Abstract
Limbal stem cell deficiency (LSCD) is a state of malfunction or loss of limbal epithelial stem cells, after which the corneal epithelium is replaced with conjunctiva. Patients suffer from recurrent corneal defects, pain, inflammation, and loss of vision.
Previously, a murine model of LSCD was described and compared to two other models. The goal was to produce a consistent mouse model of LSCD that both mimics the phenotype in humans and lasts long enough to make it possible to study the disease pathophysiology and to evaluate new treatments. Here, the technique is described in more detail.
A motorized tool with a rotating burr has been designed to remove the rust rings from the corneal surface or to smooth the pterygium bed in patients. It is a suitable device to create the desired LSCD model. It is a readily available, easy-to-use tool with a fine tip that makes it appropriate for working on small eyes, as in mice. Its application prevents unnecessary trauma to the eye and it does not result in unwanted injuries, as often is the case with chemical injury models. As opposed to a blunt scraper, it removes the epithelium with the basement membrane. In this protocol, the limbal area was abraded two times, and then the whole corneal epithelium was shaved from limbus to limbus. To avoid stroma injury, care was taken not to brush the corneal surface once the epithelium was already removed.
Keywords: Developmental Biology, Issue 117, Cornea, Stem cell, Epithelium, Neovascularization, Conjunctivalization, Mouse model, Limbal stem cell deficiency, Goblet cell
Introduction
The corneal epithelium is required to maintain the clarity and integrity of the cornea. It is constantly renewed throughout life by the epithelial stem cells residing in the limbus-a narrow zone at the junction of the cornea and the conjunctiva. These self-renewing limbal stem cells play a crucial role in regenerating the corneal epithelium, both normally and after injury. Partial or complete depletion of these stem cells will result in recurrent corneal erosions, pain, corneal scarring and neovascularization, appearance of goblet cells, and if left untreated, corneal blindness. This condition is known as limbal stem cell deficiency (LSCD) and can be idiopathic; hereditary; or acquired due to chemical or thermal injuries, long-term contact lens wear, and chronic inflammation1-6.
Research on LSCD requires a suitable animal model that not only mimics the disease in humans, but is reproducible and sustainable, with the least amount of injury to other corneal and ocular structures. This model is necessary to assess treatments and to clarify the disease mechanisms at molecular and cellular levels. As described before1, with the use of a rotating burr, one can easily develop a mouse model of LSCD that features the aforementioned advantages and persists for at least three months. The goal of this study is to present a simple, reproducible, and sustainable mouse model of LSCD.
The rotating burr is a handy tool that evenly removes the epithelium without injuring the underlying stroma1. It has been used to induce central corneal erosion7, 8 in wound healing studies. The hereby-presented technique to create LSCD in a mouse has not been reported before. Previously introduced methods of scraping the epithelium with a blunt spatula result in a less uniform injury-particularly at the limbus-and a more variable phenotype1, 9, with more restoration of normal corneal epithelium1. Unlike the blunt scraper, the rotating burr removes the epithelial basement membrane as well7, 8, 10. Other reported methods to sever stem cells involve the use of chemicals, such as sodium hydroxide, n-heptanol, and benzalkonium chloride, which not only may induce unwanted injury to underlying eye structures, but also may lead to significant inflammation and subsequent corneal opacification or epithelial squamous metaplasia11-15. The rotating burr is not associated with these severe complications. Surgically removing the limbal epithelium, alone or with the use of chemicals10, 11, 16, is more difficult to perform and is not the best option in animals with small eyes and a thin limbal epithelia (like mice). In addition, surprisingly, limbectomy may still leave some of the limbal epithelium behind10.
The below-described technique will result in LSCD with neovascularization and conjunctivalization that mimics the presentation of complete LSCD in patients and lasts for at least three months1. It is suitable for those who aim to study LSCD or wound healing pathophysiology, immunology, and potential treatments in mice. Possibly, with some modifications, this procedure can be performed in rats or rabbits10. As the rotating burr efficiently removes the epithelium, it can be utilized in any research pertaining to corneal epithelial abrasion/wounding and in any related treatment or molecular biology studies.
Protocol
All procedures performed on animals comply with the Association for Research in Vision and Ophthalmology statements for animal use in vision research. Fourteen four- to six-month-old male/female C57BL/6J wild type mice were used: ten mice for the injury model and four as control animals (unwounded corneas).
1. Animal Preparation
Anesthetize a mouse with an intraperitoneal injection of a 100 mg/kg ketamine and 5 mg/kg xylazine mixture. After the anesthesia has taken effect, pinch the toe to confirm the anesthesia depth; the animal should not react.
Inspect the eye under a slit lamp before conducting the experiments to verify the absence of any previous corneal injury.
Instill a drop of 1% tetracaine hydrochloride onto the eye. After 60 - 90 sec, test the corneal reflex to confirm the absence of sensation. Proparacaine hydrochloride 0.5% can be used instead.
When the loss of corneal sensation is certain, gently place a drop of 5% povidone-iodine on the eye and on the hair around the eye. Wipe off the drop after 1 min or spread it on the hair around the eye to prevent the hair from interfering with the tool tip.
2. Abrading the Corneal Epithelium
Wear surgical gloves and a gown. Prepare the required tools: a sterile rotating burr and a pair of sterile tweezers. For better control, use bent tweezers. Place the tools on a sterile sheet during the procedure.
Using the bent tweezers, stabilize the eye by grabbing the lids from the nasal side and gently proptosing the eye. Avoid using too much pressure.
Under a surgical microscope, shave 360° around the limbal area twice using the sterile rotating burr. Go around the limbus with a speed of 5 - 6 sec per round. NOTE: The burr has one fixed speed. However, for it to rotate at the established speed, the "end nut" should be completely tightened.
Remove the whole corneal epithelium from limbus to limbus with the rotating burr. For better results, move the tool in a circular fashion and hold it somewhat parallel to the corneal surface to work with the lateral side of its tip. Take care not to injure the stroma by not re-brushing the areas where the epithelium has already been removed. Do not apply any pressure on the eye. Clean the brush tip as required.
Instill a drop of sterile phosphate-buffered saline on the cornea and wipe away any detached epithelial sheets with a soft cleaning tissue.
Shave the remaining epithelium, if any.
Pay attention to the mouse tail during the surgery. NOTE: If any movement occurs, the anesthesia depth has faded and further injection is required. One-third to two-thirds of the primary injected volume should be sufficient. Do not overdose the animal with the anesthetic medication.
Sterilize all the tools before using them on another eye.
3. Confirm Complete Epithelial Removal
Apply a drop of 1 mg/ml fluorescein sodium onto the cornea. Clean the cornea after 30 sec and examine it under a microscope with a cobalt blue filter to verify the complete removal of the epithelium. NOTE: Corneal areas with epithelial defects are visualized in green.
4. Post-operation Care
Put the animal on a heating blanket and keep it in a safe place (e.g., in its cage) while under recovery. Do not keep an unconscious animal in the company of conscious ones.
Apply 1% erythromycin ophthalmic ointment. This will also prevent eye dryness. Continue the application the antibiotic daily for a week.
Inject 0.1 mg/kg buprenorphine subcutaneously after the procedure and again after 12 hr. Continue the injection twice per day for two days.
Observe the animal for 30 - 45 min, until complete recovery from anesthesia. NOTE: Mild movement of the respiratory muscles on the ventral surface indicates animal wellness while under recovery.
Representative Results
Within a month after performing this technique, 100% of the corneas developed superficial neovascularization (Figure 1A. Photos were taken with a camera connected to a slit lamp under a bright field and cobalt blue filter). In 18/20 (90%) of the eyes, neovascularization involved the whole corneal surface (Figure 1A). In 2/20 (10%), neovascularization was observed in three of four corneal quadrants, but it still reached the corneal center.
To validate the development of LSCD, whole mount immunostaining was performed1. This step is not a part of the protocol and can be altered depending on goals and interests. Three variations were considered as indicators of LSCD: absence of cytokeratin (CK) 12, an intermediate filament specific to normal corneal epithelial cells17, 18; appearance of CK8, a simple epithelia marker20 that is not present on the normal cornea; and presence of goblet cells on the corneal surface. To evaluate the corneal epithelial phenotype, double immunostaining for CK12 and CK8 was performed on whole mount samples three months after the injury, as described previously1, 9. Goblet cell existence was assessed by MUC5AC-goblet cell-specific mucin-immunostaining1, 15, 19 45 days following epithelial removal. The appearance of goblet cells reduces in time. For the best results, it is important to perform the staining less than two months after the injury.
Normal, uninjured corneas express CK12-a specific marker of mature corneal epithelial cells17, 18-throughout the corneal surface, but they are devoid of any CK8 or goblet cells (MUC5AC) on the corneal part of the whole mount samples (Figure 1B). In comparison to normal eyes, CK12 expression is almost absent on injury model corneas, while they significantly show positive staining for CK8 and goblet cells (Figure 1B). These observations indicate the development of LSCD.
If interested in quantifying the immunostaining results, use Image-J to separately quantify20 immunostaining densities on the original, unaltered images taken by the microscope under the same settings. Briefly, select the corneal part of the whole mount sample, measure the integrated density and area for that region, and calculate the corrected density for the background20. The ratio of CK12 to CK8 densities were previously presented on a greater number of samples1. The reduced ratio in comparison to controls (normal corneas) and the appearance of goblet cells on the corneal surface demonstrate the development of LSCD.
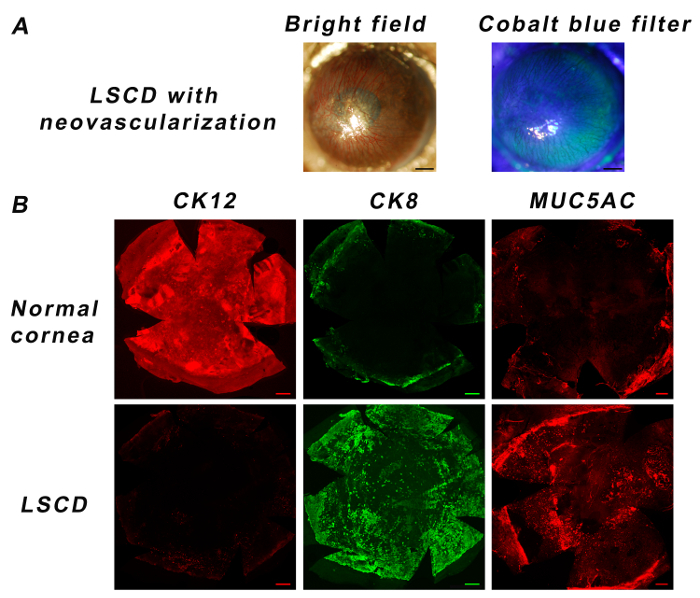 Figure 1:Corneal Phenotype After Creating LSCD in Mice. Slit lamp examination shows that all corneas develop some degree of neovascularization by one month (A, Scale bar: 0.5 mm). A normal cornea expresses CK12 all over the cornea but is devoid of any CK8 or goblet cells (MUC5AC). CK12 is absent in the LSCD model, while CK8 and goblet cells appear on the cornea (B, Scale bar: 200 µm). Please click here to view a larger version of this figure.
Figure 1:Corneal Phenotype After Creating LSCD in Mice. Slit lamp examination shows that all corneas develop some degree of neovascularization by one month (A, Scale bar: 0.5 mm). A normal cornea expresses CK12 all over the cornea but is devoid of any CK8 or goblet cells (MUC5AC). CK12 is absent in the LSCD model, while CK8 and goblet cells appear on the cornea (B, Scale bar: 200 µm). Please click here to view a larger version of this figure.
Discussion
This article describes a reproducible and relatively simple technique to create a mouse model of LSCD. There are several important aspects of this model that are worth noting. First, unlike models that use chemicals11, 12, the injury primarily involves the surface epithelium (and basement membrane), with minimal damage to the underlying corneal stroma or to other intraocular structures. Thus, there is only limited inflammation and scarring, both of which can complicate the procedure and make it more difficult to assess the outcome of any interventions aimed at the limbal stem cells. An interesting recent study by Li et al.10, which compares the use of the rotating burr with different combinations of surgical and chemical treatments, also found the burr to be safer and more effective in creating LSCD in rabbits. Second, the model seems to be stable for at least up to three months1. Creating this model involves the use of the rotating burr, an instrument that is familiar to clinicians who work with the cornea, as well as to those who do mouse wounding studies. Its advantage over using a blunt spatula is that the injury is more uniform and reproducible1.
To better destroy the limbal stem cells, the limbal area was treated twice. Based on experience working on mouse eyes, treatment of more than two rounds often abrades limbal blood vessels and causes bleeding. In case the technique is used in other animals, this step might be modified depending on the corneal epithelial thickness. In this study, a motorized tool with a 0.5-mm burr tip was utilized; burr tips in other shapes and sizes are also available and can be used instead, depending on corneal specifications and on eye size.Li et al.10 reported the 2.5-mm burr to be suitable to consistently remove rabbit corneal and limbal epithelia.
To obtain the optimal result, limbal and corneal epithelia were shaved in a circular fashion and the lateral side of the burr was used. To prevent the animal hair from interfering with the tool tip, the povidone-iodine drop was spread on the hair around the eye, instead of being wiped off. The tool should not be operated statically in one spot, as this may cause perforation. Treating the limbus at a very slow pace can result in bleeding; a speed of almost 6 sec per round is suitable for a C57BL/6J mouse eye. It is critical to avoid re-brushing the bare stroma, as this will induce more inflammation and stroma opacification. Pressure should not be applied to the eye while abrading the epithelium. After the procedure, the cornea was stained with fluorescein dye to make sure of complete epithelial removal. It is important to prevent eye dryness during animal recovery. Limitations are that this technique cannot remove 100% of the stem cells. It is also somewhat operator-dependent and requires practice to give consistent results.
When studying the corneal epithelial phenotype, whole mount staining is the best way to assess the outcome of this model. This is because it allows the whole corneal epithelium to be evaluated at once, whereas cross sections would only give information about each particular section.
This controllable technique can induce LSCD in mice, and probably in other animals as well. It provides a suitable model of LSCD to study novel treatments and to discover wider aspects of the disease pathophysiology. With some alterations, it can be useful in studying corneal wound healing, angiogenesis, and lymphangiogenesis as well.
Disclosures
The authors declare that they have nothing to disclose.
Acknowledgments
The authors thank Ruth Zelkha, MS, for her generous assistance in imaging. This research was supported by Clinical Scientist Development Program Award K12EY021475 to M.E., grant R01 EY024349-01A1 to A.R.D., core grant EY01792 from the National Eye Institute, NIH, and an unrestricted grant from Research to Prevent Blindness. A.R.D. is the recipient of a Career Development Award from Research to Prevent Blindness.
References
- Afsharkhamseh N, et al. Stability of limbal stem cell deficiency after mechanical and thermal injuries in mice. Exp. Eye Res. 2015;145:88–92. doi: 10.1016/j.exer.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorà NJ, Hill RE, Collinson JM, West JD. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res. 2015;15(3):665–677. doi: 10.1016/j.scr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Osei-Bempong C, Dana R, Jurkunas U. The culture and transplantation of human limbal stem cells. J. Cell Physiol. 2010;225(1):15–19. doi: 10.1002/jcp.22251. [DOI] [PubMed] [Google Scholar]
- Kolli S, Ahmad S, Lako M, Figueiredo F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 2010;28(3):597–610. doi: 10.1002/stem.276. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Figueiredo F, Lako M. Corneal epithelial stem cells: characterization, culture and transplantation. Regen. Med. 2006;1(1):29–44. doi: 10.2217/17460751.1.1.29. [DOI] [PubMed] [Google Scholar]
- He H, Yiu SC. Stem cell-based therapy for treating limbal stem cells deficiency: A review of different strategies. Saudi J. Ophthalmol. 2014;28(3):188–194. doi: 10.1016/j.sjopt.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Ghosh S, Pajoohesh-Ganji A, Tadvalkar G, Stepp MA. Removal of the basement membrane enhances corneal wound healing. Exp Eye Res. 2011;93(6):927–936. doi: 10.1016/j.exer.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, et al. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014;121:178–193. doi: 10.1016/j.exer.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirjamshidi H, et al. Limbal fibroblast conditioned media: a non-invasive treatment for limbal stem cell deficiency. Mol. Vis. 2011;17:658–666. [PMC free article] [PubMed] [Google Scholar]
- Li FJ, et al. Evaluation of the AlgerBrush II rotating burr as a tool for inducing ocular surface failure in the New Zealand White rabbit. Exp. Eye Res. 2016;147:1–11. doi: 10.1016/j.exer.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Ti SE, Anderson D, Touhami A, Kim C, Tseng SCG. Factors affecting outcome following transplantation of ex vivo expanded limbal epithelium on amniotic membrane for total limbal deficiency in rabbits. Invest. Ophthalmol. Vis. Sci. 2002;43(8):2584–2592. [PubMed] [Google Scholar]
- Ma Y, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24(2):315–321. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- Bu P, et al. Effects of activated omental cells on rat limbal corneal alkali injury. Exp. Eye Res. 2014;121:143–146. doi: 10.1016/j.exer.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Luengo Gimeno F, Lavigne V, Gatto S, Croxatto JO, Correa L, Gallo JE. Advances in corneal stem-cell transplantation in rabbits with severe ocular alkali burns. J. Cataract Refract. Surg. 2007;33(11):1958–1965. doi: 10.1016/j.jcrs.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Lin Z, et al. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest. Ophthalmol. Vis. Sci. 2013;54(9):6314–6325. doi: 10.1167/iovs.12-10725. [DOI] [PubMed] [Google Scholar]
- Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest. Ophthalmol. Vis. Sci. 1991;32(1):96–105. [PubMed] [Google Scholar]
- Liu CY, et al. Cornea-specific expression of K12 keratin during mouse development. Curr. Eye Res. 1993;12(11):963–974. doi: 10.3109/02713689309029222. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, González S, Mei H, Deng SX. Human limbal mesenchymal cells support the growth of human corneal epithelial stem/progenitor cells. Invest. Ophthalmol. Vis. Sci. 2014;55(10):6953–6959. doi: 10.1167/iovs.14-14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells. 2012;30(9):2032–2043. doi: 10.1002/stem.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13:1400–1412. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]


