Abstract
Recent advances in virus detection strategies and deep sequencing technologies have enabled the identification of a multitude of new viruses that persistently infect mosquitoes but do not infect vertebrates. These are usually referred to as insect-specific viruses (ISVs). These novel viruses have generated considerable interest in their modes of transmission, persistence in mosquito populations, the mechanisms that restrict their host range to mosquitoes, and their interactions with pathogens transmissible by the same mosquito. In this article, we discuss studies in our laboratory and others that demonstrate that many ISVs are efficiently transmitted directly from the female mosquito to their progeny via infected eggs, and, moreover, that persistent infection of mosquito cell cultures or whole mosquitoes with ISVs can restrict subsequent infection, replication, and transmission of some mosquito-borne viral pathogens. This suggests that some ISVs may act as natural regulators of arboviral transmission. We also discuss viral and host factors that may be responsible for their host restriction.
Keywords: mosquito-borne viruses, insect-specific viruses, flaviviruses, bunyaviruses, mesoniviruses, negeviruses
Introduction
Mosquito-borne viruses are objects of intense research due to their complex biology, ecology, and evolution and their potential to produce large and unpredictable outbreaks of disease.1 Indeed, explosive outbreaks of disease in many regions of the world have been caused by mosquito-borne viruses such as dengue virus (DENV), Zika virus (ZIKV), chikungunya virus (CHIKV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), Ross River virus (RRV), and Rift Valley fever virus. In the absence of safe and effective vaccines and antivirals against many of these viruses, new approaches are being explored to control these diseases and their transmissions.
Currently, there is a strong research focus on a novel group of “insect-specific” viruses (ISVs) that persistently infect mosquitoes, but do not infect vertebrates.2,3 Importantly, persistent infection of mosquitoes with some ISVs appears to interfere with the replication and transmission of medically significant viruses, such as West Nile virus (WNV).4–7 These findings suggest that ISVs may act as natural regulators of transmission of some arboviruses and may provide a new avenue for developing vector control strategies. Furthermore, the potential to genetically manipulate ISVs to develop new platforms for the production of safe diagnostic antigens and vaccines for mosquito-borne pathogens is now recognized as an innovative and a viable strategy.8
In this article, we review the key research conducted on mosquito-borne ISVs with a focus on the recent isolation and characterization of several new ISVs that group within a range of virus families including flaviviruses, alphaviruses, bunyaviruses, mesoniviruses, negeviruses, and reoviruses. We also discuss mechanistic and evolutionary features that are likely to be associated with the adaption of ISVs to a mosquito-only transmission cycle and the potential applications of these viruses as novel model systems for research, recombinant technology platforms, and agents of biological control.
Flaviviruses
The genus Flavivirus (family Flaviviridae) includes many important mosquito-borne human pathogens such as DENV, WNV, YFV, and ZIKV.9 These viruses cycle between mosquitoes and human or animal hosts with replication in both the arthropod and the vertebrate required to maintain transmission and persistence of the virus. In contrast, insect-specific flaviviruses (ISFs) replicate only in mosquitoes and form an interesting subgroup within the Flavivirus genus.2
The first recognized ISF, cell-fusing agent virus (CFAV), was identified as an endogenous virus in a cell line derived from Aedes aegypti mosquito larvae.10 However, it was not until almost 30 years later that ISFs, including CFAV and a related virus “Kamiti River virus”, were first isolated from mosquitoes in the wild and characterized.11–13 Since then, many ISFs have been isolated or genetically detected in several mosquito species from different regions of the world.2 Studies by Saiyasombat et al.14 and Bolling et al.6 indicate that ISFs are maintained in mosquito populations by vertical transmission – a process by which the progeny of infected female mosquitoes is infected via the egg.15
In 2010, our laboratory initiated a project to assess the biodiversity of ISFs in Australian mosquitoes. This was initially performed by the detection of flavivirus RNA directly in archival samples of homogenized mosquito pools or by inoculation of the samples onto C6/36 cells prior to the detection by reverse transcription polymerase chain reaction and/or the presence of cytopathic effect (CPE) in the cells.5 Subsequently, we enhanced the speed and sensitivity of the ISF isolation protocol by the viral RNA intermediates in cells).16 This approach allowed the detection of novel and genetically diverse ISFs (as well as other novel RNA viruses, which are given in the following sections) in a sequence-independent manner and provided a relatively simple, cost-effective, and high-throughput protocol for the detection of infected cultures prior to sequencing and characterization of the isolates.
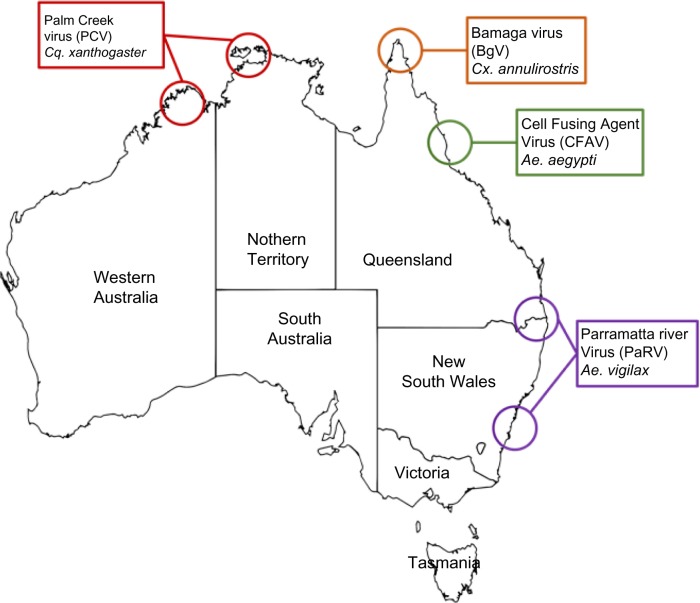
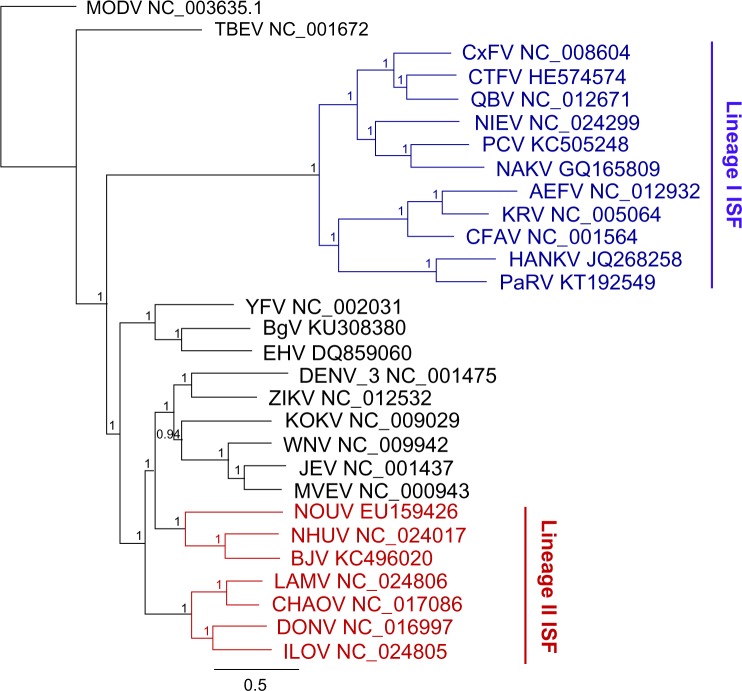
Several ISFs were subsequently isolated from Australian mosquitoes including Palm Creek virus (PCV) from Coquillettidia xanthogaster in northern Australia5,17; Parramatta River virus (PaRV) from Aedes vigilax from Sydney, Newcastle, and Brisbane17; and local isolates of CFAV from Ae. aegypti from Cairns (Harrison et al, unpublished data). Figure 1 illustrates the geographical distribution of these viruses in Australia, while Figure 2 shows the genetic relationship between these new viruses, other ISFs, and flaviviruses that infect vertebrates.
Figure 1.
Map of Australia showing general locations of insect-specific flavivirus isolations.
Figure 2.
Bayesian phylogenies of flaviviruses over the whole open reading frame nucleotide sequence. The tree was constructed in Geneious using MrBayes v3.2.2 under the Bayesian Marko chain Monte Carlo (MCMC) model with a general time reversible substitution model, gamma distribution (five discrete gamma categories), and invariant rates among sites. Horizontal branch lengths represent posterior probabilities. The tree has been rooted using the outgroup Modoc virus (MODV), a flavivirus with no known vector. The colored nodes represent insect-specific flaviviruses (ISFs), with Lineage I in blue and Lineage II in red.
ISF-like viruses
Most of the ISFs reported to date belong to a group that is phylogenetically separate from the vertebrate-infecting flaviviruses and are described as “classical” ISFs.2 For clarity, these viruses are referred to, in this article, as Lineage I ISFs (Fig. 2). However, a smaller subset of ISFs, termed dual-host affiliated ISFs, display an insect-specific phenotype but group phylogenetically with the mosquito-borne pathogenic flaviviruses.18–20 These viruses are referred to, in this article, as Linage II ISFs (Fig. 2). While Lineage II ISFs have been assessed in vitro for growth in a range of vertebrate cell lines, with no replication detected, the phylogenetic position of these viruses suggests that they may have only recently evolved from a vertebrate-infecting phenotype to an insect-specific transmission cycle.2 However, more studies are required to support this, including testing for growth in a more extensive panel of cell lines under variable growth conditions and additional in vivo experiments.
Another subset of ISF-like viruses shows some replication in vertebrate cells, but only in a limited range of cell types or under specific growth conditions. Rabensburg virus, considered to be a strain of WNV, showed little to no replication in vertebrate cell lines or live birds.21 However, further studies revealed that this virus replicates and causes CPE in vertebrate cells if they are incubated at temperatures below 35 °C.22 A recent report from our laboratory also showed that a new Australian flavivirus named Bamaga virus (BgV), which groups phylogenetically with vertebrate-infecting flaviviruses in the YFV group, displays restricted replication in vertebrates, both in vitro and in vivo.23 No replication of BgV could be detected in a range of vertebrate cells after 2 days of incubation, even when the cells were infected at a high multiplicity of infection. However, after 5 days of incubation, limited replication was detected in a subset of vertebrate cells tested.23 When a range of viral doses were inoculated into weanling mice, none of the animals injected by the intraperitoneal route seroconverted, suggesting little or no replication in vivo. Indeed, mice showed no disease, and only a few animals seroconverted when the highest viral dose (104 infectious units) was inoculated directly into the brain.
The unusual phenotype described earlier for the two viruses may be indicative of their adaption to cryptic vertebrate hosts with low optimal body temperature. Alternatively, the data may indicate that they are in transition between a vertebrate-infecting and an insect-specific transmission cycle.22,23
Interference by ISFs with the replication and transmission of flaviviral pathogens
Studies by Bolling et al.6, on a population of Culex pipiens naturally infected with the ISF Culex flavivirus (CxFV), revealed that these mosquitoes exhibited a delay in the transmission of WNV, compared to CxFV-free mosquitoes, when infected with WNV by the oral route. Suppression of WNV transmission has also recently been reported for some Culex species previously inoculated with Nhumirim virus7 or PCV.4 Furthermore, the latter study indicated that transmission interference probably occurred in the cells of the midgut; the exclusive site of localization of PCV replication as determined by immunohistochemistry labeling of mosquito sections4 and the first tissue to be infected upon oral feeding with WNV.
The effect on DENV and ZIKV transmission by Aedes species carrying ISFs, such as CFAV or PaRV, has yet to be assessed; however, in vitro studies in our laboratory revealed that the replication of DENV-3 and WNV in Aedes albopictus cells (C6/36) was strongly inhibited, in a flavivirus-specific manner, by prior infection with PaRV (McLean et al, unpublished data).
The mechanism(s) involved in both in vitro and in vivo viral interferences by ISFs at the cellular level is unknown. Current theories suggest an upregulation of an antiviral host response by the initial ISF infection that subsequently inhibits the superinfecting virus or competition for cellular resources between the resident ISF and the second flavivirus.2,4
The evolutionary origin of ISFs is an enigma. Did they evolve from vertebrate-infecting flaviviruses by adapting to replication in mosquitoes or do they represent the ancestral lineage of the dual-host flaviviruses? While it has been proposed that Lineage I ISFs represent the precursors to dual-host flaviviruses, previous attempts to address this by bioinformatics analyses were inconclusive, due to the limited number of fully sequenced ISF genomes available at the time.24 However, the discovery and full genome sequencing of several additional ISFs may now allow more meaningful bioinformatics to be undertaken to elucidate the evolutionary origins of the flavivirus genus.
The molecular basis for the restricted host range of ISFs is another poorly understood phenomenon. Alignment of the deduced amino acid sequences of Lineage I ISFs with vertebrate-infecting flaviviruses reveals significant changes in several genes including large conserved deletions in domain III of the envelope protein (EDIII).17 These deletions are of particular interest since EDIII contains the putative flavivirus receptor-binding site.25 Furthermore, four additional cysteines in the EDIII of ISFs suggest the formation of additional S–S bonds in this domain, which likely change the tertiary structure and may alter the ability of ISFs to recognize specific cell receptors or interfere with membrane fusion.17,26,27 The identification of conserved deletions in the NS5 gene and insertions in the 3′ untranslated regions of some ISFs when compared to VIFs may also indicate that additional viral factors are associated with ISF host restriction.17,28 These may be associated with the inability of ISFs to counteract the innate immune response in the vertebrate cell. Indeed, recent findings by Tree et al.29 provide the first evidence that some ISFs can infect and replicate in vertebrate cells deficient in innate immune pathways.
The use of novel methods to manipulate infectious genomes of ISFs will allow the identification of viral and host factors that restrict the replication of ISFs to mosquitoes and help us to understand the complex dynamics of the transmission of mosquito-borne viruses30,31 (Piyasena et al, unpublished data). These investigations will also provide valuable insights into the evolution of mosquito-borne viruses and underpin the future development of new strategies to genetically manipulate ISFs as new technological platforms to prevent mosquito-borne viral diseases and control their transmission.
Alphaviruses
In contrast to the ever-expanding group of ISFs discussed earlier, only a single member of the Alphavirus genus has been shown to exhibit an insect-specific phenotype. While there are many mosquito-borne alphaviruses that infect vertebrate hosts and cause disease, including CHIKV, Sindbis virus (SINV), Western equine encephalitis virus (WEEV), and RRV, only Eilat virus (EILV) appears to be restricted to replication in mosquitoes. EILV was isolated from An. constani mosquitoes trapped in Israel.32 The virus clusters phylogenetically with the WEEV complex of alphaviruses that also includes SINV and SINV-like viruses. While it shows a similar genome structure and virion morphology to other alphaviruses, it fails to infect and replicate in a range of mammalian and avian cell lines that are usually susceptible to alphavirus infection. While the precise mechanism of host restriction has yet to be defined, Nasar et al.33 have used SINV–EILV chimeric viruses to demonstrate that EILV replication is inhibited at both precell and postcell entry stages of the viral life cycle.
The construction of chimeric viruses between EILV and CHIKV, with the viral structural protein derived from the latter and the replicase components from the former, has also yielded a viable virus that is antigenically similar to the CHIKV parental virus, but unable to replicate in vertebrate cells like EILV.8 This represents a novel platform for the production of diagnostic antigens or vaccines for pathogenic alphaviruses without the need for inactivation.
Similar to findings with ISFs,4,6,7 prior infection of mosquito cells with EILV also delayed replication and reduced viral titers of other alphaviruses, including SINV, WEEV, and CHIKV.34 Furthermore, infection of Ae. aegypti with EILV prior to infection by CHIKV delayed dissemination of the latter by 3 days. These studies highlight the potential application of ISVs for the control of arbovirus transmission and disease.
Bunyaviruses
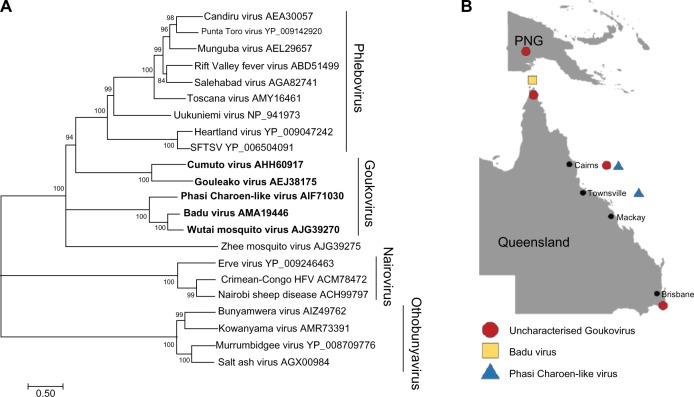
Recently, four divergent groups of bunyaviruses, which do not appear to replicate in vertebrate cells, have been identified in mosquitoes.35–37 The first of these viruses to be isolated and characterized from mosquitoes collected in Cote d’Ivoire was Gouleako virus (GOLV).35 The low genetic homology and lack of antigenic relatedness with other members of the Bunyaviridae family, in addition to its insect-restricted replication phenotype, led to the proposal of GOLV and other similar viruses to form a new genus named Goukovirus.35,38 More recently, additional viruses likely to become members of the proposed Goukovirus genus have also been identified (Fig. 3), all with a basal phylogenic relationship to the Phlebovirus genus.39 These include Badu virus (BADUV), isolated in our laboratory from mosquitoes collected from Badu island in the Torres Strait between Australia and Papua New Guinea (PNG)40; Cumuto virus (CUMV), isolated from mosquitoes collected in Trinidad38; and sequences of viruses yet to be isolated – Zhee Mosquito virus (China), Phasi Charoen-like virus (PCLV; Brazil, Thailand), and Wutai mosquito virus (China).41–43 More recently, we have obtained isolates of PCLV from Ae. aegypti mosquitoes from northern Australia (Harrison et al, unpublished data).
Figure 3.
(A) Phylogenetic analysis of representative bunyaviruses showing the taxonomic position of goukoviruses. Maximum likelihood, midpoint rooted phylogenetic tree of Bunyavirus amino acid sequences over the complete RdRP open reading frame. The tree was constructed based on an MAFFT alignment via the CIPRES gateway and using Mega v7.0.14 tree builder with the Jones–Taylor–Thornton genetic distance model. Numbers on branches represent bootstrap values. Scale bar represents the number of substitutions per site. (B) The map shows distribution of new goukoviruses isolated in Australia and Papua New Guinea (PNG).
Although the three goukoviruses most studied to date (GOLV, CUMV, and BADUV) show restricted host range in vitro, in vivo studies have only been undertaken for CUMV. While inoculation of 2-day-old mice produced no apparent disease, no detailed analyses for evidence of virus replication in these animals were performed.38 However, the association of GOLV with fatal disease in pigs in Korea flags these new viruses as potential emerging pathogens.44 Although a subsequent field study in Cote d’Ivoire found no evidence of GOLV infection in pigs,45 further in vivo investigations are required to determine the actual host range and ecology of the different goukoviruses. In this context, we isolated BADUV from Culex mosquitoes collected during incursions of JEV into northern Australia.46 In that outbreak, JEV was transmitted between Culex mosquitoes and pigs as part of the usual ecology for this virus. Whether BADUV simultaneously infected pigs is presently unknown. In addition to BADUV, we have recently isolated several novel bunyaviruses from northern Australia and PNG, which genetically cluster with the Goukovirus genus (Hobson-Peters et al, unpublished data; Fig. 3).
Mesoniviruses
The order Nidovirales comprises four genetically diverse families of enveloped positive-sense single-stranded RNA viruses, including Coronaviridae, Arteriviridae, Roniviridae, and Mesoniviridae. The Mesoniviridae is a new ISV family that was established to accommodate Cavally virus, discovered in Culex mosquitoes captured in Côte d’Ivoire, West Africa, in 2004.47 Independently, another group isolated Nam Dinh virus (NDiV) from Culex mosquitoes collected in Vietnam.48 These viruses were later classified as different strains of Alphamesonivirus-1, the prototype species in the Mesoniviridae family.49 Many other mesoniviruses have subsequently been discovered (Table 1).50–53
Table 1.
Mesoniviruses published to date.
| ISOLATE | SPECIES | ISOLATION REGION | COLLECTION DATE | MOSQUITO SPECIES OF ISOLATION | REFERENCES |
|---|---|---|---|---|---|
| Cavally | Alphamesonivirus-1 | Côte d’Ivoire | 2004 |
Culex spp. Aedes spp. Anopheles spp. Uranotaenia spp. |
47 |
| Nam Dinh Houston |
Alphamesonivirus-1 Alphamesonivirus-1 |
Vietnam U.S.A |
2002 2004, 2010 |
Culex vishnui Culex tritaeniorhynchus Culex quinquefasciatus Ae. albopictus |
48,53 |
| Karang Sari (KSaV) | Alphamesonivirus-2 | Indonesia | 1981 | Cx. vishnui | 53 |
| Bontag Baru (BBaV) | Alphamesonivirus-2 | Indonesia | 1981 |
Cx. Vishnui Cx. tritaeniorhynchus |
53 |
| Dak Nong (DKNV) | Alphamesonivirus-3 | Vietnam | 2007 | Cx. tritaeniorhynchus | 51 |
| Kamphaeng Phet (KPhV) | Alphamesonivirus-3 | Thailand | 1984–85 | Mosquito pool+ | 53 |
| Casuarina (CASV) | Alphamesonvirus-4 | Australia | 2010, 2006 |
Cq. xanthogaster Culex annulirostris |
52 |
| Hana (HanV) | Alphamesonvirus-5 | Côte d’Ivoire | 2004 |
Culex spp. Culex spp. |
50 |
| Nsé (NseV) | Mesonivirus-1 | Côte d’Ivoire | 2004 |
Aedes spp. Anopheles spp. Anopheles spp. |
50 |
| Méno (MenoV) | Mesonivirus-2 | Côte d’Ivoire | 2004 |
Culex spp. Uranotaenia chorley |
50 |
Note:
Mosquito species was not provided.
Australian mesoniviruses
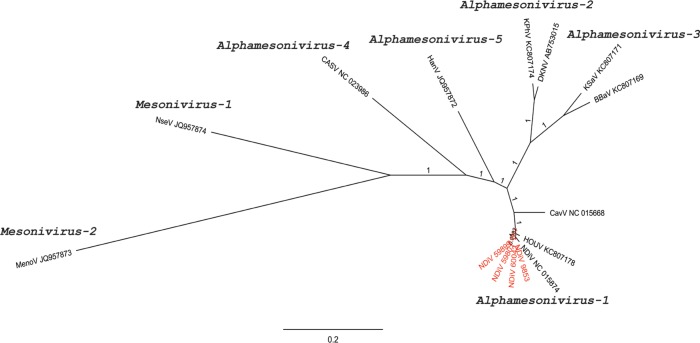
In 2014, our laboratory reported the first mesonivirus discovered in Australia. Casuarina virus (CASV) was isolated from Coquillettidia xanthogaster mosquitoes collected in Darwin, Northern Territory.52 Subsequently, we also isolated CASV from Culex annulirostris from Cairns in northern Queensland (Hobson-Peters et al, unpublished data). CASV was determined to be an ISV after the virus failed to replicate in a range of primate, rodent, human, and avian cell lines.52 Alignment of the deduced amino acid sequences of the concatenated replicase domains between CASV and the Alphamesonivirus-1 strains showed high identity of the RdRp (94.1%) and HEL (92.7–93.6%) domains. However, a lower identity (76.9–85.5%) was observed in the other four domains supporting assignment of CASV as a new species – Alphamesonivirus–4.52 We further highlighted this proposed new assignment by mapping the evolutionary distance between the mesoniviruses (Fig. 4).
Figure 4.
Phylogenetic tree showing the genetic relationship between the proposed species of the Mesoniviridae across the whole genome in nucleotides. The tree was constructed in Geneious using MrBayes v3.2.2 under the Bayesian Marko chain Monte Carlo (MCMC) model with a general time reversible substitution model, gamma distribution (five discrete gamma categories), and invariant rates among sites (Huelsenbeck and Ronquist, 2001). Horizontal branch lengths represent posterior probabilities. MenoV is used as an outgroup. Red text represents new Australian Alphamesonivirus-1 isolates. Scale bar represents substitutions per site.
Following the discovery of CASV, the first Australian isolates of Alphamesonivirus-1 (NDiV) were obtained from several mosquito species collected from various regions of the continent between 2007 and 2014 (Hobson-Peters et al, unpublished data).
Reoviruses
The family Reoviridae comprises nonenveloped, segmented dsRNA viruses that include pathogens of a wide variety of vertebrates and invertebrates including crustaceans, fish, insects, reptiles, and mammals. The genus Orbivirus is the largest genus within the family, containing 22 distinct virus species.54 Orbiviruses are characterized by a 10-segment, double-stranded RNA genome. Parry’s Lagoon virus (PLV) is a novel orbivirus that was isolated by our laboratory from Cx. annulirostris mosquitoes that were captured from northwestern Australia.55 Phylogenetic analysis of each of the viral proteins demonstrated a moderate-to-high (72.6–95.3%) amino acid similarity to another orbivirus, Corriparta virus (CORV), supporting its classification as a member of the CORV serocomplex.55 This conclusion was also confirmed by antigenic analysis that showed that PLV was recognized and cross-neutralized by CORV antisera.55
However, PLV shows a remarkably different phenotype in cell culture, failing to replicate in several mammalian and avian cell lines that supported efficient growth of CORV.55 In contrast, PLV grew well in mosquito cells, suggesting that it has developed a restricted host range, indicative of a mosquito-only life cycle. Several other reoviruses also show a restricted host range (Table 2). Aedes psuedoscutellaris reovirus replicates in various mosquito cell lines, but failed to replicate in mammalian cell lines and in vivo in mice.56 Similarly, Fako virus and Cimodo virus do not replicate in vitro in vertebrate cells.57,58
Table 2.
Putative insect-specific reoviruses published to date.
| ISOLATE | GENUS | ISOLATION REGION | COLLECTION DATE | MOSQUITO SPECIES OF ISOLATION | REFERENCES |
|---|---|---|---|---|---|
| Liao Ning# | Seadornavirus | China,Australia | 1999,2007 | Cx. spp., Ae. spp. Anopheles, Mansonia | 61, 62 |
| Parry’s Lagoon | Orbivirus | Australia | 2010 | – | 55 |
| Aedes psuedoscutellaris reovirus | Dinovernavirus | N/A | 1974 | Laboratory cell line isolation | 56 |
| Fako | Dinovernavirus | Cameroon | 2010 | Ae. spp., Eretmapodites dracaenae, inornatus | 57 |
| Cimodo | N/A* | Côte d’Ivoire | 2004 | Ae. spp., Cx. spp., An. spp. unclassified spp. | 58 |
Notes:
Australian Isolates shown to insect - specific.
Currently unclassified. The authors concluded that Cimodo virus putatively defines a novel genus within the subfamily of Spinareovirinae.
Another newly classified group of viruses in the Reoviridae family is the genus Seadornavirus.59 The genus name refers to the 12 segments of dsRNA comprising the viral genome and the geographical location of the first isolates.59 Currently, there are three members of this genus: Banna virus (BAV), Kadipiro virus, and Liao ning virus (LNV). Only BAV has been directly associated with disease in humans.60 LNV was first isolated in China in 1999. It was first detected in Australian mosquitoes by deep sequencing of mosquito samples containing unidentified viruses.61 The first Australian isolates were made in our laboratory from Ae. vigilax mosquitoes collected in Sydney in 2007 and designated LNVAu. Since then, it has been isolated from four different mosquito genera (Culex, Anopheles, Mansonia, and Aedes) collected from all over Australia (Prow et al, unpublished data). In contrast to what was reported for the Chinese LNV isolates,62 LNVAu strains do not replicate in vertebrate cell lines or in mice. Thus, at this stage, it would appear that LNVAu should be considered an ISV (Prow et al, unpublished data; Table 2). The mode of transmission between and within mosquito genera, however, still remains to be established.
Negeviruses
The taxon negevirus was originally described by Vasilakis et al.63, who reported the isolation and characterization of six viruses that formed an orphan group with no strong similarity to previously described families. Since this first report, at least six new species of negeviruses have been discovered along with a number of reisolations of previously described negeviruses often from different countries and host species (Table 3).
Table 3.
Negeviruses published to date.
| VIRUS | ISOLATION REGION | COLLECTION DATE | ISOLATION HOST | REFERENCE |
|---|---|---|---|---|
| Nelorpivirus | ||||
| Brejeira | Brazil | Not specified |
Culex spp. Psorophora ferox* |
67 |
| Castlerea | Australia | 1988–2015 |
Aedes spp. Anopheles spp. Culiseta spp. Coquillettidia spp. Anopheles albimanus |
(O’Brien et al, submitted for this supplement) |
| Loreto | Peru | 1977, 1983 |
Culex spp. Lutzomyia spp. |
63 |
| Negev virus | Israel U.S.A Portugal |
1983, 2008 |
Anopheles coustani Culex quinquefasciatus Culex coronator Culex univittatus |
63,64 |
| Ngewotan ochlerotatus | Indonesia | 1981 | Culex vishnui | 63 |
| Caspius negevirus | Portugal | 2009 | Ochlerotatus caspius | 68 |
| Okushiri | Japan Republic of Korea | 2010, 2012 |
Aedes spp. (larvae) Culex pipiens |
69,70 |
| Piura | Peru Mexico | 1996, 2008 |
Culex spp. Mansonia spp. Wyeomyia spp. Trichoprosopon spp. Coquillettidia spp. Psorophora spp. |
63,71 |
| Sandewavirus | ||||
| Dezidougou | Côte d’Ivoire | 1987 | Aedes aegypti | 63 |
| Santana | Brazil | 1992 |
Culex spp. Culex spp. |
– |
| Wallerfield | Trinidad Brazil |
2007–2009 | Psorophora ferox* | 38,67 |
| Goutanap | Côte d’Ivoire | 2004 |
Culicidae spp. Culex quinquefasciatus |
71 |
| Tanay | Philippines | 2005 |
Culex spp. Armigeres spp. |
65 |
Bioinformatic analyses have indicated that the taxon negevirus is distantly related to the mite-transmitted, plant- infecting cileviruses and is likely to form two clades (Nelorpivirus and Sandewavirus). This group of viruses comprises a 9- to 10-kb positive-sense, single-stranded RNA genome with three open reading frames, two of which are believed to encode highly divergent structural proteins.63–65
Research into these viruses to date has been mostly in vitro and suggests that these viruses are restricted to insects with no growth reported in any vertebrate cell lines tested.63,64 The discovery of a new species of negevirus, tentatively named Castlerea virus, from Australian mosquitoes is described in a separate article in this supplement (O’Brien et al, submitted for this supplement).
Conclusions
With the application of deep sequencing approaches to virus discovery, many novel viral genomes have been detected in arthropod samples.42,61,66 While the outcomes of these studies have redefined the taxonomy and phylogenetics of mosquito-borne viruses at the genus and family level and provided a wealth of data for the analysis of their evolution, additional efforts to isolate these new viruses are essential to determine their phenotypic properties. These properties include host range, mode of transmission, potential for pathogenesis, as well as interaction with the mosquito host and viral pathogens vectored by the host.
Our studies and those of several other groups demonstrate that many ISVs are carried by mosquitoes and occur at high prevalence in some populations, apparently transmitted vertically. Furthermore, some of these viruses can regulate the transmission of pathogenic arboviruses in coinfected mosquitoes. Future studies should be directed at determining the mechanisms by which ISFs interfere with arbovirus transmission and the viral and host factors associated with their restriction to mosquito hosts and efficient vertical transmission. These studies will also require the development of essential ISV-specific research tools, including antibodies, molecular detection reagents, and reverse genetics systems.
Acknowledgments
The authors would like to thank Dr Sonja Hall-Mendelin for proof reading the manuscript.
Footnotes
ACADEMIC EDITOR: Liuyang Wang, Associate Editor
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 711 words, excluding any confidential comments to the academic editor.
FUNDING: This study was funded by the Australian Research Council (ARC DP120103994). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: RAH, HBO, NAP, JH-P. Analyzed the data: RAH, HBO, BJM, CAO, AMGC, JJH, NDN, NAP, JMD, MGM, JH-P. Wrote the first draft of the manuscript: RAH. Contributed to the writing of the manuscript: RAH, HBO, CAO, AMGC, TBHP, JJH, NDN, RTB, NAP, JH-P. Agreed with the manuscript results and conclusions: RAH, HBO, BJM, CAO, AMGC, TBHP, JJH, NDN, RTB, NAP, JMD, MGM, JH-P. Jointly developed the structure and arguments for the article: RAH, HBO, CAO, AMGC, JJH, NDN, NAP, JH-P. Made critical revisions and approved the final version: RAH, HBO, BJM, CAO, AMGC, TBHP, JJH, NDN, RTB, NAP, JMD, MGM, JH-P. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Hall RA, Macdonald J. Synthetic biology provides a toehold in the fight against Zika. Cell Host Microbe. 2016;19(6):752–4. doi: 10.1016/j.chom.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Blitvich BJ, Firth AE. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses. 2015;7(4):1927–59. doi: 10.3390/v7041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-specific virus discovery: significance for the Arbovirus Community. Viruses. 2015;7(9):4911–28. doi: 10.3390/v7092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall-Mendelin S, McLean BJ, Bielefeldt-Ohmann H, Hobson-Peters J, Hall RA, van den Hurk AF. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit Vectors. 2016;9(1):414. doi: 10.1186/s13071-016-1683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson-Peters J, Yam AW, Lu JW, et al. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS One. 2013;8(2):e56534. doi: 10.1371/journal.pone.0056534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, Blair CD. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427(2):90–7. doi: 10.1016/j.virol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goenaga S, Kenney JL, Duggal NK, et al. Potential for co-infection of a mosquito-specific flavivirus, Nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses. 2015;7(11):5801–12. doi: 10.3390/v7112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erasmus JH, Needham J, Raychaudhuri S, et al. Utilization of an Eilat virus-based chimera for serological detection of chikungunya infection. PLoS Negl Trop Dis. 2015;9(10):e0004119. doi: 10.1371/journal.pntd.0004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierson TC, Diamond MS. Fields Virology. 6th. ed. Philadelphia: Lippincott Williams & Wilkins; 2013. Flaviviruses. [Google Scholar]
- 10.Stollar V, Thomas VL. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology. 1975;64(2):367–77. doi: 10.1016/0042-6822(75)90113-0. [DOI] [PubMed] [Google Scholar]
- 11.Sang RC, Gichogo A, Gachoya J, et al. Isolation of a new flavivirus related to cell fusing agent virus (CFAV) from field-collected flood-water Aedes mosquitoes sampled from a dambo in central Kenya. Arch Virol. 2003;148(6):1085–93. doi: 10.1007/s00705-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree MB, Sang RC, Stollar V, Dunster LM, Miller BR. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch Virol. 2003;148(6):1095–118. doi: 10.1007/s00705-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 13.Cook S, Bennett SN, Holmes EC, De Chesse R, Moureau G, de Lamballerie X. Isolation of a new strain of the flavivirus cell fusing agent virus in a natural mosquito population from Puerto Rico. J Gen Virol. 2006;87(pt 4):735–48. doi: 10.1099/vir.0.81475-0. [DOI] [PubMed] [Google Scholar]
- 14.Saiyasombat R, Bolling BG, Brault AC, Bartholomay LC, Blitvich BJ. Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae) J Med Entomol. 2011;48(5):1031–8. doi: 10.1603/me11043. [DOI] [PubMed] [Google Scholar]
- 15.Tesh RB, Cornet M. The location of San Angelo virus in developing ovaries of transovarially infected Aedes albopictus mosquitoes as revealed by fluorescent antibody technique. Am J Trop Med Hyg. 1981;30(1):212–8. doi: 10.4269/ajtmh.1981.30.212. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien CA, Hobson-Peters J, Yam AW, et al. Viral RNA intermediates as targets for detection and discovery of novel and emerging mosquito-borne viruses. PLoS Negl Trop Dis. 2015;9(3):e0003629. doi: 10.1371/journal.pntd.0003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLean BJ, Hobson-Peters J, Webb CE, et al. A novel insect-specific flavivirus replicates only in Aedes-derived cells and persists at high prevalence in wild Aedes vigilax populations in Sydney, Australia. Virology. 2015;486:272–83. doi: 10.1016/j.virol.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Junglen S, Kopp A, Kurth A, Pauli G, Ellerbrok H, Leendertz FH. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from uranotaenia mosquitoes from a tropical rain forest. J Virol. 2009;83(9):4462–8. doi: 10.1128/JVI.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huhtamo E, Putkuri N, Kurkela S, et al. Characterization of a novel flavivirus from mosquitoes in northern Europe that is related to mosquito-borne flaviviruses of the tropics. J Virol. 2009;83(18):9532–40. doi: 10.1128/JVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney JL, Solberg OD, Langevin SA, Brault AC. Characterization of a novel insect-specific flavivirus from Brazil: potential for inhibition of infection of arthropod cells with medically important flaviviruses. J Gen Virol. 2014;95(pt 12):2796–808. doi: 10.1099/vir.0.068031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aliota MT, Jones SA, Dupuis AP, II, Ciota AT, Hubalek Z, Kramer LD. Characterization of Rabensburg virus, a flavivirus closely related to West Nile virus of the Japanese encephalitis antigenic group. PLoS One. 2012;7(6):e39387. doi: 10.1371/journal.pone.0039387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aliota MT, Kramer LD. Replication of West Nile virus, Rabensburg lineage in mammalian cells is restricted by temperature. Parasit Vectors. 2012;5:293. doi: 10.1186/1756-3305-5-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colmant AM, Bielefeldt-Ohmann H, Hobson-Peters J, et al. A newly discovered flavivirus in the yellow fever virus group displays restricted replication in vertebrates. J Gen Virol. 2016 doi: 10.1099/jgv.0.000430. [DOI] [PubMed] [Google Scholar]
- 24.Cook S, Moureau G, Kitchen A, et al. Molecular evolution of the insect-specific flaviviruses. J Gen Virol. 2012;93(pt 2):223–34. doi: 10.1099/vir.0.036525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol. 2001;75(16):7769–73. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seligman SJ. Constancy and diversity in the flavivirus fusion peptide. Virol J. 2008;5:27. doi: 10.1186/1743-422X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Liu Y, Wang S, et al. Structure-based mutational analysis of several sites in the E protein: implications for understanding the entry mechanism of Japanese encephalitis virus. J Virol. 2015;89(10):5668–86. doi: 10.1128/JVI.00293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gritsun TS, Gould EA. The 3’ untranslated regions of Kamiti River virus and Cell fusing agent virus originated by self-duplication. J Gen Virol. 2006;87(pt 9):2615–9. doi: 10.1099/vir.0.81950-0. [DOI] [PubMed] [Google Scholar]
- 29.Tree MO, McKellar DR, Kieft KJ, Watson AM, Ryman KD, Conway MJ. Insect-specific flavivirus infection is restricted by innate immunity in the vertebrate host. Virology. 2016;497:81–91. doi: 10.1016/j.virol.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Isawa H, Kuwata R, Tajima S, et al. Construction of an infectious cDNA clone of Culex flavivirus, an insect-specific flavivirus from Culex mosquitoes. Arch Virol. 2012;157(5):975–9. doi: 10.1007/s00705-012-1240-z. [DOI] [PubMed] [Google Scholar]
- 31.Setoh YX, Prow NA, Rawle DJ, et al. Systematic analysis of viral genes responsible for differential virulence between American and Australian West Nile virus strains. J Gen Virol. 2015;96(pt 6):1297–308. doi: 10.1099/vir.0.000069. [DOI] [PubMed] [Google Scholar]
- 32.Nasar F, Palacios G, Gorchakov RV, et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A. 2012;109(36):14622–7. doi: 10.1073/pnas.1204787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasar F, Erasmus JH, Haddow AD, Tesh RB, Weaver SC. Eilat virus induces both homologous and heterologous interference. Virology. 2015;484:51–8. doi: 10.1016/j.virol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasar F, Gorchakov RV, Tesh RB, Weaver SC. Eilat virus host range restriction is present at multiple levels of the virus life cycle. J Virol. 2015;89(2):1404–18. doi: 10.1128/JVI.01856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marklewitz M, Handrick S, Grasse W, et al. Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J Virol. 2011;85(17):9227–34. doi: 10.1128/JVI.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marklewitz M, Zirkel F, Rwego IB, et al. Discovery of a unique novel clade of mosquito-associated bunyaviruses. J Virol. 2013;87(23):12850–65. doi: 10.1128/JVI.01862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marklewitz M, Zirkel F, Kurth A, Drosten C, Junglen S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc Natl Acad Sci U S A. 2015;112(24):7536–41. doi: 10.1073/pnas.1502036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auguste AJ, Carrington CV, Forrester NL, et al. Characterization of a novel Negevirus and a novel Bunyavirus isolated from Culex (Culex) declarator mosquitoes in Trinidad. J Gen Virol. 2014;95(pt 2):481–5. doi: 10.1099/vir.0.058412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Junglen S. Evolutionary origin of pathogenic arthropod-borne viruses – a case study in the family Bunyaviridae. Curr Opin Insect Sci. 2016;16:81–6. doi: 10.1016/j.cois.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Hobson-Peters J, Warrilow D, McLean BJ, et al. Discovery and characterisation of a new insect-specific bunyavirus from Culex mosquitoes captured in northern Australia. Virology. 2016;489:269–81. doi: 10.1016/j.virol.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Chandler JA, Thongsripong P, Green A, et al. Metagenomic shotgun sequencing of a Bunyavirus in wild-caught Aedes aegypti from Thailand informs the evolutionary and genomic history of the Phleboviruses. Virology. 2014;46(4–465):312–9. doi: 10.1016/j.virol.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguiar ER, Olmo RP, Paro S, et al. Sequence-independent characterization of viruses based on the pattern of viral small RNAs produced by the host. Nucleic Acids Res. 2015;43(13):6191–206. doi: 10.1093/nar/gkv587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li CX, Shi M, Tian JH, et al. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife. 2015;4 doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung HC, Nguyen VG, Goede D, et al. Gouleako and Herbert viruses in pigs, Republic of Korea, 2013. Emerg Infect Dis. 2014;20(12):2072–5. doi: 10.3201/eid2012.131742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Junglen S, Marklewitz M, Zirkel F, et al. No evidence of Gouleako and Herbert virus infections in pigs, Cote d’Ivoire and Ghana. Emerg Infect Dis. 2015;21(12):2190–3. doi: 10.3201/eid2112.141840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van-den-Hurk AF, Ritchie SA, Johansen CA, Mackenzie JS, Smith GA. Domestic pigs and Japanese encephalitis virus infection, Australia. Emerg Infect Dis. 2008;14(11):1736–8. doi: 10.3201/eid1411.071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zirkel F, Kurth A, Quan PL, et al. An insect nidovirus emerging from a primary tropical rainforest. MBio. 2011;2(3):e77–e11. doi: 10.1128/mBio.00077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nga PT, Parquet Mdel C, Lauber C, et al. Discovery of the first insect nidovirus, a missing evolutionary link in the emergence of the largest RNA virus genomes. PLoS Pathog. 2011;7(9):e1002215. doi: 10.1371/journal.ppat.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauber C, Ziebuhr J, Junglen S, et al. Mesoniviridae: a proposed new family in the order Nidovirales formed by a single species of mosquito-borne viruses. Arch Virol. 2012;157(8):1623–8. doi: 10.1007/s00705-012-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zirkel F, Roth H, Kurth A, Drosten C, Ziebuhr J, Junglen S. Identification and characterization of genetically divergent members of the newly established family Mesoniviridae. J Virol. 2013;87(11):6346–58. doi: 10.1128/JVI.00416-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuwata R, Satho T, Isawa H, et al. Characterization of Dak Nong virus, an insect nidovirus isolated from Culex mosquitoes in Vietnam. Arch Virol. 2013;158(11):2273–84. doi: 10.1007/s00705-013-1741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warrilow D, Watterson D, Hall RA, et al. A new species of mesonivirus from the Northern Territory, Australia. PLoS One. 2014;9(3):e91103. doi: 10.1371/journal.pone.0091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vasilakis N, Guzman H, Firth C, et al. Mesoniviruses are mosquito-specific viruses with extensive geographic distribution and host range. Virol J. 2014;11:97. doi: 10.1186/1743-422X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attoui H, Mertens PPC, Becnel J, et al. Family – Reoviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowits EJ, editors. Virus Taxonomy: Ninth Report of the International Committee for the Taxonomy of Viruses. San Diego: Elsevier; 2012. pp. 541–637. [Google Scholar]
- 55.Harrison JJ, Warrilow D, McLean BJ, et al. A new orbivirus isolated from mosquitoes in North-Western Australia shows antigenic and genetic similarity to corriparta virus but does not replicate in vertebrate cells. Viruses. 2016;8(5):141–56. doi: 10.3390/v8050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Attoui H, Mohd Jaafar F, Belhouchet M, et al. Expansion of family Reoviridae to include nine-segmented dsRNA viruses: isolation and characterization of a new virus designated Aedes pseudoscutellaris reovirus assigned to a proposed genus (Dinovernavirus) Virology. 2005;343(2):212–23. doi: 10.1016/j.virol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 57.Auguste AJ, Kaelber JT, Fokam EB, et al. A newly isolated reovirus has the simplest genomic and structural organization of any reovirus. J Virol. 2015;89(1):676–87. doi: 10.1128/JVI.02264-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hermanns K, Zirkel F, Kurth A, Drosten C, Junglen S. Cimodo virus belongs to a novel lineage of reoviruses isolated from African mosquitoes. J Gen Virol. 2014;95(pt 4):905–9. doi: 10.1099/vir.0.062349-0. [DOI] [PubMed] [Google Scholar]
- 59.Attoui H, Billoir F, Biagini P, de Micco P, de Lamballerie X. Complete sequence determination and genetic analysis of Banna virus and Kadipiro virus: proposal for assignment to a new genus (Seadornavirus) within the family Reoviridae. J Gen Virol. 2000;81(pt 6):1507–15. doi: 10.1099/0022-1317-81-6-1507. [DOI] [PubMed] [Google Scholar]
- 60.Xu PT, Wang YM, Zuo JM, Lin JW, Xu PM. New orbiviruses isolated from patients with unknown fever and encephalitis in Yunnan Province. Chin J Virol. 1990;6(1):27–33. [Google Scholar]
- 61.Coffey LL, Page BL, Greninger AL, et al. Enhanced arbovirus surveillance with deep sequencing: identification of novel rhabdoviruses and bunyaviruses in Australian mosquitoes. Virology. 2014;448:146–58. doi: 10.1016/j.virol.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Attoui H, Mohd Jaafar F, Belhouchet M, et al. Liao ning virus, a new Chinese seadornavirus that replicates in transformed and embryonic mammalian cells. J Gen Virol. 2006;87(pt 1):199–208. doi: 10.1099/vir.0.81294-0. [DOI] [PubMed] [Google Scholar]
- 63.Vasilakis N, Forrester NL, Palacios G, et al. Negevirus: a proposed new taxon of insect-specific viruses with wide geographic distribution. J Virol. 2013;87(5):2475–88. doi: 10.1128/JVI.00776-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carapeta S, do Bem B, McGuinness J, et al. Negeviruses found in multiple species of mosquitoes from southern Portugal: isolation, genetic diversity, and replication in insect cell culture. Virology. 2015;483:318–28. doi: 10.1016/j.virol.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 65.Nabeshima T, Inoue S, Okamoto K, et al. Tanay virus, a new species of virus isolated from mosquitoes in the Philippines. J Gen Virol. 2014;95(pt 6):1390–5. doi: 10.1099/vir.0.061887-0. [DOI] [PubMed] [Google Scholar]
- 66.Shi M, Lin XD, Vasilakis N, et al. Divergent viruses discovered in arthropods and vertebrates revise the evolutionary history of the Flaviviridae and related viruses. J Virol. 2016;90(2):659–69. doi: 10.1128/JVI.02036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nunes MR, Silva SP, Carvalho VL, et al. Emergence of new insect-restrictive viruses in the Amazon region. Genome Announc. 2015;3(2) doi: 10.1128/genomeA.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferreira DD, Cook S, Lopes A, et al. Characterization of an insect-specific flavivirus (OCFVPT) co-isolated from Ochlerotatus caspius collected in southern Portugal along with a putative new Negev-like virus. Virus Genes. 2013;47(3):532–45. doi: 10.1007/s11262-013-0960-9. [DOI] [PubMed] [Google Scholar]
- 69.Kawakami K, Kurnia YW, Fujita R, et al. Characterization of a novel negevirus isolated from Aedes larvae collected in a subarctic region of Japan. Arch Virol. 2016;161(4):801–9. doi: 10.1007/s00705-015-2711-9. [DOI] [PubMed] [Google Scholar]
- 70.Hang J, Klein TA, Kim HC, et al. Genome sequences of five Arboviruses in field-captured mosquitoes in a Unique Rural Environment of South Korea. Genome Announc. 2016;4(1) doi: 10.1128/genomeA.01644-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kallies R, Kopp A, Zirkel F, et al. Genetic characterization of goutanap virus, a novel virus related to negeviruses, cileviruses and higreviruses. Viruses. 2014;6(11):4346–57. doi: 10.3390/v6114346. [DOI] [PMC free article] [PubMed] [Google Scholar]