Abstract
Stromules, or "stroma-filled tubules", are narrow, tubular extensions from the surface of the chloroplast that are universally observed in plant cells but whose functions remain mysterious. Alongside growing attention on the role of chloroplasts in coordinating plant responses to stress, interest in stromules and their relationship to chloroplast signaling dynamics has increased in recent years, aided by advances in fluorescence microscopy and protein fluorophores that allow for rapid, accurate visualization of stromule dynamics. Here, we provide detailed protocols to assay stromule frequency in the epidermal chloroplasts of Nicotiana benthamiana, an excellent model system for investigating chloroplast stromule biology. We also provide methods for visualizing chloroplast stromules in vitro by extracting chloroplasts from leaves. Finally, we outline sampling strategies and statistical approaches to analyze differences in stromule frequencies in response to stimuli, such as environmental stress, chemical treatments, or gene silencing. Researchers can use these protocols as a starting point to develop new methods for innovative experiments to explore how and why chloroplasts make stromules.
Keywords: Plant Biology, Issue 117, stromules, chloroplasts, leucoplasts, plastids, redox signaling, cell signaling, plant cell biology
Introduction
Chloroplasts are dynamic organelles in plant cells responsible for photosynthesis and a host of other metabolic processes. Signaling pathways from the chloroplast also exert significant influence on plant physiology and development, coordinating plant responses to environmental stress, pathogens, and even leaf shape1-6. Recently, biologists have gained interest in a poorly understood aspect of chloroplast structure: stromules, very thin stroma-filled tubules that extend from the surface of the chloroplast7.
The biological functions of stromules remain unknown, although stromule frequency is known to vary in response to environmental stimuli7-9, and stromules may be capable of transmitting signaling molecules between organelles6. All types of plastids (not only the green, photosynthetic chloroplasts, but also clear leucoplasts, starch-filled amyloplasts, and pigmented chromoplasts, to name a few types of plastids) make stromules, and stromules are found in all land plant species that have been examined to date. Stromules can extend and retract dynamically, appearing or disappearing within seconds, or they can remain relatively stationary for long times. One of the major hurdles facing stromule biologists is that stromules are often studied using dramatically different methods, tissues, and species, making comparisons across the stromule biology literature difficult. Going forward, standard practices and thorough descriptions of the experimental systems used to study stromules will be critical to discovering the function of these ubiquitous features of chloroplast morphology.
Here we describe methods for visualizing stromule formation in the epidermal chloroplasts of Nicotiana benthamiana leaves. In the mesophyll, chloroplasts are densely packed into large, three-dimensional cells, which makes it difficult to accurately and rapidly visualize stromules by confocal microscopy. By contrast, epidermal cells are relatively flat, contain fewer chloroplasts, and are at the surface of the leaf, allowing for easy and rapid visualization of stromules. N. benthamiana is an ideal model system for these experiments because, unlike many plant species, all cells in the epidermis of N. benthamiana make chloroplasts10. In the epidermis of most plants, including Arabidopsis thaliana, only the stomatal guard cells have chloroplasts, while other epidermal cells have "leucoplasts", plastids that are clear, relatively amorphous, and nonphotosynthetic9,11,12. Thus, whereas a single field of view of an A. thaliana epidermis might show only a handful of chloroplasts in a pair of guard cells, a field of view of an N. benthamiana epidermis will include dozens or even hundreds of chloroplasts. All of the methods described here, however, can be modified to investigate other questions in stromule biology; for example, we have used the same approach to study leucoplast stromules of A. thaliana9.
Protocol
NOTE: For this protocol, we have focused on assaying stromule frequency in the epidermis of N. benthamiana leaves. Several stable transgenic lines have been generated that can be used for this purpose, including 35SPRO:FNRtp:EGFP13 and NRIP1:Cerulean6. Both of these lines show robust expression of fluorophores in the chloroplast stroma of leaves grown under a wide range of conditions. Alternatively, chloroplast-targeted fluorophores may be transiently expressed in N. benthamiana using Agrobacterium transformations13. This is less ideal than the transgenic lines, since Agrobacterium infiltrations induce some basal defense responses in N. benthamiana and interactions with Agrobacterium can alter stromule frequency in the leaf14, potentially complicating interpretation of results. Finally, to visualize stromule formation in vitro, chloroplasts may be extracted from any plant species, using either genetically encoded fluorophores or a fluorescent dye, as described in section 5 below.9,15
NOTE: Detailed methods for plant cultivation have been previously described.16 Briefly, grow N. benthamiana plants in 4" pots filled with any professional soil mix that provides good drainage. Cover seedlings with a clear plastic dome for the first 10-14 days to provide a humid environment for germination. Add any standard fertilizer mix following manufacturer's instructions to 14-day-old plants. Grow plants under white light, using ~100 µmol photons m-2 sec-1 light intensity. Water plants regularly.
1. Preparing Leaf Samples for Visualization
NOTE: Stromule dynamics are affected by wounding8, so tissue preparation should be conducted immediately before visualizing stromules by confocal fluorescence microscopy. Ideally, a sample should be visualized with 15 min after removal from the plant.
Cut a small section of the leaf using a very sharp razor blade. Always use the same region of the leaf for consistency, and be certain to visualize the same surface (adaxial or abaxial) in all experiments.
- Immediately after cutting the leaf section, submerge the leaf section in a 5 ml needleless syringe filled with water, remove air from the syringe, and apply a vacuum by covering the syringe orifice with a finger and pulling the plunger.
- Release the plunger gently to avoid damaging the leaf section. Repeat this two or three times, or until most of the air has been removed and the leaf appears to be deep green. NOTE: This step removes air in the leaf that can interfere with accurate visualization of stromules.
Add a drop of water to a slide and place the leaf section on this drop. Then, add another drop of water to the top of the leaf, and place a cover slip over the leaf. If there are any air bubbles, gently tap the cover slip until they are removed. The slide is now ready for microscopy, and should be visualized immediately.
2. Visualizing Stromules with Confocal Fluorescence Microscopy
With transmitted light, focus on a field of view near the center of the leaf section (away from the cells damaged by the razor blade). Use a 20X objective so that many chloroplasts can be visualized simultaneously. Save an image with transmitted light so that cell types can be distinguished later, if necessary.
- Switch the illumination source to a laser with appropriate excitation and emission filters for the fluorophore being used. For example, excite GFP with a 488 nm laser and collect emissions between 500 nm and 525 nm.
- Using the microscope's software, select the GFP channel and click to adjust the pinhole aperture to 1 Airy unit. Select laser scanning, to start visualizing the sample, and then click the GFP channel to adjust the laser intensity and the detector gain to minimize the laser power while still clearly visualizing stromules. Once these settings have been determined, keep them as consistent as possible throughout all experiments.
- Prepare a z-stack experiment that will collect a series of images through the leaf epidermis in the field of view.
- For the z-stack, include all epidermal chloroplasts in the field of view to avoid bias (for example, if chloroplasts near the leaf surface have more stromules under conditions tested).
- Take images at the maximum interval possible that will include all stromules but minimize the time required for a z-stack. For example, if 1 Airy unit is equivalent to an in-focus confocal section that is 2 µm deep, taking an image every 2 µm is likely ideal. NOTE: The leaf epidermis is an uneven surface, so the total depth of the z-stack will vary depending on the sample; most z-stacks will require 10-20 images, but may include more.
- Adjust scanning speed and image resolution as necessary to make sure that the z-stack is collected quickly (typically within 5-10 min, if possible). Save the z-stack for later analysis.
3. Image Processing
Determine stromule frequency using any image analysis software. For this protocol, we recommend using ImageJ, which is publicly available from the National Institutes of Health (http://imagej.nih.gov/ij/), or the popular upgrade of ImageJ, Fiji (http://fiji.sc/Fiji).
Merge the z-stack into a single image using the maximum intensity projection in the software.
Identify and manually count all chloroplasts in the image.
For each chloroplast, visually determine whether one or more stromules are seen extending from the chloroplast in the merged z-stack image. For examples, see Figure 1. NOTE: Since the biological functions of stromules are not known, any thin, stroma-filled, tubular extension from the chloroplast can be considered a "stromule", regardless of length. As a general rule, a stroma-filled extension from the chloroplast is a stromule if it is less than approximately 1 µm in diameter along most of its length7. Ensure that one person analyzes all images to control for subjective differences in determining whether or not a chloroplast has a stromule. A second researcher should independently verify the stromule frequencies to further reduce subjective biases.
4. Experimental Design and Sampling
NOTE: Stromule frequency is highly variable between leaves, but several reports suggest that there is little variation in stromule frequency within an individual leaf9,17.
Calculate the leaf stromule frequency from a single field of view of one leaf. Discard the leaf, and consider this an independent sample. Do not consider separate fields of view from one leaf as independent samples. NOTE: There is no strong consensus on how to best measure changes in stromule activity, and until the function of stromules is more clearly defined, researchers should continue to be creative in quantifying stromule dynamics. For comparison across the literature, however, common standards of reporting should be used in addition to more creative approaches. At a minimum, always report the frequency of chloroplasts that have one or more stromules.
- Determine the frequency of chloroplasts that have stromules in a field of view. Use ImageJ to identify all chloroplasts in a field of view. Then, for each chloroplast, determine whether the chloroplast has formed at least one stromule. Report stromule frequency as the percentage of chloroplasts with a stromule. NOTE: Some researchers transform the percentage, for example with the arcsine transformation, before reporting stromule frequencies; while this is an option for statistical analysis, the arcsine of stromule frequency is not an intuitive value, and does not need to be reported in the main text or figures of a study.
- As an alternative approach, count the total number of stromules, and divide it by the total number of chloroplasts. NOTE: Under most circumstances, this will yield similar results to approach 4.2; it may overestimate the stromule frequency, however, if a single chloroplast has formed many stromules. In the example shown under representative results, for example, several chloroplasts have two or more stromules, raising the number of stromules per chloroplast to 40/87 (versus a stromule frequency of 33/87).
- Alternatively, determine the stromule length instead of stromule frequency. Length can be measured in ImageJ by tracing the stromule with the freehand line tool. NOTE: Since the biological role of stromules remains unknown, it is not clear that stromule length affects their function. Report significant differences in stromule length if they are observed, but also report the stromule frequencies (as described in 4.2). NOTE: Stromule frequency can be highly variable among different leaves under the same growth conditions. Therefore, although determining stromule frequency can be time-consuming, experiments should be carefully designed to include large sample sizes. Using datasets from our lab, we determined that a sample size of at least 16 plants for each treatment is usually sufficiently powerful to determine if there is a statistically significant difference of at least a 1.5-fold change in stromule frequency between two conditions (with standard α = 0.05 and β = 0.80).
- Statistical analysis of results.
- To compare the mean stromule frequencies of two treatments, use the Welch's t test (also known as the heteroscedastic t test or t test assuming unequal variance). NOTE: This test is not as powerful as the conventional Student's t test, but Welch's t test is advantageous because it does not assume that the variance in stromule frequency distribution is similar between treatments. NOTE: Since stromule frequency is a "proportion", its sampling distribution is predicted to be binomial rather than normal, which could theoretically skew statistical analysis if sample sizes are very low or if stromule frequency is very low (close to 0%) or very high (close to 100%). Some reports have used arcsine transformations before statistical analysis, which is intended to transform a binomial distribution to a normal distribution, and thus satisfy the standard requirements of a Student's t test or ANOVA. In practice, however, arcsine transformations have little or no effect on statistical analysis, and are therefore not recommended. Instead, repeat experiments to increase sample size, which will increase statistical power and reduce errors in interpretation of data.
- Whatever statistical approach is used, be sure to carefully report the sampling strategy, sample size, statistical test, and p value for each experiment. NOTE: As with other fields of biology, a p value less than 0.05 may be considered "statistically significant", although researchers should certainly report the precise p value obtained. If p is slightly above 0.05, increasing the sample size with two or three experiments may help to clarify whether or not the difference observed is reproducible and statistically significant.
5. Extracting Intact Chloroplasts to Visualize Stromule Dynamics
NOTE: Several methods have been used to isolate chloroplasts from leaves, including a slightly different protocol in a recent study on stromule formation in vitro15. The protocol detailed below uses a relatively simple method that does not yield biochemically pure chloroplast samples, but does instead isolate a large quantity of intact, healthy chloroplasts9,18.
Prepare cold extraction buffer: 50 mM Hepes NaOH, 330 mM sorbitol, 2 mM EDTA, 1.0 mM MgCl2, and 1.0 mM MnCl2. Adjust the pH to 6.9 with NaOH and HCl, and refrigerate before use. NOTE: Although not necessary, using cold buffers and keeping samples on ice when possible will yield a higher proportion of intact chloroplasts.
Prepare isolation buffer: 50 mM Hepes NaOH, 330 mM sorbitol, 2 mM EDTA, 1.0 mM MnCl2, 1.0 mM MgCl2, 10 mM KCl, and 1.0 mM NaCl. Adjust the pH to 7.6 with NaOH and HCl and refrigerate before use.
If the leaves are not expressing a genetically encoded stromal fluorophore, prepare carboxyfluorescein diacetate (CFDA) solution: 50 mM CFDA stock solution in dimethyl sulfoxide (DMSO) (2.3 mg CFDA per 1.0 ml DMSO). NOTE: The exact concentration is not critical. Keep CFDA stock in the dark at all times. Store small aliquots of 50 mM CFDA at -20 °C.
To isolate chloroplasts, remove ~5-10 g leaves from several plants and briefly rinse in cold water. Immediately transfer to 50 ml cold extraction buffer. Grind leaves using a blender with several short pulses. Filter through two or three layers of cheesecloth to remove leaf debris, divide the extracted chloroplasts into two 50 ml centrifuge tubes, and centrifuge for 1 min at 750 x g.
Discard the supernatant and resuspend the green chloroplasts in 10 ml isolation buffer. Centrifuge again for 1 min at 750 x g, discard supernatant, and resuspend chloroplasts in isolation buffer to bring final volume to 5 ml.
Transfer 20 µl of the chloroplasts to a slide, cover with a coverslip, and begin microscopy. NOTE: Chloroplasts from transgenic plants expressing plastid-targeted fluorescent proteins are now ready for visualization by confocal fluorescence microscopy.
- Stain chloroplasts from plants that are not expressing plastid-targeted fluorescent proteins to visualize stromules.
- Add 5 µl of 50 mM CFDA stock to the 5 ml chloroplasts-suspended in isolation buffer. Bring the final concentration to 50 µM.
- Allow chloroplasts to incubate for 5 min, and then transfer a small aliquot (~50 µl) to a slide, cover with a coverslip, and begin microscopy.
- Use a FITC or GFP filter set. Use a 20X objective to visualize many chloroplasts simultaneously, or a higher objective to visualize a single isolated chloroplast. NOTE: CFDA will fluoresce inside the stroma of intact chloroplasts.
- If there is strong background fluorescence, wash the chloroplasts again in 10 ml isolation buffer after the 5 min incubation with CFDA, centrifuge for 1 min at 750 x g, discard supernatant, and resuspend in 5 ml isolation buffer.
Representative Results
This protocol was used to visualize stromule frequency at day and at night in the cotyledons of young N. benthamiana seedlings. Slices from a z-stack were merged into a single image (Figure 1A). For visual purposes, that image was then desaturated and inverted so that stroma appears black (Figure 1B). The chloroplasts were labeled either as having no stromules (green asterisk) or having at least one stromule (magenta asterisk). Of the 87 epidermal chloroplasts visualized, 33 have stromules. Thus, the stromule frequency in this leaf is 37.9%.
Using this analysis, the protocol was repeated for another 21 plants (one leaf per plant) during the day and a total of 24 plants at night. In the day, the average stromule frequency was 20.8 ± 1.8%; at night, the average stromule frequency was 12.8 ± 0.9% (Figure 2). Stromule frequency was significantly higher during the day, as determined by the Welch's t test (n ≥ 22, p < 0.0005). Note that although over 23,000 chloroplasts and several hundred cells were observed, the sample size, n, is reported as 22, the number of independent plants examined.
Using the protocol described here, chloroplast stromules were observed in vitro after isolation from leaves of N. benthamiana expressing plastid-targeted GFP (Figure 3A), or after using CFDA to stain chloroplasts isolated from N. benthamiana (Figure 3B) or Spinacia oleracea (Figure 3C).
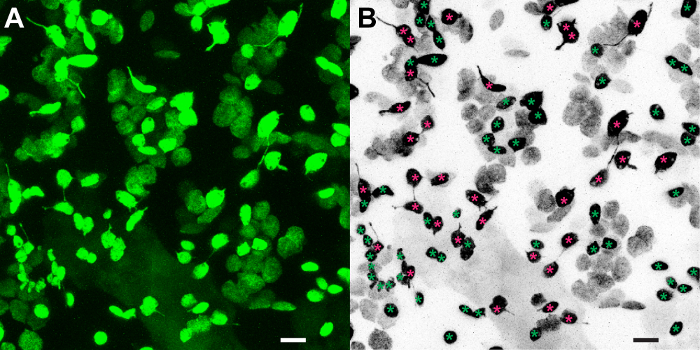 Figure 1. Quantifying stromule frequency in N. benthamiana leaves. (A) A z-stack from a partial field of view of the epidermis of N. benthamiana expressing stromal GFP was merged to show all epidermal chloroplasts and stromules in a single image. (B) This image was converted to black and white and inverted for visual purposes. Chloroplasts with stromules are indicated by a magenta asterisk; chloroplasts without stromules are indicated by a green asterisk. Scale bars represent 10 µm. Modified from Brunkard et al.9
Please click here to view a larger version of this figure.
Figure 1. Quantifying stromule frequency in N. benthamiana leaves. (A) A z-stack from a partial field of view of the epidermis of N. benthamiana expressing stromal GFP was merged to show all epidermal chloroplasts and stromules in a single image. (B) This image was converted to black and white and inverted for visual purposes. Chloroplasts with stromules are indicated by a magenta asterisk; chloroplasts without stromules are indicated by a green asterisk. Scale bars represent 10 µm. Modified from Brunkard et al.9
Please click here to view a larger version of this figure.
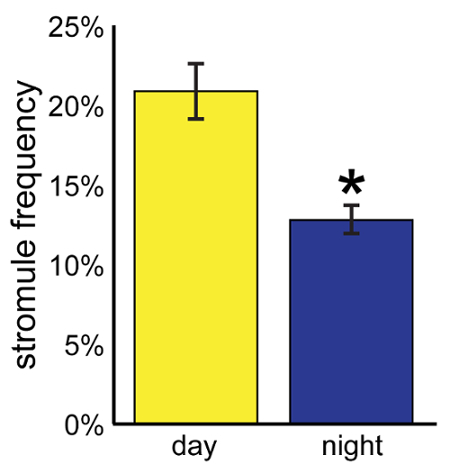 Figure 2.Comparing stromule frequency across conditions. This protocol was used to determine the stromule frequency in 22 plants in the day and 24 plants at night. Stromule frequency was significantly higher during the day than at night (Welch's t test, n ≥ 22, p < 0.0005). Error bars indicate standard error. Modified from Brunkard et al.9
Please click here to view a larger version of this figure.
Figure 2.Comparing stromule frequency across conditions. This protocol was used to determine the stromule frequency in 22 plants in the day and 24 plants at night. Stromule frequency was significantly higher during the day than at night (Welch's t test, n ≥ 22, p < 0.0005). Error bars indicate standard error. Modified from Brunkard et al.9
Please click here to view a larger version of this figure.
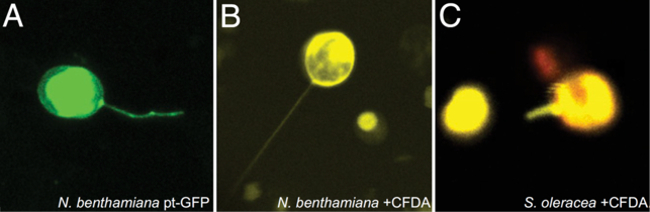 Figure 3. Visualizing stromules after extracting intact chloroplasts from leaves. (A) Chloroplasts from transgenic N. benthamiana leaves expressed GFP (green) targeted to the chloroplast were isolated using the protocols described here. (B) Chloroplasts were isolated from wild-type N. benthamiana leaves and stained with CFDA, which fluoresces only inside intact, viable chloroplasts (shown in yellow). (C) Chloroplasts can also be isolated from other plant species, such as spinach (S. oleracea), and then stained with CFDA (yellow). Chlorophyll autofluorescence is shown in red. Modified from Brunkard et al.9
Figure 3. Visualizing stromules after extracting intact chloroplasts from leaves. (A) Chloroplasts from transgenic N. benthamiana leaves expressed GFP (green) targeted to the chloroplast were isolated using the protocols described here. (B) Chloroplasts were isolated from wild-type N. benthamiana leaves and stained with CFDA, which fluoresces only inside intact, viable chloroplasts (shown in yellow). (C) Chloroplasts can also be isolated from other plant species, such as spinach (S. oleracea), and then stained with CFDA (yellow). Chlorophyll autofluorescence is shown in red. Modified from Brunkard et al.9
Discussion
When investigating stromules, three important factors must be considered throughout: (i) manipulation of the plant tissue must be kept to an absolute minimum, (ii) the experimental system must be kept consistent, and (iii) sampling strategies must be carefully planned to ensure robust, reproducible data are analyzed.
Stromules are remarkably dynamic: they can extend and retract rapidly before an observer's eyes under the microscope. Moreover, stromule frequency varies significantly in response to a wide range of treatments, including stimuli that cannot be avoided in order to visualize stromules (such as leaf wounding). The primary solution to this problem, which is addressed with the protocols described here, is to conduct well-controlled experiments, keeping all variables consistent. This includes, for example, preparing each sample for visualization immediately before bringing it to the microscope; plants should not be damaged until immediately before visualization. The second solution to this problem is to minimize the complexity of protocols: ideally, any treatments should be applied directly to the plant without removing the leaf or causing any damage.
Stromules have been studied in a dizzying array of species and cell types, including wheat root hairs, tomato fruits, and etiolated tobacco seedlings8,19. These pioneering studies advanced the understanding of stromule dynamics during plant development and explored the range of stromule activity. Drawing comparisons from these various experimental systems, however, can lead to misinterpretations, since different types of plastids are involved in considerably different biological processes.
For example, chloroplasts make stromules in response to oxidative stress caused by the photosynthetic electron chain, but since leucoplasts lack the photosynthetic machinery, they are not sensitive to the same stimuli9. Any experimental system may be used to investigate stromules, but the experimental system must be kept consistent throughout the study if any comparisons are to be drawn. Factors to consider include species, cell type, developmental stage or age of the plant, and method of staining stromules (whether through stable transgene expression, transient transgene expression, or an external fluorophore); even slight variations of these factors can confound later interpretation of results.
Finally, any study of stromule biology must use a robust, reliable sampling strategy to ensure that results are reproducible and biologically relevant. Sampling repeatedly from a single plant, e.g., taking images from several regions of one leaf, will apparently improve statistical power, but can be misleading. As shown in the representative data above, the stromule frequency of a single leaf may vary dramatically from the mean stromule frequency across many leaves: in that leaf, which was visualized during the day, 37.9% of chloroplasts made stromules, although the mean stromule frequency across leaves during the day was almost half that, only 20.8%.
Moreover, given how little is known about stromule dynamics at this time, any experiment should be entirely replicated at least three times, and more if possible, to ensure that unforeseen variables are not responsible for any changes in stromule activity that are observed. Above all else, since the stromule biology field is just beginning to take off, researchers should carefully document and report all aspects of experimental design, including creative deviations from the simple protocol outlined here.
Disclosures
The authors have nothing to disclose.
Acknowledgments
J.O.B. and A.M.R. were supported by predoctoral fellowships from the National Science Foundation.
References
- Koussevitzky S, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316(5825):715–719. [PubMed] [Google Scholar]
- Burch-Smith TM, Brunkard JO, Choi YG, Zambryski PC. PNAS Plus: Organelle-nucleus cross-talk regulates plant intercellular communication via plasmodesmata. Proc. Natl. Acad. Sci. USA. 2011;108(51):E1451–E1460. doi: 10.1073/pnas.1117226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebloom S, Brunkard JO, Cheung AC, Jiang K, Feldman L, Zambryski P. Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiol. 2012;158(1):190–199. doi: 10.1104/pp.111.186130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012;3:926. doi: 10.1038/ncomms1926. [DOI] [PubMed] [Google Scholar]
- Avendaño-Vázquez A-O, et al. An uncharacterized apocarotenoid-derived signal generated in ζ-carotene desaturase mutants regulates leaf development and the expression of chloroplast and nuclear genes in Arabidopsis. Plant Cell. 2014;26(June):2524–2537. doi: 10.1105/tpc.114.123349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, et al. Chloroplast stromules runction during innate immunity. Dev. Cell. 2015;34(1):45–57. doi: 10.1016/j.devcel.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Sattarzadeh A. Stromules: recent insights into a long neglected feature of plastid morphology and function. Plant Physiol. 2011;155(4):1486–1492. doi: 10.1104/pp.110.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, et al. Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 2012;69(3):387–398. doi: 10.1111/j.1365-313X.2011.04800.x. [DOI] [PubMed] [Google Scholar]
- Brunkard J, Runkel A, Zambryski P. Chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl. Acad. Sci. USA. 2015;112(32):10044–10049. doi: 10.1073/pnas.1511570112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P, Pwee K-H, Gray JC. Expression of photosynthesis gene-promoter fusions in leaf epidermal cells of transgenic tobacco plants. Plant J. 1991;1(1):115–120. [Google Scholar]
- Charuvi D, Kiss V, Nevo R, Shimoni E, Adam Z, Reich Z. Gain and loss of photosynthetic membranes during plastid differentiation in the shoot apex of Arabidopsis. Plant Cell. 2012;24(3):1143–1157. doi: 10.1105/tpc.111.094458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-H, et al. Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis. Plant Physiol. 2012;160(1):332–348. doi: 10.1104/pp.112.198705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattat M, Barton K, Baudisch B, Klösgen RB, Mathur J. Plastid stromule branching coincides with contiguous endoplasmic reticulum dynamics. Plant Physiol. 2011;155(4):1667–1677. doi: 10.1104/pp.110.170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JL, et al. Agrobacterium-derived cytokinin influences plastid morphology and starch accumulation in Nicotiana benthamiana during transient assays. BMC Plant Biol. 2014;14(1):127. doi: 10.1186/1471-2229-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Theg SM. The formation of stromules in vitro from chloroplasts isolated from Nicotiana benthamiana. PLoS One. 2016;11(2):e0146489. doi: 10.1371/journal.pone.0146489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JO, Burch-Smith TM, Runkel AM, Zambryski P. Investigating plasmodesmata genetics with virus-induced gene silencing and an Agrobacterium-mediated GFP movement assay. Method. Mol. Biol. 2015. pp. 185–198. [DOI] [PubMed]
- Schattat MH, Klösgen RB. Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana. BMC Plant Biol. 2011;11:115. doi: 10.1186/1471-2229-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly D, Carpentier R. Rapid isolation of intact chloroplasts from spinach leaves. Method. Mol. Biol. 2011;684:321–325. doi: 10.1007/978-1-60761-925-3_24. [DOI] [PubMed] [Google Scholar]
- Waters MT, Fray RG, Pyke KA. Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J. 2004;39(4):655–667. doi: 10.1111/j.1365-313X.2004.02164.x. [DOI] [PubMed] [Google Scholar]


