Abstract
Objective
To identify patterns of use and predictors of non-adherence to lipid lowering therapy (LLT) in commercially insured children ages 8 to 20, and the high-risk subgroup with known dyslipidemia.
Design, Setting and Participants
Commercially insured patients with a new dispensing for a LLT were included. Non-adherence was defined as a gap of ≥90 days between the last dispensing plus the medication days-supply and the next dispensing or censoring. Descriptive statistics characterize the patterns of LLT (statin, non-statin, statin combination and non-statin combination) adherence, adding, and class or specific drug switching. Kaplan-Meier curves and multivariable Cox proportional hazard models were used to identify time to, and predictors of, non-adherence for the cohort and the dyslipidemia subgroup.
Results
Of the 8,710 patients who met inclusion criteria, 87.9% were non-adherent during the study period. Statins were the most common index prescription and patients with an index statin dispensing were more likely to have multiple comorbidities and other prescription drug use. In multivariable analyses, non-adherence was inversely associated with dyslipidemia (HR 0.61, 95% CI 0.57–0.65), chronic kidney disease (HR 0.69, 95% CI 0.54–0.88) and higher outpatient (HR 0.87, 95% CI 0.77–0.98) and inpatient (HR 0.83, 95% CI 0.70–0.97) use. When limited to patients with dyslipidemia, non-adherence was related to age (HR 1.21, 95% CI 1.07–1.38) and obesity (HR 1.23 95% CI 1.02–1.49).
Conclusions and Relevance
Despite recommendations to begin continuous treatment early for high-risk children, non-adherence to LLT is frequent in this population, with modestly higher adherence in children with diagnosed dyslipidemia.
INTRODUCTION
The National Heart, Lung, and Blood Institute and American Academy of Pediatrics (AAP) recommends lipid lowering therapy (LLT) for treatment of severe dyslipidemia in children starting at ages 8 to 10 when diet and exercise have failed to sufficiently reduce low-density-lipoprotein cholesterol (LDL-C).1,2 However, these recommendations are controversial and use of LLT in children and adolescents is rare.3,4
In adults, the benefit of LLT in reducing the risk of cardiovascular disease (CVD) depends considerably on medication adherence. Yet adherence to LLT is low or moderate with long-term rates between 36% and 88%.5–11 Similar to adults, there is evidence to suggest that in children the benefits of LLT require consistent use and that the risk of CVD in children and adolescents is cumulative.12–14 For example, a recent study by Ferrence, et al., found that subjects with a gene-variant resulting in lower LDL-C from birth had significantly fewer coronary events relative to subjects who began LDL-C lowering with statins later in life.13
To date, however, the only study of adherence to LLT during childhood is a ten-year follow-up study of 214 children who participated in a 2-year clinical trial for the safety and efficacy of pravastatin.15 Although the authors found that 88.8% of study subjects were still using LLT after 10 years, the generalizability of the results to the population outside of the clinical trial setting is limited.16
Interest in population based measures of medication adherence during childhood has grown as the prevalence of chronic conditions in childhood has increased over the past two decades.17 However, studies have found mixed results with rates of adherence ranging from 25% to 88%.18,19 Further, research has tended to focus on childhood conditions in which non-adherence can result in serious acute effects, such as asthma or diabetes.19–21 In contrast, the benefits of LLT during childhood are unlikely to be realized for another 20 to 30 years, resulting in substantially different incentives for medication adherence. Thus, studies of other chronic medications during childhood may not be applicable and additional work is needed to identify possible avenues for improvement in very high-risk children, especially those with genetic dyslipidemias.
The objective of this study was to describe patterns of prescribing and adherence to LLT in a population of commercially insured children ages 8 to 20 in both the full cohort of children prescribed LLT during the study period as well as the subgroup of patients with a diagnosis for dyslipidemia. To achieve this objective, we used a large national database of private insurance claims between 2003 and 2013, a period in which the recommendations for pharmacological treatment transitioned from the 1992 NCEP and 1998 AAP guidelines recommending bile-acid sequestrants as first line agents to the 2008 AAP and 2011 NHLBI guidelines, which recommended statins as a first line treatment.
METHODS
Data sources and study population
All data are from the Marketscan Research Database for calendar years 2004 to 2013. The Marketscan Research Database is a database of employer-based health insurance claims that contains all reimbursable health care claims, including prescription medication dispensings, filled for employees and their dependents.
Our study population consisted of all enrollees ages 8 to 20 who met two criteria: 1) a new LLT dispensing, defined as the first (“index”) dispensing after a minimum of 12 months of continuous enrollment (the baseline period), and 2) a minimum 12 months of follow-up time following the index dispensing. We assessed the presence of the following clinical conditions prior to the index dispensing: dyslipidemia, diabetes, obesity, hypertension, metabolic syndrome, asthma, chronic kidney disease (CKD), depression and attention deficit hyperactive disorder (ADHD). A condition was considered present if the patient had two outpatient claims within a 24-month period, with the first occurring prior to the index dispensing, or a single inpatient claim prior to the index dispensing. Additionally, in the 12 months prior to the index dispensing, we measured the following prescription drug dispensings and measures of health care utilization in the year prior to the index dispensing; prescription dispensings for diabetes, hypertension, asthma, depression and anti-psychotics; the presence of a recorded screening for diabetes or a lipid panel; and the number of outpatient, inpatient and prescription drug dispensings other than LLT.
Classification of LLT
We classified each LLT dispensing first by class (statins, non-statins, statin combinations, and non-statin combinations which included both single pill combinations and different class dispensings which occurred within 7 days of each other) and then by the specific drug within each class. We calculated the proportion of patients with more than one dispensing during the follow up period by the type of index dispensing. Among patients with multiple dispensings we further identified the presence of subsequent switching or adding of a medication different from the index class.
Measures of Non-adherence
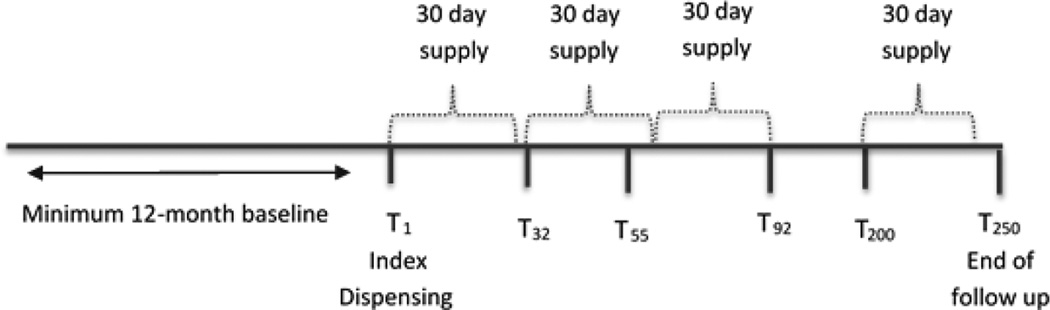
An episode of non-adherence was defined as a gap of a minimum 90 days between the last covered day of treatment and the next filled prescription or the end of follow up. Covered days of treatment were calculated by adding the days-supply for the prescription to the date the prescription was filled. If a prescription was filled early, the overlap was added to the number of days-supply based on the assumption that the patient would start the new prescription once the current prescription was finished. We defined the start of non-adherence conservatively as the date of last dispensing plus the days-supply, which assumes that the patient took the full course of LLT before discontinuing (figure 1).22 Gaps <90 days were not counted as non-adherent and although patients could reinitiate treatment, patients were censored after the first non-adherent episode. In sensitivity analyses this definition was extended to 120 days.
Figure 1. Timeline of LLT therapy illustrating definition of treatment non-adherence.
T1 represents the date of the index prescription. At T32 the patient refills the prescription, leaving a 2 day gap in the end of the first prescription and start of the second. However, because this gap is less than 90 days the patient is not considered to have had an episode of non-adherence. The patient then fills the next prescription at T55, 7 days earlier than the end of the 2nd prescription at T62. This overlap of seven days is then added to the 30 days supply and the end of covered treatment days is now T92(T55 + 7 days of overlap + 30 day supply). The next prescription is filled at T200, which is more than 90 days from the end of the last covered day of treatment (T92) and thus the patient is considered to be non-adherent on day 92.
Analysis
Patient level covariates were compared across type of index LLT using chi-square tests for comparisons. Kaplan-Meier curves were used to estimate time to non-adherence stratified by the presence of a dyslipidemia diagnosis and the index LLT class. Non-statin combinations were omitted from Kaplan-Meier analyses due to small sample size. Multivariable Cox-proportionate hazard models were used to estimate the hazard of non-adherence in the full population and the sub-population of patients with a dyslipidemia diagnosis. Multivariable Cox models were adjusted for 1) age sex, type of index LLT 2) a diagnosis of dyslipidemia, obesity, hypertension, metabolic syndrome, diabetes, asthma, CKD, depression or ADHD 3) a prescription drug dispensing within the 12 months prior to the index date for an anti-hypertensive, anti-asthmatic, anti-diabetic, anti-depressant or antipsychotic 4) a recorded diabetes screening or lipid panel 5) measures of health utilization in the 12 months prior to the index dispensing including outpatient, inpatient and prescription drugs other than LLT. All analyses were conducted using SAS 9.4 (Cary, NC).
RESULTS
Baseline characteristics and initial LLT patterns
There were a total of 19,157,497 children ages 8 to 20 between 2004 and 2012 with at least 2 years of continuous enrollment. Of these, we identified 8,710 children and adolescents with a new dispensing for LLT between 2005 and 2012 who had at least 12 months of continuous enrollment without a dispensing for an LLT prior to the index dispensing (new users) and a minimum 12 months of follow up after the index dispensing. Among children initiating LLT, the majority of patients were first prescribed a statin (51.2%) or a non-statin (44.1%), with a small proportion receiving prescriptions for combination therapy (4.5% statin and non-statin combination, and 0.2% non-statin combination) (table 1). Compared to patients with an index dispensing for a non-statin, patients with an index statin dispensing were more likely to have diagnosed comorbidities with the exception of depression. Patients with an index dispensing for a statin were also more likely to have dispensings for other studied medications (except anti-psychotics), to have been screened for diabetes or had a lipid panel, to have received more than one outpatient visit, and to have a prescription drug dispensing other than LLT in the past year (p<0.001 for all comparisons) (table 1). Results were similar with respect to comorbidities and health care utilization for patients with an index dispensing for a statin-combination as compared to a non-statin (table 1).
Table 1.
Characteristics of the study cohort by type of index lipid lowering therapy (LLT) among new users of LLT ages 8 to 20
| Non-Statin | Statin | Statin and Non- Statin Combination |
Non-Stain Combination |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| N | 3,837 | 44.1% | 4,461 | 51.2% | 393 | 4.5% | 19 | 0.2% |
| Demographics | ||||||||
| Female | 1,975 | 51.5% | 1,868 | 41.9% | 131 | 33.3% | 5 | 26.3% |
| Age ≥15 | 3,081 | 80.3% | 3,397 | 76.2% | 294 | 74.8% | 13 | 68.4% |
| Diagnosed conditions | ||||||||
| Dyslipidemia | 629 | 16.4% | 1,444 | 32.4% | 128 | 32.6% | 10 | 52.6% |
| Obesity | 117 | 3.1% | 198 | 4.4% | 12 | 3.1% | 0 | 0.0% |
| Hypertension | 149 | 3.9% | 322 | 7.2% | 25 | 6.4% | 1 | 5.3% |
| Metabolic Syndrome | 39 | 1.0% | 90 | 2.0% | 8 | 2.0% | 1 | 5.3% |
| Diabetes | 226 | 5.9% | 529 | 11.9% | 45 | 11.5% | 3 | 15.8% |
| Asthma | 341 | 8.9% | 287 | 6.4% | 24 | 6.1% | 1 | 5.3% |
| Chronic Kidney Disease | 7 | 0.2% | 90 | 2.0% | 2 | 0.5% | 0 | 0.0% |
| Depression | 261 | 6.8% | 275 | 6.2% | 20 | 5.1% | 0 | 0.0% |
| ADHD | 172 | 4.5% | 252 | 5.7% | 19 | 4.8% | 1 | 5.3% |
| Medications dispensed* | ||||||||
| Anti-Hypertensive | 639 | 16.7% | 1,333 | 29.9% | 113 | 28.8% | 5 | 26.3% |
| Anti-Asthmatic | 1,797 | 46.8% | 1,851 | 41.5% | 185 | 47.1% | 9 | 47.4% |
| Anti-Diabetic | 307 | 8.0% | 913 | 20.5% | 61 | 15.5% | 3 | 15.8% |
| Anti-Depressant | 1,199 | 31.3% | 1,037 | 23.3% | 113 | 28.8% | 3 | 15.8% |
| Anti-Psychotic | 260 | 6.8% | 296 | 6.6% | 32 | 8.1% | 2 | 10.5% |
| Procedures* | ||||||||
| Diabetes screening | 976 | 25.4% | 1,552 | 34.8% | 126 | 32.1% | 6 | 31.6% |
| Lipid panel | 1,730 | 45.1% | 2,758 | 61.8% | 227 | 57.8% | 15 | 79.0% |
| Health Care Utilization* | ||||||||
| Outpatient visits | ||||||||
| 0 | 100 | 2.6% | 247 | 5.5% | 25 | 6.4% | 1 | 5.3% |
| 1–3 | 622 | 16.2% | 1,105 | 24.8% | 134 | 34.1% | 6 | 31.6% |
| 4–5 | 515 | 13.4% | 754 | 16.9% | 63 | 16.0% | 2 | 10.5% |
| ≥6 | 2,600 | 67.8% | 2,355 | 52.8% | 171 | 43.5% | 10 | 52.6% |
| Inpatient Visits | ||||||||
| 0 | 3,318 | 86.5% | 4,046 | 90.7% | 368 | 93.6% | 16 | 84.2% |
| 1–2 | 425 | 11.1% | 323 | 7.2% | 22 | 5.6% | 3 | 15.8% |
| ≥3 | 94 | 2.4% | 92 | 2.1% | 3 | 0.8% | 0 | 0.0% |
| Rx Dispensing | ||||||||
| 0 | 184 | 4.8% | 329 | 7.4% | 30 | 7.6% | 4 | 21.1% |
| 1–2 | 438 | 11.4% | 740 | 16.6% | 82 | 20.9% | 1 | 5.3% |
| ≥3 | 3,215 | 83.8% | 3,392 | 76.0% | 281 | 71.5% | 14 | 73.7% |
In the 12 months prior to index dispensing
Among patients with an index dispensing of statins, the most common prescriptions were atorvastatin (37%) or simvastatin (35%) (Table 2). Among patients with an index dispensing of non-statins, the majority were prescribed bile acid sequestrants (59%), while approximately a quarter of patients (29%) were prescribed fibric acid derivatives. The most commonly prescribed statin-combination was statin plus ezetimibe (75%) (table 2).
Table 2.
Index Lipid Lowering Therapy (LLT) and percent that of index LLT that are not a single dispensing by type of class and type LLT
| First Recorded LLT Dispensing |
Percent of patients with more than one dispensings |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Statin | 4,461 | 51.2% | 3,366 | 75.5% |
| Simvastatin | 1,555 | 34.9% | 1,145 | 73.6% |
| Atorvastatin | 1,651 | 37.0% | 1,283 | 77.7% |
| Lovastatin | 286 | 6.4% | 205 | 71.7% |
| Fluvastatin | 21 | 0.5% | 14 | 66.7% |
| Pravastatin | 488 | 10.9% | 381 | 78.1% |
| Pitavastatin | 3 | 0.1% | 2 | 66.7% |
| Rosuvastatin | 457 | 10.2% | 336 | 73.5% |
| Non-Statin | 3,837 | 44.1% | 1,929 | 50.3% |
| Bile Acid Sequestrants | 2,273 | 59.2% | 837 | 36.8% |
| Fibric Acid Derivatives | 1,102 | 28.7% | 789 | 71.6% |
| Ezetimibe | 233 | 6.1% | 181 | 77.7% |
| Niacin | 229 | 6.0% | 122 | 53.3% |
| Stain Combination | 393 | 4.5% | 278 | 70.7% |
| Statin and Bile Acid Sequestrant | 2 | 0.5% | 0 | 0.0% |
| Statin and Fibric Acid | 36 | 9.2% | 2 | 5.6% |
| Statin and Ezetimibe | 295 | 75.1% | 211 | 71.5% |
| Statin and Niacin | 60 | 15.3% | 42 | 70.0% |
| Non Statin Combinations | 19 | 0.2% | 15 | 78.9% |
| Niacin & Ezetimibe | 1 | 5.3% | 1 | 100.0% |
| Niacin & Fibric Acid | 9 | 47.4% | 6 | 66.7% |
| Bile Acid Sequestrant & Fibric Acid | 1 | 5.3% | 0 | 0.0% |
| Ezetimibe & Fibric Acid | 6 | 31.6% | 6 | 100.0% |
| Ezetimibe & Bile Acid Sequestrant | 2 | 10.5% | 2 | 100.0% |
note: there were no niacin and bile acid sequestrant non-statin combinations
Patterns of Non-adherence
Although the proportion of patients with an initial dispensing for statins and non-statins were comparable, 76% of statin users had a second dispensing versus only 50% of non-statin users (table 2). Among those with an index dispensing for statins, there proportion receiving second dispensings varied from 66.7% for Fluvastatin to 78.1% for Pravastatin. In contrast, among patients with an index dispensing for non-statins, the proportion with second dispensings varied ranged from 36.8% for bile acid sequestrants to 78% for ezetimibe. Among patients with 2 or more dispensings, switching or adding a different type of LLT after the index prescription was uncommon, with less than 10% of patients switching from a statin to a non-statin or vice versa (supplementary table 1).
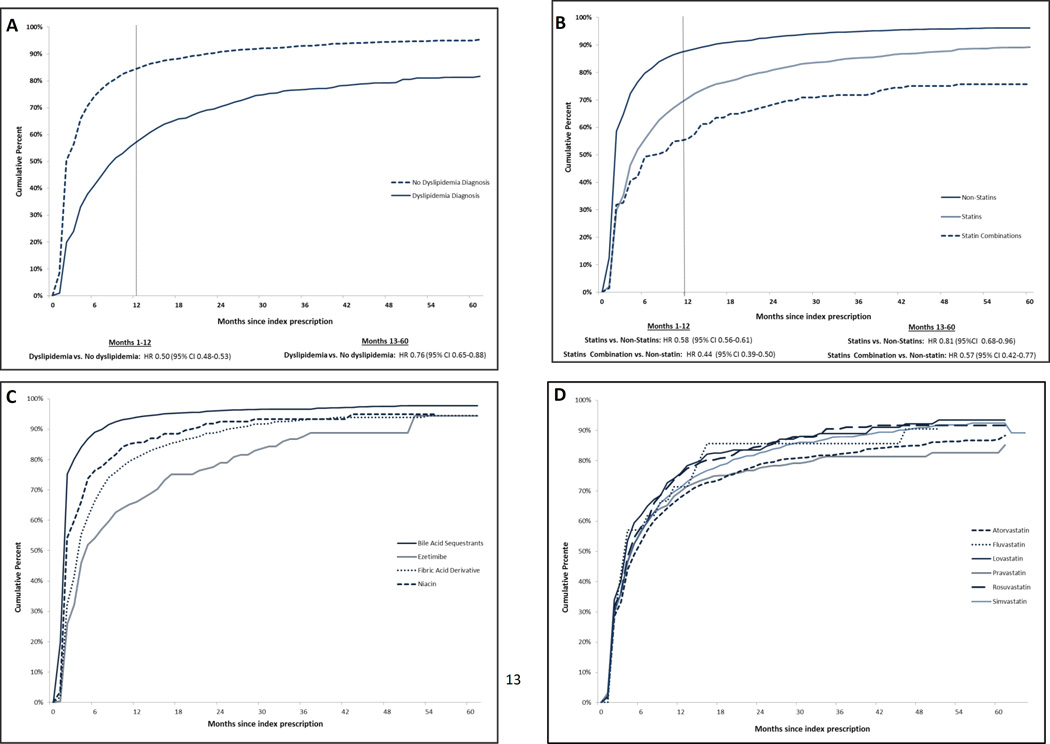
During follow-up (median 30 months, IQR 20–48), at least one episode of non-adherence occurred in 7,583 (87%) patients. Of these, 6,864 (90%) of the non-adherence episodes occurred within the first year after the index dispensing. Patients with a recorded diagnosis of dyslipidemia were less likely to have a non-adherence episode compared to patients without a dyslipidemia diagnosis (p<0.001 by the log-rank test), although this difference was slightly attenuated, yet still statistically significant, after the first year (unadjusted hazard ratio [HR] 0.50, 95% CI 0.48–0.53 in the first year, compared to HR 0.76, 95% CI 0.65–0.88 after the first year) (figure 2, panel A).
Figure 2.
Cumulative percent of patients with an episode of medication non-adherence lasting at least 90 days stratified by (A) A diagnosis of dyslipidemia (B) The index dispensing’s class of LLT (C) the type of non-statin LLT index dispensing among patients whose index dispensing was a non-statin (D) The type of statin index dispensing among patients whose index dispensing was a statin.
Compared to an index dispensing of non-statin medications, an index dispensing of statins or statin-combinations was less likely to be followed by non-adherence episode (p<0.001 by the log-rank test). Again, this effect was stronger in the first year after the index dispensing (HR comparing statin vs non-statin users: 0.58, 95%: CI 0.56–0.61 in the first 12 months vs. HR 0.81, 95% CI 0.68–0.96 after 12 months; HR comparing statin combination vs non-statin users: HR 0.44, 95% CI 0.39–0.50 in the first 12 months vs. HR 0.57 95%CI 0.42–0.77 after 12 months) (figure 2, panel B). With respect to specific medicines, bile acid sequestrants were commonly discontinued, while non-adherence did not differ by specific statin (figure 2, panels C and D).
In multivariable analyses, older adolescents (HR 1.06, 95% CI 1.01–1.13) were more likely to be non-adherent, while patients with dyslipidemia (HR 0.61, 95% CI 0.57–0.65), chronic kidney disease (HR 0.69, 95% CI 0.54–0.88), a lipid panel (HR 0.66 95% CI 0.62–0.69), six or more outpatient visits (HR 0.87 95% CI 0.77–0.98), three or more inpatient visits (HR 0.83, 95% CI 0.70–0.97), an anti-hypertensive dispensing (HR 0.90, 95% CI .85–0.96), or anti-psychotic (HR 0.89, 95% CI 0.79–0.99) in the year prior to the index dispensing and patients were less likely to be non-adherent. Additionally, patients whose index dispensing was a statin (HR 0.66, 95% CI 0.63–0.69), or statin combination (HR 0.46, 95% CI 0.40–0.52) were also less likely to be non-adherent while patients with 3 or more additional prescription dispensings in the last year were more likely experience an episode of non-adherence (HR 1.13, 95% CI 1.02–1.25) (table 3).
Table 3.
Multivariable hazard ratios for the outcome discontinuation of lipid lowering therapy among children ages 8 to 20 with at least 12 months of follow up after their index dispensing - whole population and subpopulation of patients with diagnosis of dyslipidemia prior to the index dispensing
| Total Population (n=8,710) |
Population with a dyslipidemia dianosis (n=2,211) |
|||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | |
| Demographics | ||||||
| Female | 1.03 | (0.98–1.08) | 0.26 | 0.89 | (0.80–0.99) | 0.03 |
| Age ≥15 | 1.06 | (1.01–1.13) | 0.03 | 1.21 | (1.07–1.38) | 0.00 |
| Index LLT | ||||||
| Non-statin | Ref | -- | -- | Ref | -- | -- |
| statin | 0.66 | (0.63–0.69) | <.0001 | 0.76 | (0.68–0.84) | <.0001 |
| Statin Combination | 0.46 | (0.40–0.52) | <.0001 | 0.36 | (0.27–0.47) | <.0001 |
| Non-Statin Combination | 0.86 | (0.53–1.38) | 0.52 | 1.02 | (0.51–2.07) | 0.95 |
| Diagnosed conditions | ||||||
| Dyslipidemia | 0.61 | (0.57–0.65) | <.0001 | -- | -- | -- |
| Obesity | 0.99 | (0.88–1.13) | 0.92 | 1.23 | (1.02–1.49) | 0.03 |
| Hypertension | 1.02 | (0.91–1.13) | 0.76 | 1.15 | (0.95–1.39) | 0.16 |
| Metabolic Syndrome | 0.92 | (0.76–1.12) | 0.40 | 0.99 | (0.74–1.32) | 0.92 |
| Diabetes | 1.02 | (0.92–1.14) | 0.69 | 1.12 | (0.92–1.37) | 0.27 |
| Asthma | 1.07 | (0.98–1.16) | 0.16 | 1.04 | (0.85–1.27) | 0.72 |
| Chronic Kidney Disease | 0.69 | (0.54–0.88) | 0.00 | 0.85 | (0.47–1.52) | 0.57 |
| Depression | 1.08 | (0.96–1.21) | 0.19 | 1.12 | (0.88–1.42) | 0.37 |
| ADHD | 0.93 | (0.83–1.05) | 0.24 | 0.81 | (0.64–1.03) | 0.08 |
| Medications dispensed* | ||||||
| Anti-Hypertensive | 0.90 | (0.85–0.96) | 0.00 | 0.83 | (0.72–0.97) | 0.02 |
| Anti-Asthmatic | 0.99 | (0.94–1.04) | 0.75 | 0.94 | (0.84–1.05) | 0.25 |
| Anti-Diabetic | 0.95 | (0.86–1.04) | 0.24 | 1.11 | (0.92–1.34) | 0.29 |
| Anti-Depressant | 0.96 | (0.90–1.02) | 0.16 | 0.99 | (0.86–1.14) | 0.87 |
| Anti-Psychotic | 0.89 | (0.79–0.99) | 0.03 | 0.82 | (0.64–1.03) | 0.09 |
| Procedures* | ||||||
| Diabetes screening | 0.96 | (0.90–1.02) | 0.17 | 0.97 | (0.86–1.09) | 0.59 |
| Lipid panel | 0.66 | (0.62–0.69) | <.0001 | 0.59 | (0.51–0.67) | <.0001 |
| Health Care Utilization* | ||||||
| Outpatient visits | ||||||
| 0 | Ref | -- | Ref | -- | ||
| 1–3 | 0.96 | (0.86–1.08) | 0.52 | 0.84 | (0.45–1.54) | 0.56 |
| 4–5 | 0.93 | (0.82–1.05) | 0.23 | 0.91 | (0.49–1.67) | 0.75 |
| ≥6 | 0.87 | (0.77–0.98) | 0.02 | 0.87 | (0.47–1.58) | 0.64 |
| Inpatient Visits | ||||||
| 0 | Ref | -- | Ref | -- | ||
| 1–2 | 1.02 | (0.93–1.10) | 0.72 | 1.01 | (0.80–1.27) | 0.96 |
| ≥3 | 0.83 | (0.70–0.97) | 0.02 | 1.11 | (0.67–1.84) | 0.68 |
| Drug Dispensing | ||||||
| 0 | Ref | -- | Ref | -- | ||
| 1–2 | 1.07 | (0.96–1.20) | 0.21 | 1.00 | (0.82–1.23) | 0.98 |
| ≥3 | 1.13 | (1.02–1.25) | 0.02 | 1.06 | (0.88–1.28) | 0.51 |
In the 12 months prior to index dispensing
When analyses were restricted to youth with a recorded diagnosis of dyslipidemia, older patients (HR 1.21, 95% CI 1.07–1.38) and patients with an obesity diagnosis (HR 1.23 95% CI 1.02–1.49) were less likely to be adherent. Patients with a dispensing for an anti-hypertensive (HR 0.83, 95% CI 0.72–0.97) or a lipid panel (HR 0.59, 95% CI 0.51–0.67) in the prior year, as well as patients whose index dispensing was for a statin (HR 0.76, 95% CI 0.68–0.84) or a statin combination (HR 0.36, 95% CI 0.27–0.47) were less likely be non-adherent. In sensitivity analyses we extended the definition of non-adherence to a gap of 120 days and the results were similar (supplementary table 2).
DISCUSSION
In a large, national database of private insurance claims, we found that despite recommendations to begin continuous treatment for high-risk children and adolescents, LLT use is rare and those who do initiate treatment tend to become non-adherent. Specifically, we found that among children and adolescents aged 8 to 20 years with at least one dispensing for LLT, 88% experienced an episode of non-adherence during a median follow-up time of 30 months with 91% of those patients experiencing an episode of non-adherence in the first year after the index dispensing.
Though not studied to the extent it is in adults, medication adherence in childhood is gaining prominence as the percent of children with a chronic illness has increased over the past two decades23 and studies have pointed out the need for additional information on predictors of adherence.19,20,24,25 Our results suggest that high-risk patients - those with dyslipidemia, CKD, outpatient and inpatient care and prescription drug use - are less likely to have an episode of non-adherence. Additionally, initiation with a statin-combination is associated with lower non-adherence, which may reflect that combination patients are more severely dyslipidemic, although greater tolerability of statin combination drugs cannot be ruled out. However, even among patients with the most possible to gain from LLT use, rates of adherence were low.
Although we could not confirm a diagnosis of FH among patients with dyslipidemia, 55% of patients with dyslipidemia in our sample had a claim for FH in specific (ICD-9 code 272.0), suggesting that FH makes up a substantial proportion of this group. The low rates of adherence in our analysis may explain why studies have found that among children prescribed statins both with and without a diagnosis of FH, stabilization of LDL-C at 130 mg/dL was low.27 Additionally, patients initiating use with a statin combination
When we limited the sample to only patients with dyslipidemia, only age and obesity were associated with greater adherence, suggesting that a diagnosis of dyslipidemia alone is a strong predictor of adherence, regardless of whether or not patients experience additional risk factors. Our results are consistent with those found in adults, in which patients taking LLT for primary prevention are less likely to be adherent than secondary prevention patients, such as the patients with dyslipidemia in our population.8
The distribution of index prescriptions by LLT class is notable given the relatively high frequency of non-statin dispensings in this population (44.1% of all index dispensings). Although generally efficacious, bile acid sequestrants were removed from first line therapy status in guidelines released in 2008 and 2011 due to comparatively poor tolerability.2,26,28 However, fibric acid derivatives are not approved and not widely recommended for use in children, and Niacin is explicitly cautioned against for use in children due to the high prevalence of serious side effects.2,28 The frequency of non-statin prescribing may reflect provider discomfort with prescribing statins to developing children.
Indeed, the higher rate of statin relative to non-statin dispensings in males than in females is consistent with previous hypotheses that statins might interfere with cholesterol derived sex hormones and consequent sexual maturation, although multiple studies in children with familial hypercholesterolemia have shown sex hormones and development to be largely unaffected.15,29
In addition to gender, there were significant differences in patient characteristics between patients with an index statin or statin-combination dispensing as compared to an index non-statin dispensing. In particular the lower prevalence of dyslipidemia in the non-statin group as well as the low prevalence of multiple dispensings following an index dispensing for a bile acid sequestrant suggest that some proportion of prescribing may be for conditions other than dyslipidemia, such as chronic diarrhea.30 Similarly, given the high prevalence of concomitant medications, it is possible that LLT is being prescribed to address increases in lipid levels secondary to temporary medical conditions or medication due to medications, such as corticosteroids, and thus only be prescribed for a limited time period, however appropriate or inappropriate.31
The risk of side effects associated with statin use is low, suggesting that the high rates of non-adherence are not likely due to side effects. However, it is also possible that the relatively higher rates of non-adherence among non-statin initiators may reflect the poorer tolerability in this class of LLT.1,2,28
There is no single accepted method for measuring adherence to medication, and often the choice of method depends on the goal of the analysis.22,32 Patients who discontinue medication may have been highly persistent users up until that point, while other patients may have much more intermittent use but never fully discontinue medication use. Interventions to increase medication adherence are increasingly focused on identifying characteristics associated with non-adherence and tailoring approaches towards individuals.33 Our finding that patients with greater physician contact have higher rates of adherence suggests that there may be an important role for providers in developing interventions.
Medication adherence in childhood can also be influenced by the parent-child dynamic. For example parental concern over asthma medication is associated with lower rates of adherence,34,35 and as children gain more autonomy during adolescence, rates of adherence to chronic medication decline, which is in-line with our finding that older patients were more likely to be non-adherent.36,37 Indeed studies demonstrating the influence of parental beliefs on child adherence to medication would suggest that identifying parental characteristics associated with non-adherence may be important in increasing adherence in children.34
Because of their large size and comprehensive information on prescription medications that are filled, administrative databases are frequently used in the study of medication adherence. However, it is important to note their limitations. First, it is not possible to confirm that patients are actually taking medication, only that they are refilling prescriptions. However, a study comparing prescription refill rates with electronic devices that monitor the date and time when a medication container is opened, found concordance of the measures.32 Prescription refill rates are also notably better for measure adherence to chronic medications, such as statins, than to acute medication such as a short course of antibiotics.23 Second, we used a measure of non-adherence based on a minimum gap of 90 days after the last covered day of treatment. A previous study of adherence to statins in Ontario adults used a gap period of 120 days, however, the maximum prescription available was 100 days, allowing for a 20% grace period.8 The days-supply for prescriptions in our database ranged from 30 to 90 days, and thus our measure is as, or more, conservative than prior studies. Moreover, sensitivity analyses using the 120-day definition did not substantially change identified predictors. Although there are limitations to our study, our methods are consistent with previous studies of adherence to LLT in adults8,10 and our sample came from a population of over 19 million privately insured children ages 8 to 20, making it generalizable to the commercially insured population.
Notwithstanding the controversy over LLT use in children and adolescents, the benefits of treatment cannot be realized unless medication is used consistently. Unlike adults, where even short-term adherence of 1 to 2 years can significantly reduce the risk of an acute cardiovascular event, the benefits of LLT initiated in childhood are based on the assumption of continued use over a long period of time.38 Our results show high rates of non-adherence in this population, suggesting that in order to realize the benefits of LLT, interventions to increase adherence are needed.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by the Reagan-Udall Foundation for the FDA, project RUF-IMEDS-SA-0022. Nina Joyce’s time was supported by a pre-doctoral grant from the American Heart Association-Founders Affiliate and by the National Institute Of Mental Health of the National Institutes of Health under Award Number T32MH019733. Dr. Zachariah’s time was supported by a NHLBI K23 award-HL111335. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Reagan-Udall foundation
Nina Joyce has worked as a Global Health and Economics Outcome Research fellow for Pfizer, Inc. in a capacity unrelated to the manuscript. Dr. Wellenius received consulting fees from Environmental Health & Engineering, Inc. for work unrelated to this research.
Abbreviations
- AAP
American Academy of Pediatrics
- LLT
Lipid Lowering Therapy
- LDL-C
Low Density Lipoprotein–Cholesterol
- CVD
Cardiovascular Disease
- CKD
Chronic Kidney Disease
- ADHD
Attention Deficit Hyperactivity Disorder
- HR
Hazard Ratio
- CI
Confidence Interval
Footnotes
Financial Disclosure: None of the authors have financial disclosures relevant to this article.
Conflict of Interest: The other authors have no conflicts of interest relevant to this article to disclose.
Contributors Statements:
Nina Joyce: Conceptualized and designed the study, analyzed and interpreted data and drafted the initial manuscript.
Gregory Wellenius: Conceptualized the study and supervised the design, analysis and interpretation of the data, and critically reviewed the manuscript.
Charles Eaton: Supervised the design and analysis of the study, provided critical support in the interpretation of the data, and critically reviewed the manuscript.
Amal Trivedi: Contributed to the conceptualization of the study, provided valuable feedback of the interpretation of the data and critically reviewed the manuscript.
Justin Zachariah: Conceived the initial study question, conceptualized the study and supervised the design, analysis and interpretation of the data, and critically reviewed the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Stephen RD, Frank RG Committee on N. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 2.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. PEDIATRICS. 2011 Dec;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyce N, Wellenius GA, Dore DD, Newburger JW, Zachariah JP. Patterns of Lipid Lowering Therapy among Children Ages 8–20 Years. The Journal of Pediatrics. 2015 Jul;167(1):113–119. e111. doi: 10.1016/j.jpeds.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ferranti S, Ludwig DS. Storm over statins--the controversy surrounding pharmacologic treatment of children. The New England Journal of Medicine. 2008 Sep 25;359(13):1309–1312. doi: 10.1056/NEJMp0805953. [DOI] [PubMed] [Google Scholar]
- 5.Jeppe NR, Alice C, David AA. Relationship Between Adherence to Evidence-Based Pharmacotherapy and Long-term Mortality After Acute Myocardial Infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 6.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009 Jun 16;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 7.Mitka M. Cardiologists like statins-more than patients do. JAMA. 2001 Dec 12;286(22):2799–2800. [PubMed] [Google Scholar]
- 8.Cynthia AJ, Muhammad M, Jack VT. Adherence With Statin Therapy in Elderly Patients With and Without Acute Coronary Syndromes. JAMA. 2002 doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 9.Newby LK, Nancy MAL, Anita YC, et al. Long-Term Adherence to Evidence-Based Secondary Prevention Therapies in Coronary Artery Disease. Circulation. 2006;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 10.Avorn J, Monette J, Lacour A, Bohn RL, Monane M. Persistence of use of lipid-lowering medications: a cross-national study. Persistence of use of lipid-lowering medications: a cross-national study. 1998 doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 11.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88(3):229–233. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessica R, Maud NV, Albert W, et al. Statin Treatment in Children With Familial Hypercholesterolemia The Younger, the Better. Circulation. 2007;116(6):664–668. doi: 10.1161/CIRCULATIONAHA.106.671016. [DOI] [PubMed] [Google Scholar]
- 13.Brian AF, Wonsuk Y, Issa A, et al. Effect of Long-Term Exposure to Lower Low-Density Lipoprotein Cholesterol Beginning Early in Life on the Risk of Coronary Heart Disease : A Mendelian Randomization Analysis. Journal of the American College of Cardiology. 2012;60(25) doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Jobst BC, Holmes GL. Prescribing antiepileptic drugs: should patients be switched on the basis of cost? CNS drugs. 2004;18(10):617–628. doi: 10.2165/00023210-200418100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Wiegman A, Hutten BA, de Groot E, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004 Jul 21;292(3):331–337. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 16.Braamskamp MJ, Kusters DM, Avis HJ, et al. Long-term statin treatment in children with familial hypercholesterolemia: more insight into tolerability and adherence. Paediatr Drugs. 2015 Apr;17(2):159–166. doi: 10.1007/s40272-014-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010 Feb 17;303(7):623–630. doi: 10.1001/jama.2010.104. [DOI] [PubMed] [Google Scholar]
- 18.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Medical Care. 2004 Mar;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 19.McGrady ME, Hommel KA. Medication Adherence and Health Care Utilization in Pediatric Chronic Illness: A Systematic Review. PEDIATRICS. 2013;132(4):730740. doi: 10.1542/peds.2013-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pai AL, McGrady M. Systematic review and meta-analysis of psychological interventions to promote treatment adherence in children, adolescents, and young adults with chronic illness. Journal of pediatric psychology. 2014 Sep;39(8):918–931. doi: 10.1093/jpepsy/jsu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child. 2010 Sep;95(9):717–723. doi: 10.1136/adc.2009.175125. [DOI] [PubMed] [Google Scholar]
- 22.Susan EA, Kristijan HK, Feride F, Arnold KC. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiology and Drug Safety. 2006;15(8):565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 23.Kaczorowski J. Chronic health conditions and obesity among children and youth. JAMA. 2010 May 19;303(19):1915. doi: 10.1001/jama.2010.585. author reply 1915-1915-1916. [DOI] [PubMed] [Google Scholar]
- 24.Smith BA, Shuchman M. Problem of non-adherence in chronically ill adolescents: strategies for assessment and intervention. Curr Opin Pediatr. 2005 Oct;17(5):613–618. doi: 10.1097/01.mop.0000176443.26872.6e. [DOI] [PubMed] [Google Scholar]
- 25.Hanghoj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: a systematic review. J Adolesc Health. 2014 Feb;54(2):121–138. doi: 10.1016/j.jadohealth.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007 May-Jun;22(3):218–253. doi: 10.1097/01.JCN.0000267827.50320.85. [DOI] [PubMed] [Google Scholar]
- 27.John CC, Regier MD, Lilly CL, Aly S. Long-term Pharmacotherapy For Elevated Low Density Lipoprotein Levels In Children: A Retrospective Analysis. J Clin Lipidol. 2015 doi: 10.1016/j.jacl.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller ML, Wright CC, Browne B. Lipid-lowering medications for children and adolescents. J Clin Lipidol. 2015 Sep-Oct;9(5 Suppl):S67–S76. doi: 10.1016/j.jacl.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Avis H, Kusters D, Vissers M, et al. Follow-Up of Children Diagnosed with Familial Hypercholesterolemia in a National Genetic Screening Program. The Journal of Pediatrics. doi: 10.1016/j.jpeds.2011.12.037. 2012-07-01T04:00:00Z 2012;161(1) [DOI] [PubMed] [Google Scholar]
- 30.Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol. 2010 Nov;3(6):349–357. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000 Apr;16(4):505–511. [PubMed] [Google Scholar]
- 32.Hansen RA, Kim MM, Song L, Tu W, Wu J. Comparison of methods to assess medication adherence and classify non-adherence. Annals of ‥‥. 2009 doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 33.Cutler DM, Everett W. Thinking outside the pillbox—medication adherence as a priority for health care reform. Thinking outside the pillbox—medication adherence as a priority for health care reform. 2010 doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 34.Kelly MC, Jill SH, Susan GF, Lorrie HY, Nancy PC, Peter GS. Parental beliefs about medications and medication adherence among urban children with asthma. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 2005;5(5):306–310. doi: 10.1367/A05-004R1.1. [DOI] [PubMed] [Google Scholar]
- 35.Chan PWK, DeBruyne JA. Parental concern towards the use of inhaled therapy in children with chronic asthma. Pediatrics International. 2000;42(5) doi: 10.1046/j.1442-200x.2000.01278.x. 2000. [DOI] [PubMed] [Google Scholar]
- 36.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. Journal of pediatric psychology. 2003 Jul-Aug;28(5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 37.Bruce B, Henry M, Cynthia R, Lynn A. Psychological Factors Associated with Medication Non-adherence in Asthmatic Children. Journal of Asthma. 1998 [Google Scholar]
- 38.Kusters MD, Avis HJ, Groot E, et al. Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA. 2014;312(10):1055–1057. doi: 10.1001/jama.2014.8892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.