Abstract
Many animal models have been established for the study of myocardial remodeling and heart failure due to its status as the number one cause of mortality worldwide. In humans, a pathologic occlusion forms in a coronary artery and reperfusion of that occluded artery is considered essential to maintain viability of the myocardium at risk. Although essential for myocardial recovery, reperfusion of the ischemic myocardium creates its own tissue injury. The physiologic response and healing of an ischemia/reperfusion injury is different from a chronic occlusion injury. Myocardial ischemia/reperfusion injury is gaining recognition as a clinically relevant model for myocardial infarction studies. For this reason, parallel animal models of ischemia/reperfusion are vital in advancing the knowledge base regarding myocardial injury. Typically, ischemia of the mouse heart after left anterior descending (LAD) coronary artery occlusion is confirmed by visible pallor of the myocardium below the occlusion (ligature). However, this offers only a subjective way of confirming correct or consistent ligature placement, as there are multiple major arteries that could cause pallor in different myocardial regions. A method of recording electrocardiographic changes to assess correct ligature placement and resultant ischemia as well as reperfusion, to supplement observed myocardial pallor, would help yield consistent infarct sizes in mouse models. In turn, this would help decrease the number of mice used. Additionally, electrocardiographic changes can continue to be recorded non-invasively in a time-dependent fashion after the surgery. This article will demonstrate a method of electrocardiographically confirming myocardial ischemia and reperfusion in real time.
Keywords: Medicine, Issue 117, Heart, Electrocardiogram, Ischemia/Reperfusion, Ischemia/Reperfusion Injury, Myocardial Infarction
Introduction
Heart disease remains the leading cause of death worldwide1,2. Not only is the left ventricle (LV) the most muscular chamber, responsible for pumping blood from the heart to the entire body3, it is a common cardiac injury site post-myocardial infarction4. Left ventricular tissue death often results in systolic heart failure. Animal models of heart disease are imperative for the advancement of biomedical cardiovascular research. The C57Bl/6 strain of mice have been a popular choice for animal models due to their quick breeding time, low cost and ease in genetic alterations. Most murine surgical models for the study of heart disease involve occlusion of the LAD branch of the left coronary artery. The LAD is sometimes called the left obtuse marginal5,6. The LAD supplies blood to the left ventricular anterior and antero-lateral walls. LAD occlusion studies are aimed at inducing anterior infarctions, sometimes extending into the inferior and lateral wall regions7.
Two models that are used frequently for myocardial infarction studies include chronic occlusion myocardial infarction and myocardial ischemia/reperfusion injury. The chronic occlusion is created by surgically suturing around and permanently blocking blood flow through the LAD. The ischemia/reperfusion injury is created much in the same way only with a transient, usually 30-60 min, ischemic period. To achieve transient ischemia, the occluding suture ties around the LAD and a small PE-10 tube which is placed parallel to the LAD on the epicardial surface of the heart, followed by a reperfusion period where the tubing and occluding suture is removed and blood is allowed to once again flow through the artery and into the myocardium. The ischemia/reperfusion surgery has been deemed to be clinically relevant due to the nature of reperfusion injury paralleling the treatment of human infarctions which includes prompt coronary angioplasty and stenting of the artery, or coronary artery bypass. Typically, during these surgeries, ischemia of the LV in a mouse heart is confirmed by visible pallor of the myocardial wall. However, by simply performing the surgeries on an electrocardiogram (ECG) pad under constant monitoring conditions, visible changes can be observed in the ECG waveform, thereby confirming ischemia and reperfusion of the mouse myocardium.
Although the murine heart is similar to the human heart in many respects, including its four-chambered structure, the hearts also have differences. One obvious difference is the average resting heart rate of adult mice is 600 - 700 beats per min (bpm) whereas that of adult humans is ~60-100 bpm8,9. Additionally, in mice the repolarization waves, J and T, often merge with the depolarization QRS-complex making a clear ST-segment difficult to discern10. To complicate the process of electrocardiographically confirming myocardial ischemia, it is the elevation of the T-wave and the ST-segment which are used as markers for the diagnosis of ischemia and myocardial infarction injury in humans, clinically referred to as ST elevation myocardial infarction or STEMI. One of the key differences between human and murine waveforms is that S-wave is immediately followed be a J-wave that transfers directly into a negative T-wave. During acute myocardial ischemia in mice the amplitude of S-wave decreases and is directly followed by an abnormal J-wave and an inverted T-wave11. The T-wave does not seem to represent a significant portion of the repolarization in mice11. Despite nomenclature and mouse vs. human differences, ECG confirmation of murine myocardial ischemia and reperfusion is still feasible and relatively simple. For the sake of simplifying waveform interpretation, the segment between the S-J-T is referred to as ST-segment herein.
STEMI guidelines published in 2013 recommend a patient door-to-balloon time of less than 90 min12.This means that the time frame from the identification of the patient's coronary artery occlusion until the artery is reopened should be less than 90 min. The beating heart is constantly working and therefore, has a high oxidative metabolism and a high level of oxygen consumption3. To provide for this, a network of capillaries is available to each myocyte3. It only takes a heart a few beats to exhaust its oxygen and nutrient supply. In a 90 min window, an ischemic heart region in a human will have been blocked from receiving between 5,400 and 9,000 heart beats worth of oxygen-rich blood. In that same 90 min window, a mouse would have 54,000 to 63,000 heart beats. Experimental time points for murine ischemia/reperfusion injury are typically between 30 and 60 min.
The importance of developing a supplemental method of confirming myocardial ischemia and reperfusion in a murine model has profound implications on the consistency and reproducibility of data in myocardial ischemia/reperfusion studies. The current practice of visually observing the heart for a change in tissue color is not adequate as a stand-alone diagnostic. Additionally, reperfusion after removal of the tubing and suture is not guaranteed. Although the artery is no longer tied off, the artery may have sustained damage during the procedure and may become impossible to reperfuse. It would be beneficial to have a record of electrocardiographic changes to confirm reperfusion rather than relying on observations of myocardial pallor and rubor (red color). Hearts that do not show the markers of ischemia/reperfusion injury can then quickly be flagged and a decision on how to proceed can be made by the investigators.
Lastly, establishing a record of ECG changes from baseline throughout the ischemic and reperfusion periods allows investigators to continue to monitor the heart after the initial surgery. Investigators currently lose sight of the heart as soon as the surgery is completed. ECG is a simple way to gain insight into changes occurring in the myocardium hours to days after the surgery. ECG recorded at time points after surgery could reveal late-developing Q-waves indicating continued or worsening tissue death. However, to effectively gage new or worsening electrocardiographic markers, a baseline ECG must be available for comparison.
This protocol will demonstrate how to prepare, obtain, and interpret the ECG to confirm ischemia and reperfusion of the mouse heart using 8 - 12 week old male C57Bl/6 mice.
Protocol
All surgical procedures performed on animals should be carried out in accordance with Guide for the Care and Use of Laboratory Animals13 or other appropriate ethical guidelines. Protocols should be approved by the animal welfare committee at the appropriate institution before proceeding.
1. Preparing for the ECG
NOTE: Before beginning, don personal protective equipment including gloves, eyewear and a clean laboratory coat or disposable gown.
Clean the ECG pad using a non-alcohol and non-bleach based decontamination solution. Gently use a delicate task wipe to blot off excess solution to ensure that the electrode pad does not become damaged.
If the ECG pad has a heating feature, use it. Anesthetized mice tend to lose body heat rapidly. Heat the pad to 40-42 °C to maintain normothermic body temperature of 37 °C throughout the surgery14. Monitor body temperature using a rectal thermometer. Adjust pad temperature as necessary to maintain the body temperature ~37 °C.
As most thoracotomies are performed with the mouse lying on its back (supine), ensure that the toggle is flipped to the "supine" setting. Many ECG pads have a function to toggle between prone and supine positions. Failure to select the right orientation can result in misrepresentation of electrocardiographic events.
Anesthetize mouse using 5% inhaled isoflurane and 1 L/min oxygen. Once mouse is anesthetized, transfer mouse to ECG pad equipped with an anesthesia nose-cone and reduce isoflurane to 2% and 1 L/min oxygen. Confirm proper anesthesia by ensuring mouse does not react when the mouse's foot is pinched with forceps.
Apply a thin coat of eye lubrication ointment over the mouse's eyes to prevent dryness and corneal damage while anesthetized.
Clean the mouse's paws with a wet wipe to remove all visible bedding that may be stuck to the paws or may interfere with the transmission of electrical impulses from the paws to the ECG pad. Dry paws with a wipe.
Apply a small amount (slightly smaller than a USD dime) of highly conductive electrolyte gel to each of the four metallic electrodes on the ECG pad. Note: Be sure to only apply a small amount of gel as too much gel makes it difficult to restrain the paws to the pad using tape. Additionally, paws are likely to slip out of the restraint during surgery if they were wet before applying tape.
With the mouse in supine position, use clear medical tape to restrain each paw to its corresponding electrode (Figure 1). First press each paw to its piece of tape and then adhere the tape to the ECG pad. Ensure that each restrained paw is in contact with the electrolyte gel and the electrode.
2. Acquiring the ECG
Depending on the equipment used for ECG acquisition, configure the machine so that the ECG waveform can be visualized in real time. For ECG recordings using the physiologic monitoring settings on an echocardiography machine, a live B-mode image will have the ECG waveform running along the bottom of the screen. Note: See individual machine user guides to determine how best to configure that equipment.
- Enable real time visualization of ECG waveform by pressing the B-mode key on an echocardiography machine or the equivalent on other ECG recording devices.
- Adjust the resolution to account for differences in amplitude. If the peak of the R-wave or the trough (valley) of the Q-wave are out of the visual frame, adjust the resolution until the entire height of the waveform can be observed. Note: This can be done under the physiological settings tab on an echocardiography machine. Click the increase or decrease arrows until the entire waveform is visible.
Any time that an image is to be obtained, clear the ECG pad of tools. Touching the mouse during ECG recording with forceps or fingers will disturb the waveform. Ensure that the mouse is still and untouched on the ECG pad before recording any ECGs.
Use the machine's "record" or "store" feature before making any surgical incisions on the mouse. This image will be used as a baseline for comparison later on.
3. Surgical Procedure and Recording ECG
Inject anesthetized mouse with analgesic (buprenorphine, 1.5 µg, intraperitoneal) before beginning. The details of the ischemia/reperfusion surgical procedure can also be found elsewhere5.
Remove hair around the surgical site chemically or mechanically and disinfect the area with betadine solution. Use a scalpel to make a vertical incision parallel to the esophagus and trachea. Gently move the lymph nodes to each side of the incision until the thin tissue covering the trachea is exposed. Using forceps, gently separate the tissue until the white cartilage rings of the trachea are visible. Note: We generally use Nair hair removal lotion. The lotion is applied on the surgical site for ~1 min. Nair is then thoroughly washed off using saline or water. This method is preferred in our laboratory because high resolution echocardiography (which can detect hair follicles) is performed before and after surgery. However, caution should be taken to avoid sensitive areas such as genitals, and then thoroughly washing the Nair off to avoid potential skin burns.
Quickly remove the mouse's nose from the nose-cone and insert ventilation tubing into the mouth of the mouse and towards the throat. When the tip of the ventilation tubing is visible through the exposed neck area, align the tube with the start of the trachea. Gently wiggle the tube side to side while applying upward pressure until the tubing slides into the trachea which can be confirmed visually through the translucent trachea.
Ensure that the mouse remains anesthetized during the intubation procedure. Pause from intubation and return the mouse to the nose-cone if it begins to stir.
Using a loop of string, hook the mouse's two front teeth through the loop and tape the string ends to the ECG pad to steady the head of the mouse and to ensure the ventilation tubing does not move during surgery. Quickly attach ventilation tubing to rodent ventilator and adjust ventilation settings according to the weight of the mouse. Tape ventilation tubing in place.
Cover the mouse's exposed trachea with a gauze soaked in warm saline to keep tissue from drying.
Make a vertical incision using a scalpel along the left side of the sternum.
Using forceps, gently separate the fascia layer from the muscle layer. Carefully cut the underlying muscle layers without cutting visible blood vessels.
Using forceps, grab the third rib and pull upwards gently. Maintain grip on the rib with one hand and use surgical scissors to carefully cut the intercostal tissue between the third and fourth rib. Ensure that the lungs are not damaged. Note: Lungs will retract deep into the chest cavity almost immediately after the chest cavity is punctured by the surgical incision due to the loss of the pressure gradient. Wait until the lungs have retracted before continuing.
Use forceps to grab and gently separate the thin layer of pericardium which surrounds the heart.
Insert retractors or manually use forceps as rib retractors to move the ribs into a position where the heart is visible between the ribs. Note: It is common practice to move the mouse's lower left paw so that it is overlapping the lower right paw during the placement of the ligature. This helps to position the heart so that the left atrial appendage, or auricle, is easily visible during placement of the ligature. Be aware that valid ECG waveforms will not be obtained while the lower left paw is off of the electrode. For this reason it is advisable to return the paw to its electrode after the suturing ligature is passed through the myocardial tissue but before a knot is tightened.
Locate the LAD visually, beneath the left auricle. Swiftly insert a 7-0 silk tapered suturing needle into the myocardium deeply enough to pass under the LAD but not so deep as to penetrate the LV cavity. Pull the suturing ligature through until there is about 4 cm of suturing silk left on the free (non-needle) end of the suturing ligature.
Begin to tie a simple suture knot. Once the free end of the suturing silk has been pulled through the loops to form the knot, pause.
Holding both the free and needle ends of the suturing silk with forceps, insert a ~1 cm section of PE-10 tubing underneath the forming knot and atop the epicardial surface.
If the mouse's left paw is crossed, return the paw to its proper electrode. Tighten knot so that the PE-10 tubing is sutured to the heart. Release all physical contact with the mouse to allow ECG to be recorded.
- Allow ECG waveform to cycle through for ~10 sec. Check ECG waveform visually and record waveform as "Time of Occlusion". If the T-wave does not increase in amplitude within 1 min, reassess placement of ligature.
- If the T-wave amplitude does not increase, either discard the animal from the study or attempt to correct the ligature placement.
Visually check the color of the myocardium to confirm ischemic paling of the LV.
If ECG changes and myocardial color changes indicate ischemia, double knot the suture around the PE-10 tubing.
Cover the open chest cavity with warm saline gauze.
Record ECG every 5-10 min for the duration of the ischemic period.
4. Confirmation of Reperfusion Using ECG
Remove saline gauze covering the chest cavity and visualize the heart.
Use a blade to cut the suturing silk atop the PE-10 tubing. Once the ligature is cut, remove the section of PE-10 tubing and gently remove the suturing ligature from the myocardium.
Release all physical contact with the mouse and allow the ECG waveform ~10 sec to cycle. Record waveform as "Time of Reperfusion." Continue to record ECG waveforms every 5-10 min until the desired experimental time point is reached.
- Adjust resolution for changes in amplitude as needed. If the T-wave does not change upon removal of the PE-10 tubing and ligature, reperfusion is not confirmed.
- If the T-wave does not change upon removal of tubing, either discard the animal from the study or attempt to correct the ligature placement.
Visually inspect myocardium to additionally confirm reperfusion by return to red color.
Close chest cavity by suturing the intercostal space with a 5-0 silk suture while applying gentle pressure to the mouse's chest to expel excess air that has entered during surgery. Then suture the muscle layers and finally, skin. Note: Applying pressure to the chest cavity may not be sufficient to evacuate the chest cavity of air in all mice. Therefore, the syringe and needle method of evacuation should be employed to ensure that all air has been expelled.
Record the last ECG before turning inhaled anesthesia off and removing the mouse's paws from the electrodes. Increase oxygen to 2 L/min and maintain ventilation until the mouse regains consciousness.
Allow mouse to recover in a constant temperature controlled environment, e.g. heating pad or warm incubator, to avoid infarct variability. Treat mouse with buprenorphine 24 hr after the surgery and then as needed as indicated by the mouse grimace scale. Note: Procedure for reperfusion is also discussed in detail by Xu et al.5
Representative Results
A normal murine ECG is displayed in Figure 2 with alphabetic markers for electrical events P, Q, R, S, J and T. P is the initial atrial depolarization. QRS is the wave of depolarization over the ventricles. J is early repolarization and T represents heterogeneous repolarization also known as recovery11. It should be noted that many labs do not use the J-wave nomenclature and instead refer to the SJT-segment as the ST-segment10,15-17. Here, results and analyses are representative and based off of laboratory observations of 40 mice. Most mice exhibited similar waveform progressions over the course of the surgery. Mice that did not exhibit similar waveforms were flagged for further analysis and were considered non-infarcted animals. Similar waveform results have also been reported by Jong et al.15.
Murine hearts suffering from regional ischemia due to LAD occlusion typically show increased amplitude of the R-wave as well as hyperacute peaking of the JT-segment followed by eventual elevation of the ST-segment. Figure 3 shows the first sign of acute myocardial ischemia; hyperacute peaking of the T-wave. As can be seen in this figure, the T-wave has increased in amplitude from baseline conditions. However, this is not yet ST-segment elevation because the S-wave is still projecting deeply and negatively as it does on the baseline waveform.
Baseline ECG configuration displays a negatively projecting S-wave (Figure 2). As time progresses, ECG changes are noted. The ST-segment is defined as the segment between the end of the S-wave and the start of the T-wave. This ST-segment is clear in humans. Due to high heart rate, this segment is merged in mice and an additional, early repolarization "J-wave" separates the S- and T-waves. Therefore, the elevation of the S-wave to the isoelectric line or higher should be considered as the murine version of ST-segment elevation. In Figure 4 the progression of ischemia to early infarction can be seen by ST-segment elevation. Here the S-wave is displaying at elevated amplitude, above the isoelectric line. The J-wave is also elevated, especially when compared to the baseline waveform (Figure 2). Therefore, the ST-segment is elevated which is indicative of injury/infarction10.
Figure 5 follows the progression of one mouse from baseline all the way through reperfusion. The first waveform displays a normal sinus rhythm which was the recorded baseline. The second waveform displays the ECG 1 min after the ligature was tied and the artery became occluded. The red circle on this line indicates hyperacute T-wave peaking. If compared with "Baseline," it is clear that the T-wave is elevated. The third waveform shows the complete ST-segment elevation at the 5 min time point. In the "1 min ischemia" image, the S-wave was still projecting negatively, passing the isoelectric line. However, at 5 min the S portion of the complex does not reach as far negative as it should before progressing into the J- and T-waves. This is described as ST-segment elevation because the segment between the S- and T-waves is elevated from the isoelectric line. Another electrophysiological marker of regional ischemia is widening of the QT-interval16 which extends from the beginning of the QRS complex and continues until the end of the T-wave. At 20 min of ischemia, the QT-interval has widened and the ST-segment is still elevated. After 45 min of ischemia, the QT-interval remains widened and the ST-segment remains elevated.
Reperfusion of myocardium that has been ischemic for 30 min or less should result in the ECG returning back to baseline conditions in a mouse. Preda and Burlacu established a correlation between murine electrocardiographic changes, ischemic time, and infarct severity17. It was observed that ischemic periods of 30 min did not cause permanent ECG changes whereas ischemic periods of 1 hr did cause permanent ECG changes. Additionally, reperfusion of the occluded artery after 24 hr of ischemia had no salvage effect17. Normally, Q-waves can be identified as the slight downward projection just before the depolarization QRS-complex. Significant, or pathologic Q-waves can develop shortly after onset of myocardial ischemia as considerable muscle death begins to set in10. Significant Q-waves are defined as at least 1/3 of the height of the corresponding R-wave or by their elongated time, resulting in a wide Q-wave. The significant Q-wave results from a region of dead myocardium deflecting electrical currents away from the electrode7. After 5 min of reperfusion, evidence of deep, significant Q-waves begin to appear (Figure 5). Additionally, the T-wave returns to the isoelectric line (Figure 5). After 30 min of reperfusion, the negative Q-waves remain and are likely showing permanent damage. At this time point, the Q-waves are wide and deep, and indicate that the dying heart tissue is deflecting electrical currents around the damaged area (Figure 5).
After continuous ischemia, the progression to injury and infarction leads to enhanced negative T-wave projection (Figure 5, 30 min reperfusion, second red circle). This enhanced T-wave projection due to a true infarction will usually be permanent7. The second red circle in the 30 min reperfusion waveform shows what appears to be an inverted T-wave (Figure 5). If the 5 and 30 min T-waves are compared it is clear that the T-wave is projecting more negatively. This, coupled with the significant Q-waves provides evidence for permanent tissue damage to this heart. It should be noted that inhaled isoflurane anesthesia reduces heart rate, and therefore increases QT-intervals. However, amplitude of recovery T-waves remains unaffected18.
The aforementioned changes can be quantitatively analyzed in terms of voltage. Figure 6 shows that exporting physiological data as a .csv file will provide a very large amount of data. In addition to offering ECG values (amplitudes) at fractions-of-millisecond rates there may be options to include other data from respirations, temperature probes, blood pressure cuffs etc. if so desired. These quantitative data can be graphed as shown in Figure 7. Graphing a series of waveforms from P-wave to P-wave helps visualize an ECG configuration trend. The time period of 500 ms is a good time frame to visualize since any less time may not result in enough waveforms and any additional time will make the graph appear cluttered and electrophysiological events may be missed or difficult to recognize when viewed on a standard computer monitor.
 Figure 1: Correct Placement of Mouse on ECG Pad. This mouse is positioned in supine position. Each one of the mouse's paws are taped to the corresponding electrodes on the ECG surface pad. Please click here to view a larger version of this figure.
Figure 1: Correct Placement of Mouse on ECG Pad. This mouse is positioned in supine position. Each one of the mouse's paws are taped to the corresponding electrodes on the ECG surface pad. Please click here to view a larger version of this figure.
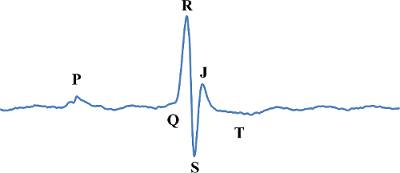 Figure 2:Normal Murine Baseline ECG Waveform. The normal murine baseline ECG is labeled with the letters P, Q, R, S, J and T which are used to describe electrical events in the heart. Please click here to view a larger version of this figure.
Figure 2:Normal Murine Baseline ECG Waveform. The normal murine baseline ECG is labeled with the letters P, Q, R, S, J and T which are used to describe electrical events in the heart. Please click here to view a larger version of this figure.
 Figure 3:T-wave Elevation. Also known as hyperacute T-wave or peaking. The T-wave is amplified and higher than the baseline T-wave (Figure 2). Please click here to view a larger version of this figure.
Figure 3:T-wave Elevation. Also known as hyperacute T-wave or peaking. The T-wave is amplified and higher than the baseline T-wave (Figure 2). Please click here to view a larger version of this figure.
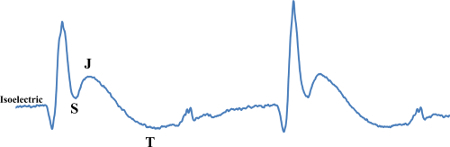 Figure 4:ST-segment Elevation. This figure displays ST-segment elevation which can be observed as the ST-segment is higher than the isoelectric point. Please click here to view a larger version of this figure.
Figure 4:ST-segment Elevation. This figure displays ST-segment elevation which can be observed as the ST-segment is higher than the isoelectric point. Please click here to view a larger version of this figure.
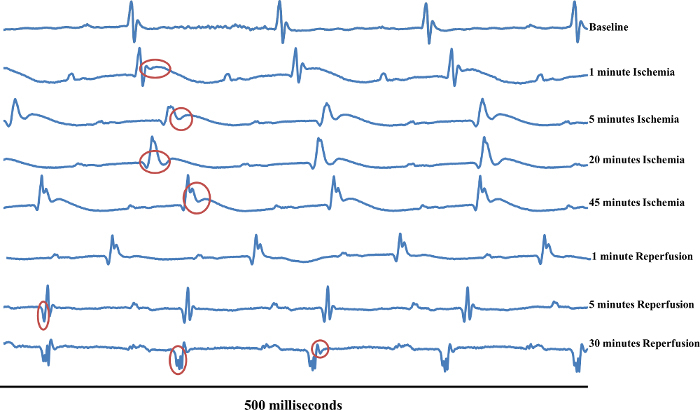 Figure 5:Typical ECG Changes Over the Course of an Ischemia/Reperfusion Surgery. This figure follows one mouse over the duration of myocardial Ischemia/Reperfusion surgery. The first waveform displays a normal sinus rhythm which was the recorded baseline (Baseline). The second waveform (1 min ischemia) displays the waveform 1 min after the ligature was tied and the artery became occluded. The red circle on this line shows hyperacute T-wave peaking. The red circle on the third waveform (5 min Ischemia) displays the complete ST-segment elevation. The red circle on the fourth waveform (20 min Ischemia) displays a widened QT-interval and the S-wave is still elevated. The red circle on the fifth waveform (45 min Ischemia) displays widened QT-segment and elevated ST-segment. The sixth waveform (1 min Reperfusion) displays no significant changes versus 45 min ischemia.The red circle on the seventh waveform (5 min Reperfusion) displays deep, significant Q-wave form. The first red circle in the eighth waveform (30 min Reperfusion) displays significant Q-waves, while the second red circle displays possible T-wave enhancement. Please click here to view a larger version of this figure.
Figure 5:Typical ECG Changes Over the Course of an Ischemia/Reperfusion Surgery. This figure follows one mouse over the duration of myocardial Ischemia/Reperfusion surgery. The first waveform displays a normal sinus rhythm which was the recorded baseline (Baseline). The second waveform (1 min ischemia) displays the waveform 1 min after the ligature was tied and the artery became occluded. The red circle on this line shows hyperacute T-wave peaking. The red circle on the third waveform (5 min Ischemia) displays the complete ST-segment elevation. The red circle on the fourth waveform (20 min Ischemia) displays a widened QT-interval and the S-wave is still elevated. The red circle on the fifth waveform (45 min Ischemia) displays widened QT-segment and elevated ST-segment. The sixth waveform (1 min Reperfusion) displays no significant changes versus 45 min ischemia.The red circle on the seventh waveform (5 min Reperfusion) displays deep, significant Q-wave form. The first red circle in the eighth waveform (30 min Reperfusion) displays significant Q-waves, while the second red circle displays possible T-wave enhancement. Please click here to view a larger version of this figure.
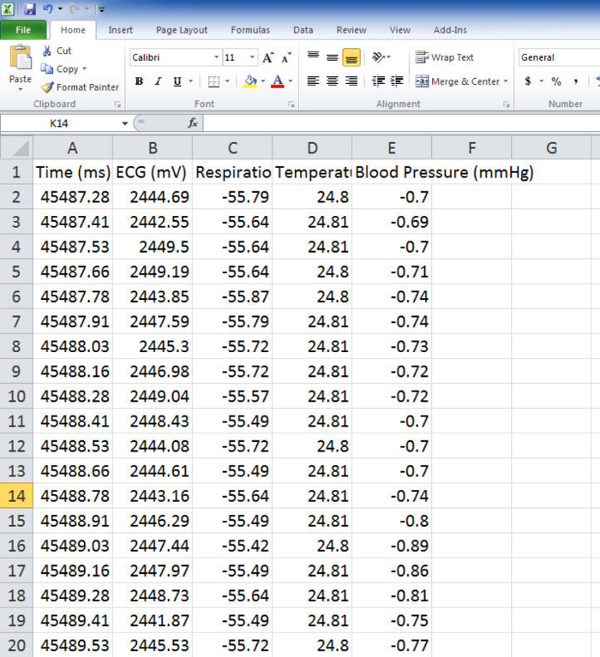 Figure 6:Physiological Data. This figure shows the physiological data as it is exported as .csv file to spreadsheet. Please click here to view a larger version of this figure.
Figure 6:Physiological Data. This figure shows the physiological data as it is exported as .csv file to spreadsheet. Please click here to view a larger version of this figure.
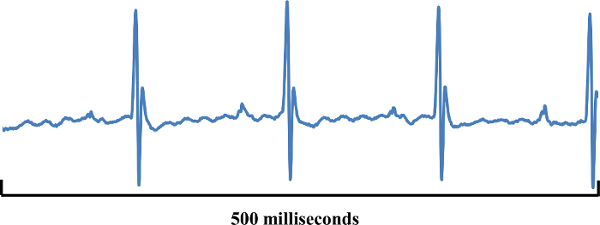 Figure 7: Graphed mV Values. The graph displayed in this figure shows mV values from three consecutive and complete waveforms using the physiological data file (Figure 6). The graph is a simple line graph using points from the physiological data file. Please click here to view a larger version of this figure.
Figure 7: Graphed mV Values. The graph displayed in this figure shows mV values from three consecutive and complete waveforms using the physiological data file (Figure 6). The graph is a simple line graph using points from the physiological data file. Please click here to view a larger version of this figure.
Discussion
Using ECG changes as a supplemental method for confirming myocardial ischemia and reperfusion ensures the accurate placement of the occluding ligature. Accuracy of ligature placement is critical to reducing data variability among animals. The LAD in a mouse heart is a difficult artery to visualize. Therefore, supplementing visual pallor with electrocardiographic changes will help ensure the correct placement of the ligature and resulting tissue damage.
Since the ECG pad offers a non-invasive view of the heart, multiple ECGs can be obtained during the course of the study. This can help provide a better understanding of cardiac changes that occur during and after the surgery. It is critical to obtain a baseline ECG to use for comparison after the surgical procedure. Later stage tissue death and even ventricular aneurysm can be observed by deflections of electrophysiological signals and ECG configuration changes. This may provide insight into the progression of heart failure.
The advantages of measuring ECG using echocardiographic machine include the simultaneous measurement of structural and functional parameters of the heart prior to or after the ischemia/reperfusion surgery. The limitations of the system to record ECG include the high cost to purchase an echocardiography machine. However if the experiments require constant ECG monitoring over multiple days, there are a variety of apparatuses available for ECG recording including remote telemetric ECG units with corresponding software that can be programed to record and analyze waveforms at various time intervals. However, many of the ECG telemetry units require an implantation procedure or specialized habitats. Additionally, many alterative electrode options exist including electrode clips and needles. Ischemia/Reperfusion surgery via thoracotomy is a highly invasive procedure. Advantages to using the ECG pad with an echocardiography machine include non-invasive procedure with no wires connected to the animal during surgery and no extra surgical procedure. However, investigators should determine the best equipment for their laboratory and experimental needs.
As mentioned previously, isoflurane anesthesia decreases heart rate. Additionally, isoflurane may be cardioprotective via activation of KATP channels and therefore reduce infarct size, as has been found in dogs19. General anesthesia in mice can be induced using injectable agents. Inhalation anesthesia does provide greater safety, particularly for prolonged procedures. However, inhalation anesthesia requires complex and expensive equipment such as precision vaporizers and flowmeters, specific breathing systems, and efficient scavenging systems to prevent pollution. The disadvantages of injectable anesthetics include difficulty in choosing an initial dose, no chance of accurately modulating the depth of prolonged anesthesia, prolonged recovery, etc. The choice of anesthesia must be adapted according to the length of the procedure and aim of the study20.
Using ECG as a supplemental method for confirming ischemia/reperfusion injury in mice will help improve consistency and reproducibility of infarctions but also opens the possibility for future applications of the technique through establishing quantitative trends. Investigators may notice similar ECG configurations within certain experimental groups. For instance, a genetically modified animal group may exhibit unusually wide QT-intervals after surgery when compared to the wild type. This informative data would have been missed if investigators use myocardial color changes as a sole confirmation of ischemia and reperfusion injury. For comparative studies between wild type and transgenic mice, considerations to the Lambeth Conventions guidelines may also be valuable, especially with respect to age, sex, blinding and randomization of animals21.
In conclusion, supplemental confirmation of myocardial ischemia/reperfusion injury offers multiple benefits. Using ECG as a supplemental technique can help establish consistency in surgery. This may help decrease the number of animals used, while providing higher quality data. It also allows investigators to monitor cardiac injury, tissue death and/or remodeling non-invasively over time. Lastly, using ECG as a confirmation of myocardial ischemia/reperfusion offers the possibility of establishing quantitative electrophysiological trends.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by Merit Review awards (BX002332 and BX000640) from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development, National Institutes of Health (R15HL129140), and funds from Institutional Research and Improvement account. The project is supported in part by the National Institutes of Health grant C06RR0306551.
References
- Kochanek KD, Murphy SL, Xu J. Deaths: Final Data for 2011. Natl Vital Stat Rep. 2015;63(3):1–120. [PubMed] [Google Scholar]
- World Health Organization. The 10 leading causes of death in the world. 2012. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/http://www.who.int/mediacentre/factsheets/fs310/en/
- Klabunde RE. Cardiovascular Physiology Concepts 2edn. Philadelphia: Wolters Kluwer Health Lippincott Williams & Wilkins; 2012. p. 243. [Google Scholar]
- Bhardwaj R, Kandoria A, Sharma R. Myocardial infarction in young adults-risk factors and pattern of coronary artery involvement. Niger Med J. 2014;55(1):44–47. doi: 10.4103/0300-1652.128161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Alloush J, Beck E, Weisleder N. A murine model of myocardial ischemia-reperfusion injury through ligation of the left anterior descending artery. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Fernández B, et al. The coronary arteries of the C57BL/6 mouse strains: implications for comparison with mutant models. J Anat. 2008;212(1):12–18. doi: 10.1111/j.1469-7580.2007.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler MS. The Only EKG Book You'll Ever Need. Philadelphia: Lippincott Williams & Wilkins; 2003. 4 edn. [Google Scholar]
- Poirier P. Exercise, heart rate variability, and longevity: the cocoon mystery? Circulation. 2014;129(21):2085–2087. doi: 10.1161/CIRCULATIONAHA.114.009778. [DOI] [PubMed] [Google Scholar]
- Boudoulas KD, Borer JS, Boudoulas H. Heart Rate, Life Expectancy and the Cardiovascular System: Therapeutic Considerations. Cardiology. 2015;132(4):199–212. doi: 10.1159/000435947. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Kirchhoff S, Doevendans PA. Mouse electrocardiography: an interval of thirty years. Cardiovasc Res. 2000;45(1):231–237. doi: 10.1016/s0008-6363(99)00335-1. [DOI] [PubMed] [Google Scholar]
- Boukens BJ, Rivaud MR, Rentschler S, Coronel R. Misinterpretation of the mouse ECG: 'musing the waves of Mus musculus. J Physiol. 2014;592(21):4613–4626. doi: 10.1113/jphysiol.2014.279380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara PT, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82(1):E1–E27. doi: 10.1002/ccd.24776. [DOI] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. Washington DC: National Academies Press; 2011. 8 edn. [PubMed] [Google Scholar]
- Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in Mice. Curr Protoc Mouse Biol. 2011;1:71–83. doi: 10.1002/9780470942390.mo100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong WM, et al. Reduced acute myocardial ischemia-reperfusion injury in IL-6-deficient mice employing a closed-chest model. Inflamm Res. 2016;65(6):489–499. doi: 10.1007/s00011-016-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, et al. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009;46(6):960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preda MB, Burlacu A. Electrocardiography as a tool for validating myocardial ischemia-reperfusion procedures in mice. Comp Med. 2010;60(6):443–447. [PMC free article] [PubMed] [Google Scholar]
- Speerschneider T, Thomsen MB. Physiology and analysis of the electrocardiographic T wave in mice. Acta Physiol (Oxf. 2013;209(4):262–271. doi: 10.1111/apha.12172. [DOI] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87(2):361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Gargiulo S, et al. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J. 2012;53(1):E55–E69. doi: 10.1093/ilar.53.1.55. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, et al. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther. 2013;139(2):213–248. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]


