Abstract
Objectives
To provide a critical review of a multipronged recruitment approach used to identify, recruit and enroll a diverse community-based sample of persons with memory disorder into an 18 month randomized controlled dementia care coordination trial.
Design
Descriptive analysis of a recruitment approach comprised of 5 strategies: 1) Community Liaison (“gatekeepers”) method; (2) letters sent from trusted community organizations; (3) display and distribution of study materials in the community; (4) research registries; and (5) general community outreach and engagement activities.
Setting
Baltimore, MD.
Participants
55 community organizations and 63 staff of community organizations.
Intervention
None
Measurements
Participant referral sources, eligibility, enrollment status, demographics, and loss to follow-up were tracked in a relational Access database.
Results
1275 referrals were received and 303 socioeconomically, cognitively, and racially diverse community-dwelling persons with cognitive disorders were enrolled. Most referrals came from letters sent from community organizations directly to clients on the study’s behalf (39%) and referrals from community liaison organizations (29%). African-American/Black enrollees were most likely to come from Community Liaison organizations.
Conclusions
A multipronged, adaptive approach led to the successful recruitment of diverse community residing-elders with memory impairment for an intervention trial. Key factors for success included employing a range of evidence-supported outreach strategies; forming key strategic community partnerships; seeking regular stakeholder input through all research phases; and obtaining “buy-in” from community stakeholders by aligning study objectives with perceived unmet community needs.
Keywords: Dementia, recruitment, community outreach, casefinding, memory disorders, detection
Alzheimer’s disease and other forms of dementia affect over 5 million Americans, and this may grow to 16 million by 2050 (1). These are likely underestimates however, since up to 50% of dementia cases go unrecognized and undiagnosed (2, 3). As the burden of disease increases and the availability of effective treatment options remain limited, the ability to conduct large, well-powered intervention trials is essential (4). Yet, recruitment of persons with dementia is difficult (5, 6), and there is a scarcity of detailed empirical descriptions of effective recruitment strategies for this population (7, 8). Understanding how to effectively and efficiently recruit diverse and representative samples of persons with dementia is especially important now, in light of increasing older population racial diversification as well as national initiatives such as the National Alzheimer’s Plan Act (Public Law 111–375) and The Center for Medicare & Medicaid Innovation awards that call for development and testing of new models of dementia care. Effective recruitment of diverse samples also allows for more meaningful data analyses, particularly how interventions may have differential effects in different groups of persons, and subsequently how they might be tailored for maximum benefit.
In general, recruiting older adults with mental health disorders into research or service programs is challenging, and patients with dementia represent a large segment of this vulnerable, at-risk population (5,9). Barriers inherent to recruitment of persons with dementia include: (a) perceived stigma associated with dementia (10,11); (b) high rates of undetected cases (2,3); (c) social isolation (11); (d) restrictive research criteria and high participation burden (4,11); (e) complicated informed consent procedures (e.g., need for consent capacity evaluation; lack of a legal proxy) (13,14); (f) and lack of a study partner (i.e. a reliable proxy source of information) (15).
Recruitment of racially diverse populations with dementia adds another layer of difficulty. Additional barriers to recruitment of minority persons may be fear and mistrust of science and research (16); different definitions, views or stigmas regarding normal versus abnormal aging or behavior and low awareness of dementia (e.g., expectations that “normal” aging is associated with significant memory loss; lack of knowledge of the symptoms of dementia) (17,18); higher burden of participation in research (19,20); lack of sensitivity to culture and language barriers in recruitment efforts; lack of culturally-sensitive medical and psychosocial interventions; and disparities in access to healthcare and access to clinical trial programs (16).
To date, there have been very few data-driven evaluations of methods to recruit persons with dementia into research (21). Studies on enrollment of older adults into community mental health service programs support several approaches including traditional referral sources (e.g., free health screenings, referrals from health care providers and social service agencies) (21,22), as well as the “gatekeeper model” (23–25), in which front-line community service staff (i.e., gatekeepers) are trained to identify and refer potential participants for assessment (21,22), effectively reaching individuals who may not yet be diagnosed, those who live alone, or are socially or economically isolated (11). Examples of gatekeepers may include bus drivers, postal workers, or senior public housing staff (10,21,22). Multimodal approaches, those that incorporate a variety of strategies and recruitment sources, as well as elements of community-based participatory research (e.g., partnering with community organizations and stakeholders during all research phases) (26) and/or social marketing (e.g., application of commercial marketing techniques for clinical trial recruitment) (27–28), are potentially superior to a unilateral approach, but can also be resource intensive (28–31).
The current paper provides a unique contribution to the literature by undertaking a detailed, critical analysis of a successful, multipronged approach to recruit a diverse group of 303 community-residing older adults with memory disorders into a randomized dementia care coordination trial in Baltimore, MD. We aim to [1] describe the five main recruitment strategies used, [2] compare the relative effectiveness of each strategy in terms of overall participant yield and yield of racially diverse participants, [3] and review facilitators and barriers encountered during implementation. By providing a careful review, we hope this effort can inform researchers in both the planning, design and implementation of future dementia trials.
METHODS
Overview of study design and procedures
These data come from an 18-month randomized controlled trial designed to identify community-living older adults with memory disorders and to test whether a community-based, multicomponent, dementia care coordination intervention (Maximizing Independence (MIND) at Home) could delay transition from home and improve clinical and quality of life outcomes for participants. The main results for the larger study are detailed elsewhere (32). Participants were recruited from Baltimore, MD (i.e., a 28 postal code area), were community-residing, age 70+, English-speaking, met criteria for dementia or cognitive disorder not otherwise specified, and had a reliable study partner. Most (95%) of study partners were caregivers (i.e., had regular contact, at least once per week, and were relied on by the participant for assistance in daily activities).
The recruitment process included study referral, a telephone screen (20 minutes), an in-home clinical assessment to confirm eligibility, and performed the Johns Hopkins Dementia Care Needs Assessment (JHDCNA) (33, 34) (2 hours), and a baseline quantitative outcome measure visit (1 hour). Participants were then randomized to the MIND intervention or augmented usual care (1:2 allocation). Augmented usual care participants’ study partners and primary care physicians (PCPs) received the written results of the JHDCNA, including recommendations for each identified unmet need as well as a brief resource guide that provided program and contact information for local and national aging service organizations. MIND participants received the written results of the JHDCNA and then up to 18 months of care coordination by an interdisciplinary care team comprised of non-clinical community workers (Coordinators) linked to a nurse and a geriatric psychiatrist. Outcome data were collected every 4.5 months for 18 months (32).
The study (clinicaltrials.gov; NCT01283750) was approved by the Johns Hopkins Medicine Institutional Review Board. Informed consent was provided by primary participants (i.e., person with cognitive disorder) and their study partners. If primary participants had impaired consent capacity, proxy consent was obtained from a legally authorized representative. Participants and study partners received $30 each per in-home visit.
Recruitment plan
The multi-strategy recruitment approach for this study combined principles of community-based participatory research (28, 35) and gatekeeper outreach models (21–22), with the goal of assembling a cognitively, economically and racially diverse cohort of community-living elders with memory disorders. The study consulted with a Community Advisory Board during all stages of the project (quarterly). The Community Advisory Board was coordinated and hosted by the Associated: Jewish Federation of Baltimore. The Associated plays a large role in the local Baltimore Jewish community and was instrumental in connecting the study team to its local network of community partners. The team also consulted with the Hopkins’ Institute for Clinical and Translational Research Director of Outreach and Engagement to develop a minority recruitment plan and relied on professional networks to identify other potential community partners. These were nearly all new connections and relationships between community partners and the investigative team; the vast majority of identified potential community partners had not directly worked with, or partnered with the study’s investigative team prior to this research effort.
Referrals were ascertained through five recruitment strategies/sources: (1) Community Liaison (i.e., “gatekeeper”) organizations, (2) community organizations that sent letters about the study to their client lists, (3) community organizations that displayed/distributed study brochures/flyers/bookmarks (4) Johns Hopkins dementia research registries, and (5) general community outreach activities. Each partner organization chose it’s level of participation in the study, which ranged from higher commitment (i.e., serving as a community liaison and allowing staff to be trained as gatekeepers) to lower commitment (i.e. allowing display of flyers in a patient waiting area). Organizations were exclusively either a Community Liaison Organization, a community organization that sent letters, or a community organization that distributed or displayed recruitment material. The recruitment plan was implemented in stages, beginning with the training of Community liaison organizations and general community outreach activities, followed by letters from organizations to their clients and display/distribution of brochures/flyers/bookmarks by another set of community organizations, and finally use of JHU research registries. Referral sources, eligibility, enrollment status, and loss to follow-up were tracked over time in a custom Access database. Three FTE staff (1 study coordinator, 2 research assistants) were responsible for recruitment and data collection and met weekly with investigators to report recruitment flow, progress, barriers/challenges, and logistical issues. The recruitment approach was adapted over time based on near real-time evaluation of recruitment flow, sources, and input from the Community Advisory Board.
Community Liaison training
Each organization that took part in the gatekeeper training identified a supervisory staff member as the main point of contact and this person identified employees who would serve as “community liaisons” (CL) based on their contact with at risk elders (e.g., front desk staff, Meals on Wheels drivers, housing maintenance staff) and helped coordinate a 1-hour training meeting. The study’s lead clinical investigator (D.J.) then provided the CL training which included: (1) a 30-minute, in-person, presentation (i.e., What is dementia?, 10 Warning signs, Impact on individuals and families, Common types of dementia-related needs, What is the MIND study, The role of the CL, How to refer to MIND, MIND contact information); and (2) distribution of packets to trained CLs containing a summary of the training, flyers/brochures, and coordinator business cards. CLs completed surveys immediately after initial training and then 4 months later to assess dementia knowledge, MIND study knowledge, past referral behaviors related to memory concerns, perceived study benefits, obstacles to referrals (4 months) and recommendations for improving referrals (4 months). CLs received a $5 gift card for completed surveys. CL training was implemented in 3 waves: in-person training for organizations with greatest expected number of eligible clients (Wave 1); in-person training for smaller organizations (Wave 2); and a brief booster (email or in-person), by contacting individual trained CL, for all organizations (Wave 3).
RESULTS
Study start-up took place over a 5 month period (01/2008—06/2008) and included assembly of the Community Advisory Board, development of community partnerships, IRB approvals, and Wave 1 CL organization training. Over a 2 year period (07/2008—05/2010), a total of 1,275 persons were referred to the study. At initial contact, 209 individuals did not meet inclusion criteria (i.e., outside catchment area, non-English speaker, <70 years old), 206 were unreachable, 150 declined, and 46 had died. Of the remaining 664 referrals, 284 were ineligible at phone screen and 380 had a positive phone screen (9 were excluded because of being unable to perform the home visit). 360 completed the in-home screening visit, and 303 were subsequently enrolled and randomized. The overall drop-out rate over the 18-month follow-up period was low (4%,11/303).
Participant and caregiver baseline demographics are in Table 1. Participants had a range of cognitive impairment severity, with 88% of participants meeting criteria for dementia and the remaining 12% having cognitive disorder not otherwise specified (i.e., mild cognitive impairment), Of those with dementia, 49% were mild stage, 37% moderate, and 14% severe (36). A quarter had annual household incomes below the 25 percentile of the annual US household income in 2010 (< $25,000), and 29% were Black/African American or Asian. Caregiver demographics in this sample were similar to other national caregiver surveys (37).
Table 1.
Baseline Characteristics of MIND Participants with a Memory Disorder and their Caregivers
| Characteristic | n | Mean (SD) or n (%) | |
|---|---|---|---|
| Primary Participant Characteristics | |||
| Age, No. (%) | 303 | ||
| 70–79 | 77 (25) | ||
| 80–89 | 181 (60) | ||
| 90 and older | 45 (15) | ||
| Female, No. (%) | 303 | 193 (64) | |
| Black/African American or Other Non-White Race, No. (%) |
303 | 87 (29) | |
| Education, mean (SD), y | 300 | 13.2 (3.6) | |
| Living with Caregiver, No. (%) | 303 | 211 (70) | |
| Time living at residence, means (SD), y | 297 | 21.1 (18.3) | |
| Annual household income, No. (%) | 209 | ||
| Less than $25,000 in last year | 56 (27) | ||
| Greater than or equal to $25,000 in last year | 153 (73) | ||
| Currently receiving public assistance benefits, No. (%) |
303 | 35 (12) | |
| Had dementia, No. (%) | 303 | 265 (88) | |
| Mild dementia | 265 | 130 (49) | |
| Moderate dementia | 265 | 98 (37) | |
| Severe dementia | 265 | 37 (14) | |
| Caregiver Characteristics | |||
| n | Mean (SD) or n (%) | ||
| Age | 275 | ||
| 59 or younger | 101 (37) | ||
| 60–69 | 51 (18) | ||
| 70–79 | 53 (19) | ||
| 80 and older | 70 (26) | ||
| Female, No. (%) | 278 | 207 (75) | |
| Relationship | 278 | ||
| Spouse (%) | 123 (44) | ||
| Child (%) | 130 (47) | ||
| Other person (%) | 25 (9) | ||
| Education, mean (SD), y | 278 | 15.4 (3.0) | |
| Currently Employed, No. (%) | 276 | 127 (46) | |
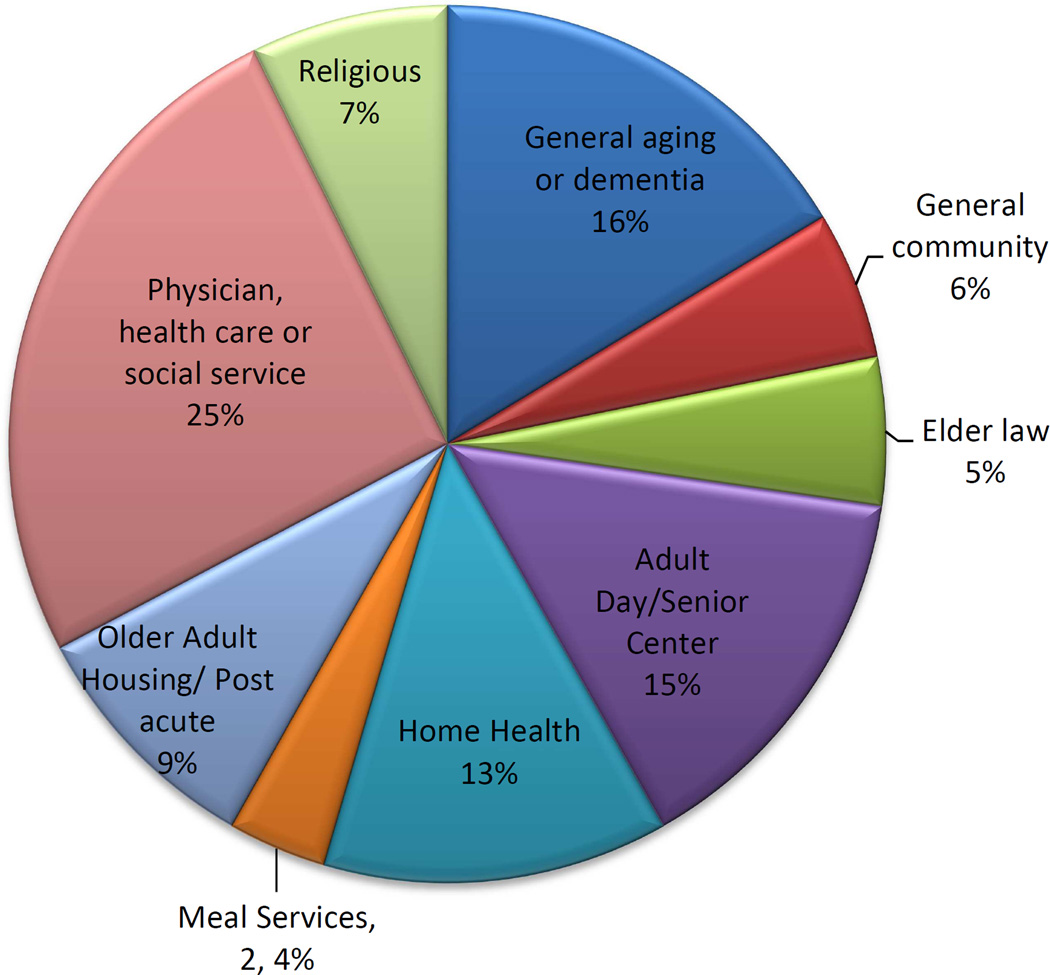
Overall 55 community organizations in the Baltimore area provided referrals, representing health professional practices, religious institutions, social, aging, nutritional services, and housing providers. Figure 1 shows that referrals were fairly well distributed by community partner type.
Figure 1.
Percent of referrals (n=1275) by community partner type
Recruitment Strategies Findings
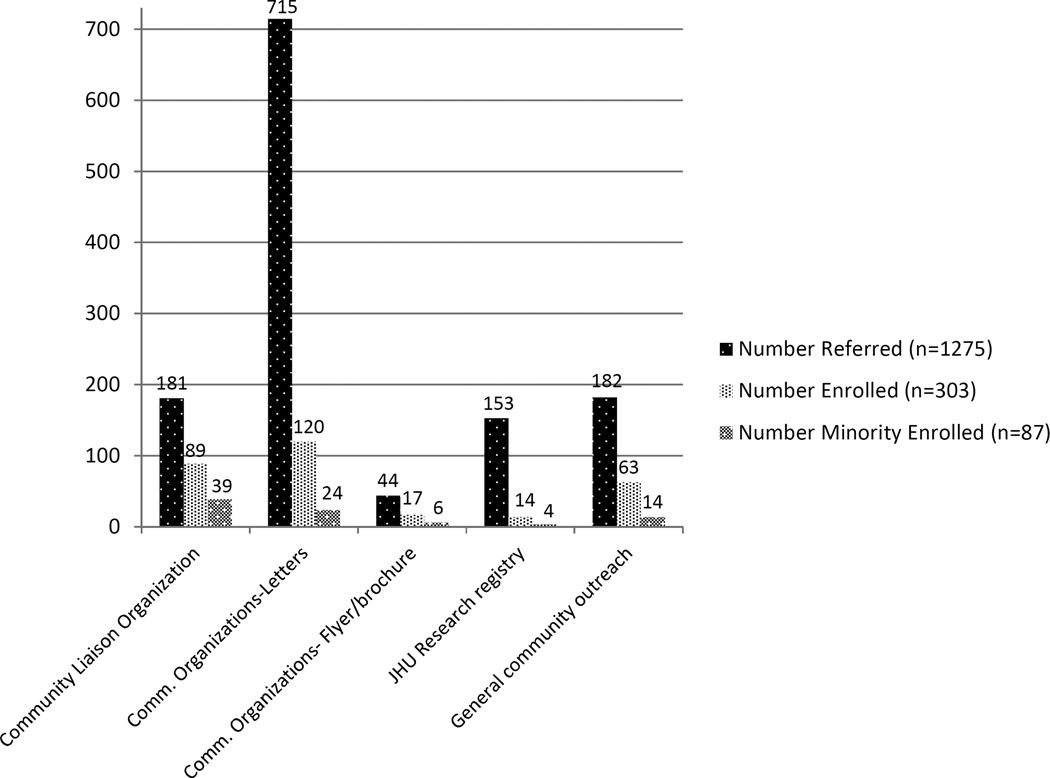
Number of referrals and disposition by recruitment strategies are in Table 2. Letters produced the greatest number of referrals (n=715), with an overall enrollment yield of 17%. CL organizations and general community outreach resulted in fewer referrals (n=181 and n=182, respectively), however referrals were higher quality (enrollment yield of 49% and 35%, respectively). CL organization referrals in particular had the highest yield for enrollment of persons from minority groups (22%). Considering raw numbers of enrollees overall (Figure 2), targeted letters produced the highest number of enrollees (120/303; 39%). For minority enrollees, the majority were from CL organizations (39/87; 45%) (Figure 2).
Table 2.
Number of referrals and disposition by the five main recruitment strategies
| Source | Number Referred |
Number cognitive screened-phone a |
Overall Screening Yield by Source (screened/referred *100) |
Number Enrolled b | Overall Enrollee Yield by Source (enrolled/referred *100) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Minority | All | Minority a | All | Minority | All | Minority | |||
| Community Liaison Organizations c | 181 | 122 | 43 | 67% | 24% | 89 | 39 | 49% | 22% | |
| Community organizations-Letters d | 715 | 353 | 29 | 49% | 4% | 120 | 24 | 17% | 3% | |
| Community organizations-display Flyer/brochure/bookmark e |
44 | 35 | 6 | 80% | 14% | 17 | 6 | 39% | 14% | |
| JHU Research Registry | 153 | 31 | 6 | 20% | 4% | 14 | 4 | 9% | 3% | |
| General community outreach | 182 | 123 | 17 | 68% | 9% | 63 | 14 | 35% | 8% | |
| TOTALS | 1275 | 664 | 101 | 52% | 8% | 303 | 87 | 24% | 7% | |
Notes: Minority refers to individuals who identified their racial background as African American/Black or Asian. Organizations were categorized as exclusively being either a Community Liaison Organization, a community organization that sent letters, or a community organization that distributed or displayed recruitment material.
Missing race data on 195
Missing race data on 4
Community Liaison Organizations included adult day/senior centers (n=2), general aging/dementia (n=3), home health agencies (n=5), meal service (n=1), older housing/post acute (n=1), and physician, allied health, or social service agencies (n=3)
Community organizations that sent letters included adult day/senior centers (n=3), general community organizations (n=1), general aging or dementia focused organizations (n=2), and older housing/post acute (n=1).
Community organizations displaying/providing Flyers/brochure/bookmarks included adult day/senior centers (n=2), general community organizations (n=2), religious (n=2), older housing/post acute (n=3), elder law (n=1), and physician, allied health, or social service agencies n=7).
Figure 2.
Number of referrals, enrollees, and minority enrollees by method
Community Liaison Organizations
A total of 15 organizations participated including adult day/senior centers (n=2), general aging/dementia (n=3), home health (n=5), nutrition services (n=1), housing (n=1), and physician, allied health, or social service agencies (n=3), with most participating on the Community Advisory Board. Overall, 63 CLs were trained and they had a mean age of 44 (SD 12), were mostly women (89%) and mostly white (80%). They were well educated (92% had undergraduate or graduate training) and had been employed by the agency for about 4 years (SD 4).
CL survey results are in Table 3 and reflect a 4-month response rate of 43%. In the immediate post-training survey, 41% of CLs surveyed reported that they had referred at least one client to a supervisor or welfare agency for concern about memory problems in the past year, with a median of 2.5 referrals (IQR = 1.0 - 8.5 referrals). In general, the majority of CLs agreed that the training provided knowledge of the MIND at Home study and referral process, that it could improve their case-finding skills, and had positive expections for the study’s ability to produce positive change. There were few changes on these ratings 4 months later. At 4 months, 40% of responders reported referring ≥ 1 potential participants to the study (median 4.5). Most referrals involved CLs providing study information to the client or caregiver (over 50%). Only 20% of CLs reported providing client contact information directly to the study team for contact initiation. The most common barriers to referral were not working with clients who would be eligible (non-English speaking, outside of postal code areas), and being unsure of client eligibility (e.g., study partner availability) (Table 4). The most common recommendations for improving referrals were expanding eligibility criteria (e.g., geographic reach, study partner); increasing reminders about the study and eligibility criteria; and improving case identification knowledge (Table 4).
Table 3.
Results from Community Liaison (CL) training survey at immediate post CL training (n=63) and 4 months post CL training (n=27)
| Immediate Post Training |
4 Months Post Training | Related Samples Wilcoxon Signed Rank Test Sig. |
||||
|---|---|---|---|---|---|---|
| N | Mean (SD) |
N Pairs | Time 1 Mean (SD) |
Time 2 Mean (SD) |
||
| Current knowledge of dementia | 58 | 7.8 (1.7) | 25 | 7.8 (1.7) | 7.6 (2.2) | 0.646 |
| Current confidence in ability to recognize person with significant memory problems |
59 | 7.7 (2.1) | 26 | 7.6 (2.1) | 7.8 (1.8) | 0.682 |
| Improved recognition of dementia signs | 57 | 1.9 (0.8) | ||||
| Improved understanding of dementia | 57 | 1.9 (0.7) | ||||
| Recognition of areas of need for assistance | 55 | 2.2 (0.7) | ||||
| More likely to recognize clients with dementia | 59 | 1.6 (0.7) | ||||
| Clear understanding of project goals | 61 | 1.5 (0.5) | 27 | 1.6 (0.5) | 1.7 (0.6) | 0.366 |
| Understand role as community liaison | 61 | 1.5 (0.5) | ||||
| Understanding of referral process | 60 | 1.6 (0.5) | 25 | 1.6 (0.5) | 1.8 (0.8) | 0.305 |
| Expectations of MIND study (range 1–4)b | ||||||
| My role referring to study can bring positive change |
58 | 1.6 (0.6) | 21 | 1.7 (0.8) | 1.8 (0.8) | 0.796 |
| Study will help identify adults with dementia | 58 | 1.6 (0.6) | ||||
| Study is a useful way to find adults whose needs may have gone unnoticed |
59 | 1.5 (0.7) | 26 | 1.9 (0.7) | 1.8 (0.8) | 0.796 |
Values range 1 = no understanding to 10 = advanced understanding
Values range from 1 = Strongly Agree to 4 = Strongly Disagree
Table 4.
Community Liaison Survey on results on Study Referrals at 4 months (n=28)
| Reasons for not referring more clients to the study | n | Yes % |
No % |
|---|---|---|---|
| Have not worked with eligible clients | 28 | 50 | 50 |
| Worried study would affect client relationship | 28 | 7 | 93 |
| Client declined referral | 28 | 11 | 89 |
| Unsure if study would help client | 28 | 11 | 89 |
| Worried about client’s privacy | 28 | 11 | 89 |
| Worried referral would violate agency policy | 28 | 4 | 96 |
| Unsure of how to make a referral | 28 | 7 | 93 |
| Unsure of client’s eligibility | 28 | 29 | 71 |
| Unsure if client had a study partner | 27 | 26 | 74 |
| Too busy with other responsibilities for client | 28 | 11 | 89 |
| Difficulty contacting Study Coordinator | 28 | 0 | 100 |
| Possible improvements to increasing referrals | |||
| Expanding eligibility criteria to more geographic areas | 27 | 67 | 33 |
| Increasing knowledge of recognizing dementia | 27 | 33 | 67 |
| Greater availability of team staff | 27 | 30 | 70 |
| More support from agency leaders | 27 | 22 | 78 |
| Giving monetary incentives for referrals | 27 | 19 | 82 |
| Increasing reminders about study and eligibility criteria | 26 | 50 | 50 |
Community organizations sending targeted letters
Seven organizations collectively mailed out over 25,000 letters through two mailing rounds (12,580 total letters per round). This method produced the greatest number of referrals (Table 2) and allowed establishment of a fairly predictable a referral flow because mailings were planned and staggered. It produced the volume needed to achieve recruitment in 24 months. Median overall response rate was 11% (ranged from 3% to 41%). Agencies with highest response rates were general aging advocacy organizations and Adult Day programs. Not surprisingly, more general, not-aging specific organizations had the lowest response. Nearly all agencies made minor stylist changes to the content of the letters to better engage their individual client base. The method was costly, averaging about $0.50 per piece (for material, staff time, and postage), and was labor intensive for study staff managing large volumes of referrals and phone screenings, though with a relatively low enrollment yield rate of 17% (Table 2).
Community organizations displaying and distributing study brochures/flyers/bookmarks
IRB approved flyers/brochures/bookmarks were displayed or distributed by 17 community organizations. This included placement of materials in doctor’s offices, senior apartments lobbies, library bulletin boards, or handed out with meeting minutes at religious events. This strategy produced the fewest overall number of referrals but ones that were high quality, meaning that a high proportion of referrals become enrollees (Table 2, 39% enrollee yield). Staff time and printing costs were not insignificant; for example, it required in-person delivery and set up at diverse sites on a monthly basis.
Hopkins research registries
Potentially eligible participants from two Hopkins research registries (the Johns Hopkins Alzheimer’s Disease Research Center, the ADAPT study) were used to identify and recruit participants who had given permission to be contacted about future research. This approach was employed in the latter third of the recruitment period to ensure the study met its recruitment goals. Letters were sent from the MIND team to potentially eligible participants, followed by a telephone call. It produced the lowest enrollee yield, both overall and for minorities (Table 2). Competing concurrent recruitment efforts likely led to low yield.
General community outreach
This included (a) 5 newspaper articles or public radio segments (i.e., “Medical Minute”); (b) 9, 8-week newspaper advertisements; and (c) in-person participation in 13 community events/health fairs. Community events were specifically selected to maximize the exposure of the study to the older African American community and to potential caregivers. Overall, this yielded 21% (63/303) of all enrollees (Figure 2). Though labor intensive and requiring work hour flexibilty, this approach enabled a “background” awareness of the study in the local community and helped make make personal connections with older adults and caregivers, as well as network with other community aging providers.
CONCLUSIONS
Over a 2.5 year startup and enrollment period, we received 1275 referrals and successfully met our recruitment targets by enrolling 303 socioeconomically, cognitively, and racially diverse community-dwelling persons with cognitive disorders. Overall, the majority of enrollees came from two sources: letters sent from community organizations directly to clients on the study’s behalf (39%) and referrals from community liaison organizations (29%). We almost met our minority recruitment goal of 30%, meant to be representative of the proportion of older minorities in Baltimore, MD (33%) (38). African-American/Black enrollees were most likely to come from Community Liaison organizations, suggesting perhaps the added utility of recruitment through personal relationships with trusted “gatekeepers.” Caregiver demographics here were similar to national reports (37).
Keys to success
Consistent with prior reports (28–31), we attribute overall recruitment success to: (a) forming strategic community partnerships with diverse aging and advocacy organizations; (b) obtaining “buy-in” from community stakeholders by aligning study objectives with unmet community needs and seeking input through all research phases; and (c) employing a staged and adaptive recruitment plan that included a range of evidence-supported outreach strategies. Keys to overcoming barriers (e.g., underdiagnosis, misinformation about dementia, stigma, isolation, cultural insensitivity) and reaching a diverse set of persons with dementia specifically, included targeted outreach to potential enrollees (e.g. through training Meals on Wheels drivers as gatekeepers), as well as caregivers (e.g. letters sent from AARP); partnerships with non-traditional, non-medical, and cultural-specific organizations (e.g. churches and synagogues); providing dementia education in local forums; and use of proactive strategies (i.e. gatekeeper model) to counteract underdiagnosis. Other potentially helpful strategies, though not specifically tested here, may be having a specifically dedicated staff to work exclusively on community outreach and recruitment. Ideally, this staff person would be an “insider” with prior experience working in the relevant community network and someone who already has excellent, pre-existing relationships with potential partners. Another potentially useful strategy is use of innovative recruitment materials that contain educational information or public health tips (e.g., 5 tips for home safety), as opposed to relying on traditional study flyers that simply describe the study, who is eligible, and who to contact.
Challenges, considerations, and limitations
The project faced several challenges. First, though crucial, development of community partnerships was slow and resource-consuming. We initially planned a 5-month start-up, but in the absence of ready-built networks, 10–12 months is more realistic. Creating strategic partnerships early on is likely helpful. For example, The Associated augmented our efforts by championing the study in its network of local aging providers, bringing them online more quickly than we could have alone. We emulated this strategy with other non-affiliated organizations, by first partnering with larger organizations, then requesting help in connecting with relevant organizations in their networks. In some cases obtaining buy-in from organizations was a challenge because of the perception that the research intervention (dementia care coordination) was duplicating services already available. For instance, one care management agency felt as though they already offered services highly similar to the experimental model being tested, and implicitly sent the message that we may be encroaching on their “turf.” This challenge could often, but not always, be overcome through clear communication of research goals and the final ‘product’, and through framing the partnership in ways that emphasized mutual benefits (e.g., that the intervention model was not a ‘competitor’ but enhancement of current practices). Identifying and enlisting a supervisory-level “champion” also helped, but did not always translate to buy-in at the front-line staff level, which required direct contact to gain trust and project enthusiasm.
Implementation of the multipronged recruitment plan was resource-consuming and required constant monitoring so strategies could be adapted as needed. Financially, in accounting exclusively for recruitment costs, we retrospectively estimate that it took effort from 3 FTE research staff, 1.5 FTE faculty investigators, $11,000 for study mailings, $7,500 for paid advertising, and $10,000 for recruitment flyers/brochures, promotional, and educational materials (FY 2009 dollars; excluding participant remuneration).
Despite initial expectations of high referral rates, the community liaison strategy produced fewer than hoped and was an inconsistent referral flow. Yet, it produced the highest quality referrals, meaning that 49% of referrals became enrollees, and 22% of referrals who became enrollees were African-American/Black. In retrospect, we likely brought on CL agencies too rapidly and became overextended, making it difficult to maintain contacts and stay highly visible to encourage referrals. The CL training also overemphasized the project’s eligibility criteria, producing a barrier to referral for CLs.
Mailings from trusted community organizations are a valuable tool in this population (39), and here produced the largest number of referrals of all strategies employed (n=715), and ultimately the leading source of study enrollees (39%). It was crucial that these letters came from familiar, trusted community organizations themselves, versus Hopkins, in which the response level would likely have been much lower. Also, since timing of mailings could be controlled, this helped create consistency in referral flow. However, the referral quality was low (17%), meaning many of the referrals screened ineligible. This, in turn, required significant staff time. Finally, general community outreach was another important contributor and yielded 21% of all enrollees. However advertisement costs and staff time and flexibility to attend community events on nights and weekends is a consideration.
There are several limitations to the study design. While this analysis serves as a quantitative case example of successful recruitment into a dementia care clinical trial, use of mixed methods to understand participants’ perceptions would have augmented our understanding of outreach strategies and how they might be improved. Further, each research project is unique in its goals, timelines, constraints, targeted population and geographic locale, and these factors will dictate how a plan is developed and implemented, though we believe the underlying principles described here serve valid starting point. Also, we did not randomly select our 55 partner community organizations in Baltimore and therefore do not know how representative they may be of all the possible potential aging services organizations. Further we did not randomize matched agencies to specific types of recruitment strategies, and therefore we cannot definitively disentangle which of the five strategies worked most effectively or the impact of individual strategies in isolation. For example, the types and sizes of agencies participating in each kind of strategy may have contributed to observed results, including better or worse enrollment rates, and differences in enrollment rates of racial subgroups. Also, the recruitment strategies focused on enrolling participants who were proficient in English and may not generalize to non- or limited- English speakers. Finally, the sample obtained through this recruitment plan may not be clinically representative of the underlying dementia population, though they appear to be demographically similar to the older Baltimore, MD population.
Final thoughts
Overall, this analysis contributes important empirical data to address an increasingly important problem: how to efficiently recruit diverse older adults with dementia into research. Detailed analyses of these kind, that systematically report on recruitment methods and strategies, and their strengths and limitations, can serve as an important source of information for both researchers and grant makers alike and can aid in the successful and practical planning and implementation of new research programs in the future.
Acknowledgments
Conflicts of Interest and Source of Funding:
Under an agreement between DEMeasure and Drs. Black and Rabins, Dr. Black and Dr. Rabins are entitled to a share of income received by DEMeasure from sales of the Alzheimer’s Disease Related Quality of Life questionnaire and scale used in the study described in this article. Drs. Black and Rabins have ownership interests in DEMeasure. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Black has grant support from NIA (R01AG038440). Dr. Lyketsos has grant support from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, Functional Neuromodulation Inc. Janssen. He is a consultant/Advisor for Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genetech, Elan, NFL Players Association, NFL Benefits office, Avanir, Zinfandel, BMS. He has received honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, Health Monitor. The remaining authors have no relevant conflicts or financial interests to disclose. The project donors were: The Hoffberger Family Fund; LeRoy Hoffberger; The Harry and Jeannette Weinberg Foundation; Rosenberg Foundation; Hirschhorn Foundation; Stulman Charitable Foundation; Meyerhoff Foundation; Marc and Leonor Blum; Baltimore County Department of Aging; Blum Family; Lowell Glazer; Greif Family Fund; Marvin Schapiro Family Foundation; Lois and Phillip Macht; Eliasberg Family Foundation; Richard and Rosalee Davison; Alison & Arnold Richman; Moser Family Philanthropic Fund; Richard Lansburgh; Arnold Richman; Anonymous; and other supporting contributions. Support was also provided to Dr. Samus by the National Institute of Mental Health/National Institute on Aging (K01 MH085142). We wish express our sincere gratitude to Mr. LeRoy Hoffberger who served as the community champion for this project, and to The Associated: Jewish Community Federation of Baltimore, Jewish Community Services, Levindale Hebrew Geriatric Center, and the Alzheimer’s Association of Baltimore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PREVIOUS PRESENTATION OF ABSTRACT: A limited amount of data presented in this manuscript were presented in a symposium at the American Association for Geriatric Psychiatry Annual Meeting in Washington DC, 2012. Reference:
Samus Q. Dementia Detection Training of Aging Services Agency Partners and the MIND at Home Study. Symposium: Geriatric Psychiatry and Dementia Care at Home in an Urban Setting: Interdisciplinary Experience and Research in Baltimore City. American Association for Geriatric Psychiatry, Washington DC, 2012.
References
- 1.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Ganguli M, Rodriguez E, Mulsant B, et al. Detection and management of cognitive impairment in primary care: The steel valley seniors survey. J Am Geriatr Soc. 2004;52:1668–1675. doi: 10.1111/j.1532-5415.2004.52459.x. [DOI] [PubMed] [Google Scholar]
- 3.Connolly A, Gaehl E, Martin H, et al. Underdiagnosis of dementia in primary care: Variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011;15:978–984. doi: 10.1080/13607863.2011.596805. [DOI] [PubMed] [Google Scholar]
- 4.Knebl JA, Patki D. Recruitment of subjects into clinical trials for alzheimer disease. J Am Osteopath Assoc. 2010;110:S43–S49. [PubMed] [Google Scholar]
- 5.Williams DE, Vitiello MV, Ries RK, et al. Successful recruitment of elderly community-dwelling subjects for alzheimer's disease research. J Gerontol. 1988;43:M69–M74. doi: 10.1093/geronj/43.3.m69. [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Mansfield J. Recruitment rates in gerontological research: The situation for drug trials in dementia may be worse than previously reported. Alzheimer Dis Assoc Disord. 2002;16:279–282. doi: 10.1097/00002093-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Carr SA, Davis R, Spencer D, et al. Comparison of recruitment efforts targeted at primary care physicians versus the community at large for participation in alzheimer disease clinical trials. Alzheimer Dis Assoc Disord. 2010;24:165–170. doi: 10.1097/WAD.0b013e3181aba927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napoles AM, Chadiha LA. Resource Centers for Minority Aging Research. Advancing the science of recruitment and retention of ethnically diverse populations. Gerontologist. 2011;51:S142–S146. doi: 10.1093/geront/gnr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klap R, Unroe KT, Unutzer J. Caring for mental illness in the united states: A focus on older adults. Am J Geriatr Psychiatry. 2003;11:517–524. [PubMed] [Google Scholar]
- 10.Robbins B, Rye R, German PS, et al. The psychogeriatric assessment and treatment in city housing (PATCH) program for elders with mental illness in public housing: Getting through the crack in the door. Arch Psychiatr Nurs. 2000;14:163–172. doi: 10.1053/apnu.2000.8653. [DOI] [PubMed] [Google Scholar]
- 11.Van Citters AD, Bartels SJ. A systematic review of the effectiveness of community-based mental health outreach services for older adults. Psychiatr Serv. 2004;55:1237–1249. doi: 10.1176/appi.ps.55.11.1237. [DOI] [PubMed] [Google Scholar]
- 12.Arean PA, Alvidrez J, Nery R, et al. Recruitment and retention of older minorities in mental health services research. Gerontologist. 2003;43:36–44. doi: 10.1093/geront/43.1.36. [DOI] [PubMed] [Google Scholar]
- 13.Sugarman J, McCrory D, Hubal R. Getting meaningful informed consent from older adults: A structured literature review of empirical research. Journal of the American Geriatrics Society. 1998;46:517–524. doi: 10.1111/j.1532-5415.1998.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 14.Black BS, Brandt J, Rabins PV, et al. Predictors of Providing Informed Consent or Assent for Research Participation in Assisted Living Residents. American Journal of Geriatric Psychiatry. 2008;16:83–91. doi: 10.1097/JGP.0b013e318157cabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols L, Martindale-Adams J, Burns R, et al. Social marketing as framework for recruitment: Illustrations from the REACH study. Journal of Aging and Health. 2004;16(Suppl):157S–176S. doi: 10.1177/0898264304269727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl SM, Vasquez L. Approaches to improving recruitment and retention of minority elders participating in research: Examples from selected research groups including the national institute on aging's resource centers for minority aging research. J Aging Health. 2004;16:9S–17S. doi: 10.1177/0898264304268146. [DOI] [PubMed] [Google Scholar]
- 17.Welsh KA, Ballard E, Nash F, et al. Issues affecting minority participation in research studies of alzheimer disease. Alzheimer Dis Assoc Disord. 1994;8:S38–S48. [PubMed] [Google Scholar]
- 18.Lu FG, Lim RF, Mezzich JE. Issues in the assessment and diagnosis of culturally diverse individuals. American Psychiatric Press review of psychiatry. 1995 [Google Scholar]
- 19.Mattson ME, Curb JD, McArdle R. Participation in a clinical trial: The patients' point of view. Control Clin Trials. 1985;6:156–167. doi: 10.1016/0197-2456(85)90121-7. [DOI] [PubMed] [Google Scholar]
- 20.Demi AS, Warren NA. Issues in conducting research with vulnerable families. West J Nurs Res. 1995;17:188–202. doi: 10.1177/019394599501700206. [DOI] [PubMed] [Google Scholar]
- 21.Buckwalter KC, Smith M, Zevenbergen P, et al. Mental health services of the rural elderly outreach program. Gerontologist. 1991;31:408–412. doi: 10.1093/geront/31.3.408. [DOI] [PubMed] [Google Scholar]
- 22.Bartsch DA, Rodgers VK, Strong D. Outcomes of senior reach gatekeeper referrals: Comparison of the spokane gatekeeper program, Colorado senior reach, and mid-Kansas senior outreach. Care Manag J. 2013;14:11–20. doi: 10.1891/1521-0987.14.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Florio ER, Rockwood TH, Hendryx MS, et al. A model gatekeeper program to find the at-risk elderly. J Case Manag. 1996;5:106–114. [PubMed] [Google Scholar]
- 24.Florio ER, Jensen JE, Hendryx M, et al. One-year outcomes of older adults referred for aging and mental health services by community gatekeepers. J Case Manag. 1998;7:74–83. [PubMed] [Google Scholar]
- 25.Raschko R. In: The Spokane Elder Care Program: community outreach methods and results, in Progress in Alzheimer’s Disease and Similar Conditions. American Psychopathological Association series. Heston LL, editor. Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- 26.Israel BA, Schulz AJ, Parker EA, et al. Critical issues in developing and following community-based participatory research principles. In: Minkler M, Wallerstein N, editors. Community-based participatory research for health. San Francisco, CA: Jossey-Bass; 2003. pp. 56–73. [Google Scholar]
- 27.UyBico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: A systematic review of recruitment interventions. Journal of General Internal Medicine. 2007;22:852–863. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etkin CD, Farran CJ, Barnes LL, et al. Recruitment and enrollment of caregivers for a lifestyle physical activity clinical trial. Res Nurs Health. 2012;35:70–81. doi: 10.1002/nur.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster SE, Jones L, Saxton JM, et al. Recruiting older people to a randomised controlled dietary intervention trial--how hard can it be? BMC Med Res Methodol. 2010;10:17. doi: 10.1186/1471-2288-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ejiogu N, Norbeck JH, Mason MA, et al. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist. 2011;51:S33–S45. doi: 10.1093/geront/gnr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHenry JC, Insel KC, Einstein GO, et al. Recruitment of Older Adults: Success May Be in the Details. Gerontologist. 2012 Aug 16; doi: 10.1093/geront/gns079. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samus Q, Johnston D, Black B, et al. A multidimensional home-based care coordination intervention for elders with memory disorders: the Maximizing Independence at Home (MIND) Pilot Randomized Trial. Am J Geriatr Psychiatry. 2014;22:398–414. doi: 10.1016/j.jagp.2013.12.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black B, Johnston D, Handel S, et al. Manual for the Johns Hopkins Dementia Care Needs Assessment (JHDCNA) Baltimore, MD: Johns Hopkins University; 2008. [Google Scholar]
- 34.Black B, Johnston D, Rabins PV, et al. Unmet Needs of Community-Residing Persons with Dementia and Their Informal Caregivers: Findings from the Maximizing Independence at Home Study. J Am Geriatr Soc. 2013;61:2087–2095. doi: 10.1111/jgs.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israel BA, Schulz AJ, Parker EA, et al. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 36.Perneczky R, Wagenpfeil S, Komossa K, et al. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14:139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 37.Alzheimer’s Association. [Accessed 06/01/2014];Alzheimer’s Disease facts and figures 2007. doi: 10.1016/j.jalz.2014.02.001. http://www.alz.org/national/documents/report_2007factsandfigures.pdf. [DOI] [PubMed]
- 38.US Census data. [Accessed 0324/2014];2010 Available at http://planning.maryland.gov/msdc/census/cen2010/SF1/rawdata/sf1_rawdata.shtml.
- 39.Morrison K, Winter L, Gitlin LN. Recruiting community-based dementia patients and caregivers in a non-pharmacologic randomized trials: What works and how much does it cost? Journal of Applied Gerontology. 2014 doi: 10.1177/0733464814532012. Published online 4 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]