissue-engineering constructs must be placed into the defect, which usually requires additional fixation18. In contrast, the use of the nail allows the implantation of the bone substitute over the nail, providing adequate positioning and fixation (Figure 6).
The intramedullary locking nail introduced here is comparable to nails used for the treatment of trauma patients in clinical practice. Accordingly, we feel that the nail can be used in a wide spectrum of murine bone healing research ranging from normal to defective.
Disclosures
Romano Matthys is an employee of RISystem AG, Davos, Switzerland, which produces the implants and implant-specific instruments. The other authors have no conflicts of interest.
Acknowledgments
This work was supported by RISystem AG, Davos, Switzerland.
References
- Histing T, et al. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone. 2011;49(4):591–599. doi: 10.1016/j.bone.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Claes L, Augat P, Suger G, Wilke HJ. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res. 1997;15(4):577–584. doi: 10.1002/jor.1100150414. [DOI] [PubMed] [Google Scholar]
- Histing T, et al. Characterization of the healing process in non-stabilized and stabilized femur fractures in mice. Arch Orthop Trauma Surg. 2016;136(2):203–211. doi: 10.1007/s00402-015-2367-7. [DOI] [PubMed] [Google Scholar]
- Histing T, et al. Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. J Orthop Res. 2009;27(9):1152–1156. doi: 10.1002/jor.20849. [DOI] [PubMed] [Google Scholar]
- Holstein JH, et al. Development of a stable closed femoral fracture model in mice. J Surg Res. 2009;153(1):71–75. doi: 10.1016/j.jss.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Garcia P, et al. The LockingMouseNail-a new implant for standardized stable osteosynthesis in mice. J Surg Res. 2011;169(2):220–226. doi: 10.1016/j.jss.2009.11.713. [DOI] [PubMed] [Google Scholar]
- Cheung KM, Kaluarachi K, Andrew G, Lu W, Chan D, Cheah KS. An externally fixed femoral fracture model for mice. J Orthop Res. 2003;21(4):685–690. doi: 10.1016/S0736-0266(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Garcia P, et al. A new technique for internal fixation of femoral fractures in mice: impact of stability on fracture healing. J Biomech. 2008;41(8):1689–1696. doi: 10.1016/j.jbiomech.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Histing T, et al. An internal locking plate to study intramembranous bone healing in a mouse femur fracture model. J Orthop Res. 2010;28(3):397–402. doi: 10.1002/jor.21008. [DOI] [PubMed] [Google Scholar]
- Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20(5):1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;11(2):305–312. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- Manigrasso MB, O'Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18(10):687–695. doi: 10.1097/00005131-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Lovati AB, et al. Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS One. 2013;8(6):e67628. doi: 10.1371/journal.pone.0067628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade Shantz JA, Yu YY, Andres W, Miclau T, 3rd, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma. 2014;28:10–14. doi: 10.1097/BOT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes LE, et al. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998;355(Suppl):132–147. doi: 10.1097/00003086-199810001-00015. [DOI] [PubMed] [Google Scholar]
- Gröngröft I, et al. Fixation compliance in a mouse osteotomy model induces two different processes of bone healing but does not lead to delayed union. J Biomech. 2009;18(13):2089–2096. doi: 10.1016/j.jbiomech.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Glatt V, Matthys R. Adjustable stiffness, external fixator for the rat femur osteotomy and segmental bone defect models. J Vis Exp. 2014. p. e51558. [DOI] [PMC free article] [PubMed]
- Manassero M, et al. A novel murine femoral segmental critical-sized defect model stabilized by plate osteosynthesis for bone tissue engineering purposes. Tissue Eng Part C Methods. 2013;19(4):271–280. doi: 10.1089/ten.TEC.2012.0256. [DOI] [PubMed] [Google Scholar]


