Abstract
Anxiolytic afobazole (5‐Ethoxy‐2‐[2‐(morpholino)‐ethylthio]benzimidazole dihidrochloride) has pronounced ligand properties toward Sigma‐1 receptor (σ1 receptor,SigmaR1) and MT 3 receptors. Our previous work demonstrated that afobazole possess cytoprotective effect in the in vitro model of menadione genotoxicity (Woods et al. 1997) through interaction with MT 3 receptor (Kadnikov et al. 2014). Present study utilized previously described models to address the contribution of SigmaR1 to cytoprotective action of afobazole. The reduction in afobazole cytoprotective effect observed after preincubation of cell suspension with selective SigmaR1 antagonist BD‐1047 revealed an important contribution of SigmaR1 in afobazole‐mediated effect. We confirmed our observation using selective SigmaR1 agonist PRE‐084. We conclude that pronounced cytoprotective effect of afobazole over PRE‐084 is likely achieved by additive SigmaR1 and MT 3‐mediated effects.
Keywords: afobazole, bone marrow cells, comet assay, menadione, sigma‐1 receptor
Abbreviations
- Afafobazole
5‐Ethoxy‐2‐[2‐(morpholino)‐ethylthio]benzimidazole dihidrochloride
- M‐11
2‐[2‐(3‐oxomorpholin‐4‐il)‐ethylthio]‐5‐ethoxybenzimidazole hydrochloride
- men
menadione
- MT1
melatonin receptor type 1A
- MT3
melatonin binding site of NQO2 enzyme
- NQO1
quinone reductase 1
- NQO2
quinone reductase 2
- SCGE‐assay
single‐cell gel electrophoresis assay
Introduction
Anxiolytic drug afobazole (5‐Ethoxy‐2‐[2‐(morpholino)‐ethylthio]benzimidazole dihidrochloride) (Fig. 1) was designed and pharmacologicaly studied FSBI “Research Zakusov Institute of Pharmacology”, Russia. Previous pharmacological studies have identified ligand interaction of afobazole with MT1 (K i = 1.6E‐05 M), MT3 (K i = 9.7E‐07 M), σ1 (SigmaR1) (K i = 5.9E‐06 M) receptors and MAO A (K i = 3.6E‐06 M) and defined its main effects as anxiolytic and neuroprotective (Seredenin and Voronin 2009). Further studies revealed its cardioprotective action (Stoliaruk et al. 2010; Kryzhanovskyi et al. 2011) and protective action in various toxicological models (Durnev et al. 2009). These findings confirmed previously postulated hypothesis of cytoprotective potential of this anxiolytic drug (Seredenin and Voronin 2009).
Figure 1.
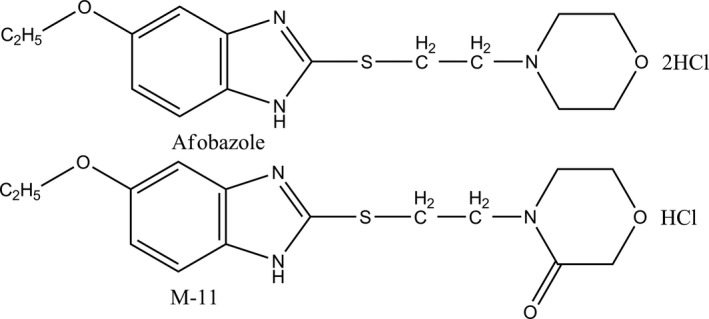
Chemical structure of afobazole and compound M‐11.
Multiple literary sources characterizing cell effects mediated by ligand activation of SigmaR1 (Behensky et al. 2013a,b; Cuevas et al. 2011a; Katnik et al. 2013) and our own findings (Seredenin et al. 2009) suggest possible dependence of afobazole cytoprotective effects on SigmaR1. However, the presence of MT3 receptor, one of the cellular targets of a drug, which serves as a regulatory site of quinone reductase 2 enzyme (NQO2), does not preclude it contribution to cytoprotection. Especially, since this enzyme promotes generation of reactive oxygen species (ROS) (Reybier et al. 2011) and, therefore, its inhibition by afobazole (Kadnikov et al. 2014) may be cytoprotective. The combination of afobazole and its main metabolite M‐11 (2‐[2‐(3‐oxomorpholin‐4‐il)‐ethylthio]‐5‐ethoxybenzimidazole hydrochloride) (Fig. 1) became a very convenient tool to test this hypothesis (Seredenin et al. 2008). Importantly, among all four molecular targets of afobazole M‐11 has significant affinity only to MT3 receptor (K i = 3.9 × 10−7 M for MT3 receptor; K i = 7.9 × 10−4 M for SigmaR1; K i = 4.4 × 10−4 M for MT1 receptor; K i = 1.8 × 10−4 M for MAO‐A) (Seredenin and Voronin 2009). We utilized SCGE‐assay (comet assay) to evaluate DNA damage in mouse bone marrow cells exposed to menadione as a marker of oxidative stress in vitro (Kadnikov et al. 2015). We found that in this model M‐11 possess less cytoprotective potential than afobazole. These findings suggest the contribution of the MT3 receptor in the cytoprotective effect of afobazole and provide rationale for further investigation of SigmaR1 role regarding this effect of the drug.
Materials and Methods
Chemicals
The following chemicals were used: afobazole (5‐Ethoxy‐2‐[2‐(morpholino)‐ethylthio]benzimidazole dihidrochloride), M‐11 (2‐[2‐(3‐oxomorpholin‐4‐il)‐ethylthio]‐5‐ethoxy benzimidazole hydrochloride) (Fig. 1) (FSBI “Research Zakusov Institute of Pharmacology”,Russia); BD‐1047, PRE‐084, RPMI‐1640 (Sigma‐Aldrich, St. Louis, Missouri, USA); NaCl, EDTA‐Na2, Tris, DMSO, TritonX‐100 (Amresco, Solon, Ohio, USA); light melting agarose type 4, high melting agarose type 1, NaOH (Panreac, Barcelona, Spain); fetal calf serum (PanEco, Moscow, Russia).
Experimental animals
The experiments were performed on single‐cell suspension extracted from the bone marrow of male CD‐1 mice (18–20 g, n = 8) obtained from Pushchino Breeding Center (Branch of the Institute of Bioorganic Chemistry, Russian Academy of Sciences). The animals were kept under standard housing conditions (20–22°C, relative humidity 30–70%, 12 h light/dark cycle) in plastic cages with stainless steel upper lid and dust‐free wood sawdust bedding, 10 mice per cage, with constant access to food and water.
Animals were killed by cervical dislocation. Epiphyses of the femurs were cut off; bone marrow cells were flushed with 3 mL of RPMI‐1640 containing 10% fetal calf serum. This study consisted of two independent sets of experiments using similar incubation conditions with examined compounds. Each experimental series included the material obtained from 4 mice. Aliquots of cell suspension from each animal were placed into microtubes for further incubation in control conditions, incubation with ligands of SigmaR1 or MT3 receptors and exposition to menadione.
Model of menadione genotoxicity
Mechanisms of cytoprotective action of afobazole were studied using previously described model of menadione genotoxicity (Kadnikov et al. 2015; Woods et al. 1997). Incubation of cell suspension with menadione (exogenous substrate of quinone reductase 1 (NQO1) and 2 (NQO2) enzymes) leads to concentration‐dependent increase in DNA oxidative damage. Dicoumarol inhibits NQO1 further enhancing DNA damage. This effect can be explained by the capability of NQO2 to single electron reduction in quinones to semiquinones, which readily potentiate oxidative stress (Reybier et al. 2011). This model provides all necessary conditions to investigate and compare effects of afobazole mediated by SigmaR1 and MT3 receptors.
We studied the effect of afobazole (10 μmol/L) on induced genomic DNA damage on cells preincubated with selective SigmaR1 antagonist BD‐1047. Effect of afobazole was compared to the effect of selective SigmaR1 agonist PRE‐084 and antagonist BD‐1047, which were used in final concentrations 1 and 10 μmol/L both. Concentration of afobazole used in our experiments corresponds to its K i for sigma‐1 (K i = 5.9 × 10−6 M) and MT3 (K i = 9.7 × 10−6 M) receptors. Concentration of PRE‐084 and BD‐1047 were chosen in respect to effective concentration of afobazole and data from scientific periodic where these prototype ligands were used in 1–10 μmol/L range in in vitro experiments (Katnik et al. 2006; Cuevas et al. 2011b; Behensky et al. 2013c).
Suspension of bone marrow cells was incubated with BD‐1047 at 37°C for 30 min, following by the addition of afobazole and dicoumarol (10 μmol/L) and subsequent 30 min incubation. Afterward cells were exposed to menadione in final concentration of 10 μmol/L for 1 h at 37°C. In appropriate time identical quantities of vehicle solutions were added to the control cell suspension. Effects of afobazole and PRE‐084 were compared at the same conditions, but without BD‐1047 addition (Fig. 2).
Figure 2.
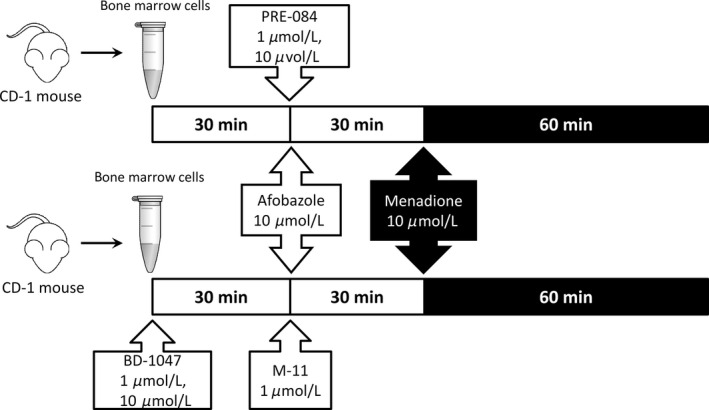
Design of the menadione genotoxicity model experiment.
The verification of the obtained results was performed using preincubation of bone marrow cells with BD‐1047 followed by incubation with metabolite of afobazole M‐11 (50 μmol/L).
Single‐cell gel electrophoresis assay (Comet assay)
Induced DNA damage was measured using the comet assay as previously described (Burlinson 2012) with some modifications (Sirota et al. 2014). After final incubation with menadione 70 μL of bone marrow cells suspension was mixed with 350 μL of 0.9% light melting agarose solution. The same amount of obtained mixture was dropped on slides precoated with 1% high melting agarose. The slides were covered with coverslips and placed on ice for 5 min. After gel solidification coverslips were gently removed. All following steps were conducted under dim light to prevent the occurrence of additional DNA damage. The slides were placed into Schifferdecker type glass cuvette filled with lysis solution (10 mmol/L Tris‐HCl, 2.5 mol/L NaCl, 100 mmol/L EDTA‐Na2, 1% Triton‐X 100, 10% DMSO, pH 10, 4°C) and incubated at 4°C for at least 1 h. After lysis step the slides were washed with deionized water and placed into electrophoresis chamber (BioRad, Hercules, California, USA) filled with 2.2 L of alkaline electrophoretic solution (300 mmol/L NaOH, 1 mmol/L EDTA‐Na2, 8°C, pH>13,) for alkali treatment during 20 min. Electrophoresis was performed in the same solution for 20 min at electric field strength of 1 V/cm, the applied voltage was 32 V and the current was 300 mA. After electrophoresis, the slides were washed in 1 × PBS and fixed in 70% ethanol, dried at room temperature and stored until staining. Immediately prior to microscopic analysis, the slides were stained with SYBR Green I (1:10,000 in TE buffer) for 30 min in the dark. Analysis was performed on a Mikmed‐2 12T epifluorescence microscope (‘LOMO’, St. Petersburg, Russia) combined with a high‐resolution digital camera (VEC‐335, St. Petersburg, Russia), at 200× magnification. The images of comets were analyzed using CASP v.1.2.2 software (www.casplab.com).
DNA damage was evaluated by the percent of DNA in the tail of comet (%TDNA). Each experimental group was characterized by median and quartiles of %TDNA obtained as a result of analysis of at least 100 cells per slide. Median of spontaneous DNA damage did not exceed 3%TDNA.
Statistical analysis
To evaluate the type of experimental data distribution D'Agostino‐Pearson normality test was used. Whereas experimental data did not fit Gaussian distribution to evaluate statistical significance of obtained data, we used Kruskal–Wallis test with Dunn's post‐test. Data are presented as median with minimum and maximum values (min‐max). To perform statistical analysis and plotting of graphs GraphPad Prism v.5.02 (GraphPad Software, San Diego CA, (www.graphpad.com) was used.
Results
Statistical analysis of experimental data has shown that the impact of menadione on bone marrow cells was individual for each mouse. Therefore, further analysis of effects of the assayed compounds was performed for each animal individually.
First, we have assayed the influence of 30‐min preincubation of cells suspension with BD‐1047 prior afobazole addition. After incubation of cell suspension with menadione and dicoumarol divergence of %TDNA medians falls within 17.29–24.28 range (Table 1). As it is shown on Figure 3, afobazole decreases menadione induced DNA damage to 4.9–9.92 range (P < 0.0001) (Table 1). Preincubation of cell suspension with SigmaR1 selective antagonist at 1 μmol/L or 10 μmol/L leads to significant decrease in afobazole cytoprotective action to 8.99–15.52%TDNA and 8.61–15.4%TDNA, respectively (P < 0.05 for each animal) (Table 1). Meanwhile incubation of cell suspension with BD‐1047 at both concentrations has no impact on menadione induced DNA damage (P > 0.1 for each animal) (Table 1, Fig. 3). Preincubation of cell suspension with BD‐1047 prior adding M‐11 at 50 μmol/L concentration has no impact on it cytoprotective effect. Obviously, it is due to lack of ligand properties of M‐11 toward Sigmar‐1 (Table 1).
Table 1.
The influence of bone marrow cells preincubation with BD‐1047 on effects of afobazole and M‐11
| Mouse, # | Menadione | Afobazole, menadione | BD‐1047 (1 μmol/L), afobazole, menadione | BD‐1047 (10 μmol/L), afobazole, menadione | BD‐1047 (1 μmol/L), menadione | BD‐1047 (10 μmol/L), menadione | M‐11 (50 μmol/L), menadione | BD‐1047 (1 μmol/L), M‐11, menadione | BD‐1047 (10 μmol/L), M‐11, menadione |
|---|---|---|---|---|---|---|---|---|---|
| 1 |
24.28 (2.76–69.81) n = 115 |
9.92 (0.19–43.76) n = 129 P men < 0.0001 |
15.52 (0.37–43.7) n = 118 P men = 0.0029 P af = 0.035 |
15.40 (0.36–56.23) n = 100 P men = 0.0071 P af = 0.042 |
21.49 (0.12–65.67) n = 101 P men > 0.999 |
17.62 (0.14–66.3) n = 110 P men = 0.38 |
10.98 (0.24–48.24) n = 101 P men < 0.0001 |
7.89 (0–37.65) n = 104 P men < 0.0001 P m–11 = 0.33 |
8.721 (0.002–62.22) n = 100 P men < 0.0001 P m–11 > 0.999 |
| 2 |
18.46 (0.59–68.46) n = 156 |
8.99 (0–69.29) n = 165 P men < 0.0001 |
11.56 (0.67–70) n = 130 P men = 0.006 P af = 0.04 |
14.46 (0.0007–63.9) n = 103 P men = 0.039 P af = 0.027 |
15.88 (0.002–76.89) n = 102 P men = 0.58 |
14.2 (0.56–71.22) n = 108 P men = 0.17 |
9.13 (0.001–38.17) n = 102 P men < 0.0001 |
10.5 (0.003–65.72) n = 100 P men < 0.0001 P m–11 > 0.999 |
9.23 (0.39–39.4) n = 101 P men < 0.0001 P m–11 > 0.999 |
| 3 |
17.29 (0.003–70.52) n = 113 |
6.27 (0.003–52.44) n = 100 P мeн < 0.0001 |
10.9 (0–40.83) n = 114 P men = 0.028 P af = 0.042 |
11.38 (0.001–60.82) n = 102 P men = 0.049 P af = 0.041 |
14.91 (0.0003–76.07) n = 105 P men > 0.999 |
13.29 (0.001–74.73) n = 103 P men = 0.18 |
8.78 (0.003–82.03) n = 104 P men < 0.0001 |
7.38 (0.0005–62.54) n = 103 P men < 0.0001 P m–11 > 0.999 |
7.41 (0–39.66) n = 105 P men<0.0001 P m–11 > 0.999 |
| 4 |
19.43 (0.26–68.93) n = 127 |
4.9 (0.002–60.52) n = 120 P men < 0.0001 |
8.99 (0.11–50.26) n = 138 P men = 0.0053 P af = 0.038 |
8.61 (0–86.85) n = 103 P men = 0.037 P af = 0.029 |
11.24 (0–86.82) n = 103 P men > 0.999. |
17.16 (1.01–75.11) n = 111 P men > 0.999. |
5.83 (0.03–59.29) n = 109 P men < 0.0001 |
5.98 (0–86.68) n = 107 P men < 0.0001 P m–11 > 0.999 |
3.59 (0–76.95) n = 101 P men < 0.0001 P m–11 > 0.999 |
Data are presented as median (min‐max); n, the number of analyzed cells from the slide; P men , statistical significance versus Menadione group; P af, statistical significance versus Afobazole, Menadione group; P m‐11, statistical significance versus M‐11, Menadione.
Figure 3.
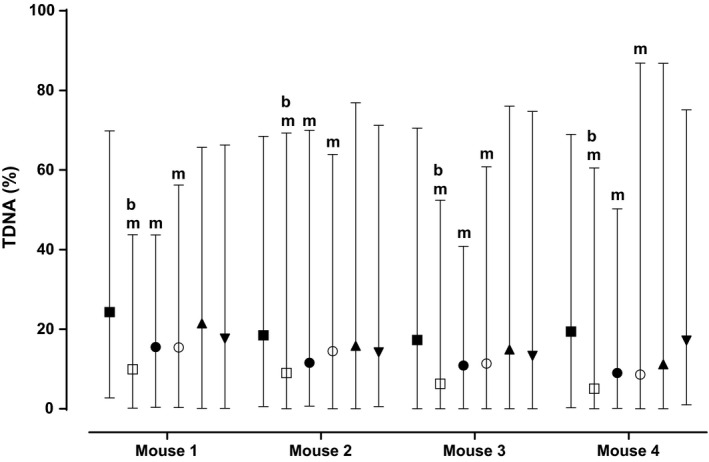
Effect of preincubation of CD‐1 mice bone marrow suspension with BD‐1047 on cytoprotective action of afobazole. Bone marrow cells were extracted from femur bones of mouse.Cells from each animal were divided as following: control group (data not shown, %TDNA was lower than 3%); (■ – Menadione; (□) – Afobazole, Menadione; (●) – BD‐1047 1 μmol/L, Afobazole, Menadione; (○) – BD‐1047 10 μmol/L, Afobazole, Menadione; (▲) – BD‐1047 1 μmol/L, Menadione; (▼) – BD‐1047 10 μmol/L, Menadione. At least 100 cells from each group were assayed. Data are presented as median with minimum and maximum. m ‐ statistical significance versus Menadione (■)group (P < 0.05, Kruskal–Wallis test, Dunn's post hoc) b ‐ statistical significance versus BD‐1047, Afobazole, Menadione (●,○) groups (P < 0.05, Kruskal–Wallis test, Dunn's post hoc).
Next, the effects of afobazole were compared with effects of selective SigmaR1 agonist PRE‐084. Induced DNA damage for this experimental set was in range of 11.74–16.74% TDNA (Table 2). As it is shown on Figure 4 afobazole decreased DNA damage to 4‐57–8.13 range of medians (P < 0.0001 for each animal) (Table 2). PRE‐084 at 1 μmol/L and 10 μmol/L also decreased DNA damage to 9.86–12.88%TDNA and 8.16–12.8%TDNA, respectively (P < 0.05 for each animal) (Table 2, Fig. 4). Therefore, cytoprotective effect of PRE‐084 was much weaker as compared to afobazole (P < 0.05 for each animal) (Table 2, Fig. 4).
Table 2.
Comparison of afobazole and PRE‐084 effects of menadione‐induced DNA damage of bone marrow cells extracted from CD‐1 mice
| Mouse, # | Menadione | Afobazole, menadione | PRE‐084 (1 μmol/L), menadione | PRE‐084 (10 μmol/L), menadione |
|---|---|---|---|---|
| 1 |
12.92 (0.00003–58.45) n = 117 |
4.57 (0.00002–47.85) n = 187 P men < 0.0001 |
9.86 (0.0001–52.53) n = 195 P men = 0.026 P a f = 0.043 |
8.19 (0.0001–47.49) n = 195 P men = 0.039 P af = 0.014 |
| 2 |
16.74 (0.002–64.45) n = 127 |
8.13 (0.0004–41.26) n = 102 P men < 0.0001 |
12.35 (0.001–42.04) n = 134 P men = 0.042 P af = 0.0024 |
11.58 (0.002–50.68) n = 130 P men = 0.0031 P af = 0.047 |
| 3 |
16.39 (0.29–27.12) n = 109 |
6.02 (0.0002–37.36) n = 118 P men < 0.0001 |
12.15 (0.001–62.63) n = 112 P men = 0.021 P af = 0.04 |
12.8 (0.0005–70.11) n = 110 P men = 0.019 P af = 0.049 |
| 4 |
17.92 (0.34–57.12) n = 107 |
9.65 (0.0004–39.36) n = 102 P men < 0.0001 |
12.88 (0.001–42.14) n = 108 P men = 0.046 P af = 0.019 |
12.56 (0.0008–50.07) n = 101 P men = 0.035 P af = 0.027 |
Data are presented as median (min‐max); n, the number of analyzed cells from the slide; P men , significant difference versus Menadione; P af , significant difference versus Afobazole, Menadione.
Figure 4.
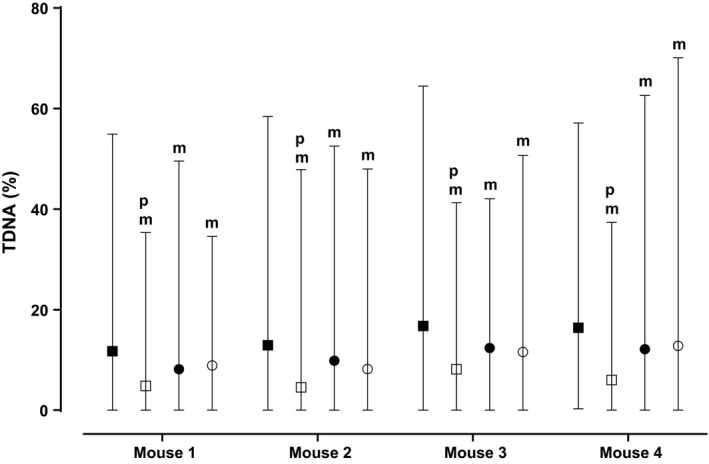
Effect of afobazole and PRE‐084 on menadione induced DNA damage of CD‐1 mice bone marrow cells. Bone marrow cells were extracted from femur bones of mouse. Cells from each animal were divided as following: control group (data not shown, %TDNA was lower than 3%); (■) – Menadione; (□) – Afobazole, Menadione; (●) – PRE‐084 1 μmol/L, Menadione; (○) – PRE‐084 10 μmol/L, Menadione. At least 100 cells from each group were assayed. Data are presented as median with minimum and maximum. m ‐ statistical significance versus Menadione (■) group (P < 0.05, Kruskal–Wallis test, Dunn's post hoc). p ‐ statistical significance versus PRE‐084, Menadione (●,○) groups (P < 0.05, Kruskal–Wallis test, Dunn's post hoc).
Discussion
Our findings demonstrate that afobazole within frameworks of menadione genotoxicity model exerts potent cytoprotective effect. Decreased cytoprotective potential of afobazole in response to preincubation with selective SigmaR1 antagonist BD‐1047 suggests contribution of Sigmar‐1to the effect of the drug. This conclusion is confirmed by the experiments using selective agonist of Sigmar‐1 PRE‐084 (Bucolo et al. 2006). More pronounced cytoprotective effect of afobazole as compared with PRE‐084 corresponds to concept of multitarget mechanism of the drug action and presumably achieved by additive effects of SigmaR1 and MT3 (Kadnikov et al. 2015).
Participation of SigmaR1 in cytoprotective effect of afobazole defined in this study corresponds to our previous results (Zenina et al. 2005) and results of other in vitro studies (Nguyen et al. 2015; Ruscher and Wieloch 2015). Selective agonist of SigmaR1 PRE‐084 prevents cell death in in vitro model of Huntington disease (Hyrskyluoto et al. 2013). In in vivo experiments PRE‐084 restores motor functions and increases neuron survivability in models of motor neuron degeneration (Peviani et al. 2013) and Parkinson disease in mice (Francardo et al. 2014). Intraperitoneal injection of PRE‐084 to newborn mice decreases the area of neonatal excitotoxic brain damage (Griesmaier et al. 2012). Other SigmaR1 ligands also demonstrate neuroprotective activity in the model of glutamate toxicity (Luedtke et al. 2012). Some serotonin reuptake inhibitors with nonselective SigmaR1 agonist action, such as fluvoxamine and fluvoxetine, also show neuroprotective activity (Hashimoto 2015).
Contribution of MT3 to cytoprotective action of afobazole is linked to ligand‐dependent inhibition of NQO2 (Reybier et al. 2011). It is known, that inhibitors of NQO2 S26695 and S29434 (NMDPEF) (Pegan et al. 2011) increase survivability in concentration‐dependent manner and decrease the amount of apoptotic hippocampal cells after exposition to menadione. in vitro effects of these compounds correlate with their antiamnesic action in scopolamine model of memory impairment in rats (Benoit et al. 2010). In paraquat‐induced toxicity in vitro model in a variety of cell lines including human astrocytoma (U373), human embryonic kidney (HEK293), and rat pneumocytes NQO2 inhibitor NMDPEF have significant cytoprotective effect. This compound has antidote activity both at systemic administration of paraquat and at substantia nigra microinfusion (Janda et al. 2013). Moreover, recent study devoted to uncovering effects of combined administration of resveratrol and PRE‐084 in mouse model of amyotrophic lateral sclerosis (Mancuso et al. 2014). Authors of this paper, however, did not discuss interaction of resveratrole, a potent NQO2 inhibitor (Buryanovskyy et al. 2004), with MT3 receptor. However, no enhancement of neuroprotection at combined administration of resveratrol and PRE‐084 was observed compared to the effects of individual drug administration.
The alternative mechanism of cytoprotective effect of afobazole is related to inhibition of ROS generation achieved by regulation of SigmaR1 (Hayashi 2015; Mori et al. 2013; Meunier and Hayashi 2010; Pal et al. 2012). SigmaR1 stabilizes ER stress sensor – IRE1 protein, which prolongs activation of signaling cascade associated with activation of XBP1 protein. In turn, activation of XBP1 protein triggers subsequent expression of genes responsible for resistance of cells to damage (Liu et al. 2009). Perhaps, this mechanism may be initiated by afobazole or PRE‐084 before addition of menadione into incubation medium, decreasing genomic DNA damage as a result.
Therefore, our in vitro experiments using the model of menadione genotoxicity demonstrate that the cytoprotective mechanism of afobazole action includes ligand activation of SigmaR1.
Disclosures
None declared.
Acknowledgements
This study was supported by The Russian Foundation for Basic Research grant (13‐04‐01014).
Voronin M. V. , Kadnikov I. A.. Contribution of Sigma‐1 receptor to cytoprotective effect of afobazole, Pharma Res Per, 4(6), 2016, e00273, doi: 10.1002/prp2.273
References
- Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J (2013a). Afobazole activation of sigma‐1 receptors modulates neuronal responses to amyloid‐beta25‐35. J Pharmacol Exper Ther 347: 468–477. [DOI] [PubMed] [Google Scholar]
- Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J (2013b). Stimulation of sigma receptors with afobazole blocks activation of microglia and reduces toxicity caused by amyloid‐beta25‐35. J Pharmacol Exper Ther 347: 458–467. [DOI] [PubMed] [Google Scholar]
- Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J (2013c). Stimulation of sigma receptors with afobazole blocks activation of microglia and reduces toxicity caused by amyloid‐beta25‐35. J Pharmacol Exper Ther 347: 458–467. [DOI] [PubMed] [Google Scholar]
- Benoit CE, Bastianetto S, Brouillette J, Tse Y, Boutin JA, Delagrange P, et al. (2010). Loss of quinone reductase 2 function selectively facilitates learning behaviors. J Neurosci 30: 12690–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Drago F, Lin LR, Reddy VN (2006). Sigma receptor ligands protect human retinal cells against oxidative stress. NeuroReport 17: 287–291. [DOI] [PubMed] [Google Scholar]
- Burlinson B (2012). The in vitro and in vivo comet assays Pp 143–163 in: Parry J. M. and Parry E. M., eds. Genetic toxicology: principles and methods. Springer Science+Business Media, LLC, New York. [Google Scholar]
- Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, et al. (2004). Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry 43: 11417–11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Behensky A, Deng W, Katnik C (2011a). Afobazole modulates neuronal response to ischemia and acidosis via activation of sigma‐1 receptors. J Pharmacol Exper Ther. 339: 152–160. [DOI] [PubMed] [Google Scholar]
- Cuevas J, Rodriguez A, Behensky A, Katnik C (2011b). Afobazole modulates microglial function via activation of both sigma‐1 and sigma‐2 receptors. J Pharmacol Exper Ther 339: 161–172. [DOI] [PubMed] [Google Scholar]
- Durnev AD, Zhanataev AK, Shreder OV, Seredenin SB(2009). Antimutagenic and antiteratogenic properties of afobazole. Eksperimental'naia i klinicheskaia farmakologiia 72: 46–51. [PubMed] [Google Scholar]
- Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA (2014). Pharmacological stimulation of sigma‐1 receptors has neurorestorative effects in experimental parkinsonism. Brain 137:1998–2014. [DOI] [PubMed] [Google Scholar]
- Griesmaier E, Posod A, Gross M, Neubauer V, Wegleiter K, Hermann M, et al. (2012). Neuroprotective effects of the sigma‐1 receptor ligand PRE‐084 against excitotoxic perinatal brain injury in newborn mice. Exp Neurol 237: 388–395. [DOI] [PubMed] [Google Scholar]
- Hashimoto K (2015). Activation of sigma‐1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J Pharmacol Sci 127: 6–9. [DOI] [PubMed] [Google Scholar]
- Hayashi T (2015). Sigma‐1 receptor: the novel intracellular target of neuropsychotherapeutic drugs. J Pharmacol Sci 127: 2–5. [DOI] [PubMed] [Google Scholar]
- Hyrskyluoto A, Pulli I, Tornqvist K, Ho TH, Korhonen L, Lindholm D (2013). Sigma‐1 receptor agonist PRE084 is protective against mutant huntingtin‐induced cell degeneration: involvement of calpastatin and the NF‐kappaB pathway. Cell Death Dis 4: e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda E, Parafati M, Aprigliano S, Carresi C, Visalli V, Sacco I, et al. (2013). The antidote effect of quinone oxidoreductase 2 inhibitor against paraquat‐induced toxicity in vitro and in vivo. Br J Pharmacol 168: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadnikov IA, Voronin MV, Seredenin SB (2014). Effect of afobazole on activity of quinone reductase 2. Pharm Chem J 47: 514–516. [Google Scholar]
- Kadnikov IA, Voronin MV, Seredenin SB (2015). Cytoprotective effect of afobazole and its main metabolite M‐11. Bull Exp Biol Med 159: 44–47. [DOI] [PubMed] [Google Scholar]
- Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevas J (2006). Sigma‐1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J Pharmacol Exper Ther 319: 1355–1365. [DOI] [PubMed] [Google Scholar]
- Katnik C, Garcia A, Behensky AA, Yasny IE, Shuster AM, Seredenin SB, et al. (2013). Treatment with afobazole at delayed time points following ischemic stroke improves long‐term functional and histological outcomes. Neurobiol Dis 62C: 354–364. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskyi SA, Sorokina AV, Stolyaruck VN, Vititnova MB, Miroshkina IA, Tsorin IB, et al. (2011). Study of anti‐ischemic effect of afobazole in experimental myocardial infarction. Bull Exp Biol Med 150: 316–319. [DOI] [PubMed] [Google Scholar]
- Liu Y, Adachi M, Zhao S, Hareyama M, Koong AC, Luo D, et al. (2009). Preventing oxidative stress: a new role for XBP1. Cell Death Differ 16: 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke RR, Perez E, Yang SH, Liu R, Vangveravong S, Tu Z, et al. (2012). Neuroprotective effects of high affinity sigma 1 receptor selective compounds. Brain Res 1441: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R, Del Valle J, Morell M, Pallas M, Osta R, Navarro X (2014). Lack of synergistic effect of resveratrol and sigma‐1 receptor agonist (PRE‐084) in SOD1G(9)(3)A ALS mice: overlapping effects or limited therapeutic opportunity? Orphanet J Rare Dis 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Hayashi T (2010). Sigma‐1 receptors regulate Bcl‐2 expression by reactive oxygen species‐dependent transcriptional regulation of nuclear factor kappaB. J Pharmacol Exper Ther 332: 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Hayashi T, Hayashi E, Su TP (2013). Sigma‐1 receptor chaperone at the ER‐mitochondrion interface mediates the mitochondrion‐ER‐nucleus signaling for cellular survival. PLoS ONE 8: e76941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Lucke‐Wold BP, Mookerjee SA, Cavendish JZ, Robson MJ, Scandinaro AL, et al. (2015). Role of sigma‐1 receptors in neurodegenerative diseases. J Pharmacol Sci 127: 17–29. [DOI] [PubMed] [Google Scholar]
- Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE (2012). The sigma‐1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol 682: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegan SD, Sturdy M, Ferry G, Delagrange P, Boutin JA, Mesecar AD (2011). X‐ray structural studies of quinone reductase 2 nanomolar range inhibitors. Protein Sci 20: 1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peviani M, Salvaneschi E, Bontempi L, Petese A, Manzo A, Rossi D, et al. (2013). Neuroprotective effects of the Sigma‐1 receptor (S1R) agonist PRE‐084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol Dis 62C: 218–232. [DOI] [PubMed] [Google Scholar]
- Reybier K, Perio P, Ferry G, Bouajila J, Delagrange P, Boutin JA, et al. (2011). Insights into the redox cycle of human quinone reductase 2. Free Radical Res 45: 1184–1195. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Wieloch T (2015). The involvement of the sigma‐1 receptor in neurodegeneration and neurorestoration. J Pharmacol Sci 127: 30–35. [DOI] [PubMed] [Google Scholar]
- Seredenin SB, Voronin v (2009). Neuroreceptor mechanisms of the afobazole effect. Eksperimental'naia i klinicheskaia farmakologiia 72: 3–11. [PubMed] [Google Scholar]
- Seredenin SB, Viglinskaia AO, Mozhaeva T, Kolyvanov GB, Litvin AA, Avdiunina NI, et al. (2008). Afobazole metabolism in rats. Eksperimental'naia i klinicheskaia farmakologiia 71: 50–52. [PubMed] [Google Scholar]
- Seredenin SB, Antipova TA, Voronin MV, Kurchashova SY, Kuimov AN (2009). Interaction of afobazole with sigma1‐receptors. Bull Exp Biol Med 148: 42–44. [DOI] [PubMed] [Google Scholar]
- Sirota NP, Zhanataev AK, Kuznetsova EA, Khizhnyak EP, Anisina EA, Durnev AD (2014). Some causes of inter‐laboratory variation in the results of comet assay. Mutat Res, Genet Toxicol Environ Mutagen 770: 16–22. [DOI] [PubMed] [Google Scholar]
- Stoliaruk VN, Vititnova MB, Tsorin IB, Kryzhanovskii SA (2010). Afobasol antifibrillation activity in animals with the intact and denervated myocardium. Vestnik Rossiiskoi akademii meditsinskikh nauk/Rossiiskaia akademiia meditsinskikh nauk 65: 45–48. [PubMed] [Google Scholar]
- Woods JA, Young AJ, Gilmore IT, Morris A, Bilton RF (1997). Measurement of menadione‐mediated DNA damage in human lymphocytes using the comet assay. Free Radical Res 26: 113–124. [DOI] [PubMed] [Google Scholar]
- Zenina TA, Gavrish IV, Melkumyan DS, Seredenina TS, Seredenin SB (2005). Neuroprotective properties of afobazol in vitro. Bull Exp Biol Med 140: 194–196. [DOI] [PubMed] [Google Scholar]


