Abstract
Although liposomal amphotericin B (AmBisome) is considered as the first‐line treatment for New Kala‐azar, there is not enough evidence on the dosage formulation in children and its effect on them. Being considered as the safest drug for treatment of Kala‐azar, this case of AmBisome‐induced avascular necrosis now gives rise to the question; whether it is actually safe enough and if a dosage modification is needed in case of children. This so far, to the best of our knowledge, is the first instance of such severe adverse event due to AmBisome administration.
Keywords: Avascular necrosis, liposomal amphotericin B, Paediatric, leishmania, kala‐azar
Abbreviation
- RES
reticulo‐endothelial system
- VL
visceral Leishmaniasis
Introduction
Case Report
A one and a half year‐old girl, weighing 8 kg hailing from Trishal, Mymensingh was referred to SKKRC as a diagnosed case of Kala‐azar, with a history of fever for 4 months and abdominal swelling for 15 days. On examination, she was severely anemic, spleen was found 3 cm below the costal margin, febrile (101°F), pulse rate −112/min. She was rk‐39 positive and initially anemia was corrected by transfusing 160 mL of blood. Initially, we decided to treat the girl with inj. liposomal amphotericin B (AmBisome Gilead Sciences International Ltd. Granta Park, Abington, Cambridge, UK), in total of 15 mg/kg in three equally divided doses on every alternate day for 3 days. Following the second dose of liposomal amphotericin B (AmBisome), she developed blisters on the nose tip and on the perianal area. Gradually, they turned into blackish discolored areas and the girl developed high fever. Considering these occurrences, the third dose of AmBisome was postponed and necessary steps were taken to prevent sepsis on those areas. Four days later, alae of the nose sloughed out (Fig. 1), and the histopatholgical finding of the redounding part of the tissue revealed fibro‐fatty tissue and muscular elements with mature hyaline cartilage within the tissue. In some areas, surface was sloughed out but still could be identified as squamous cell epithelium. The patient was symptomatically treated and the raw area of the nose gradually healed. Then, she was referred to pediatric plastic surgery department for further management.
Figure 1.
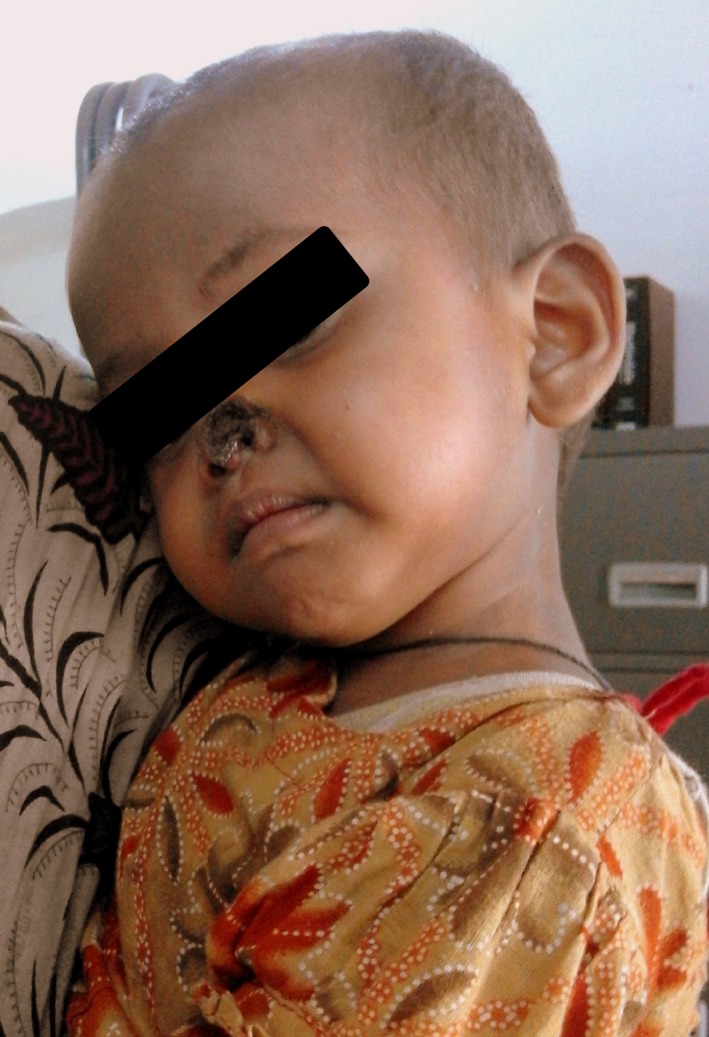
Avascular necrosis of the alae of the nose (blackish discoloration) after AmBisome administration.
Discussion
Particle‐based product AmBisome is made of nonrigid solid particles and much smaller than capillary diameter. For this reason, it offers the possibility of injecting solid, nondeformable particle without risking capillary occlusion. The easy surface modification to bear the targetable properties makes liposomes the attractive candidates for to be used as drug‐delivery vehicles (Campbell 1982). There are evidence that liposomal formulations enhance the therapeutic efficacy of the drugs in preclinical models and in humans compared to conventional formulations, due to the alteration of biodistribution (Tolentino et al. 2004). Although infusion‐related side effects such as fever, rigors, and chills are common in patients who receive therapy with AmBisome, multi‐dose studies of AmBisome continued to show a significant reduction in toxicity even at 10 times the standard dose of conventional amphotericin B in mice and four times the standard dose in rabbits (Clark et al. 1991; Olsen et al. 1991).
The exact mechanism of this event is unknown. Vasospasm due to endothelial edema, intravascular thrombosis, and chemical endarteritis are the proposed pathophysiological mechanisms (Knill and Evans 1975). The pharmacokinetics of amphotericin B after administration of AmBisome in pediatric patients has not yet been studied, although the pathogenesis of this symptom complex is believed to involve mechanical obstruction and/or chemical injury. The microaggregate of neutral fat may cause occlusion of capillary vasculature of the nose and anus. The free fatty acids released from fat globules may result in toxic injury to the endothelium (Campbell 1982). The disposition of lipid‐based amphotericin B depends on particle size, electrostatic charge, bilayer rigidity, and amount of lipid material. Smaller liposomes (e.g., LAmB) tend to have a longer circulating half‐life in blood than the larger lipid‐based products (multilamellar vesicles, amphotericin B lipid complex) as they are not as readily recognized and entrapped by phagocytes of the reticulo‐endothelial system (RES) (Tolentino et al. 2004). A few literatures suggest particle‐related factors, the administered dose, route of administration, and extent of tissue distribution are important parameters in cytotoxicity. Highly membrane soluble drug causes swelling and disruption of premature capillary endothelial cells. Many hypotheses have been proposed for the arterial hypoperfusion or spasm which is the final event leading to ischemia (Tolentino et al. 2004). Infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid mixed amphotericin in capillaries, which could lead to embolism and vascular occlusion (Campbell 1982).
AmBisome is widely used for treatment of Kala‐azar and considered as the safest option for treating visceral Leishmaniasis (VL)/Kala‐azar. Incidents as such may hinder the Kala‐azar elimination program in endemic regions. There is no concrete evidence on the dose, frequency, and rate of administration of AmBisome to be used in pediatric patients. Clinicians should be more cautious and efforts should be taken to focus on pediatric dose fixation of AmBisome in Kala‐azar management.
Disclosure
All the authors of this manuscript have no conflict of interest. None of the authors received any financial benefits from anywhere for this article. No gifted or unauthorized drugs were used for this study. None of the authors worked in any pharmaceutical company previously or currently and there is no scope for the authors of any kind of gain or lose financially through publication of the paper.
Ethical consideration
For performing all necessary laboratory investigations in SKKRC and also using photograph for publication, informed written consent was obtained from the patient's guardian.
Maruf S., Nath P., Aktar F., Basher A.. AmBisome Induced Avascular Necrosis of the Alae of the Nose of a very young girl suffering from Kala‐azar – a Case Report, Pharma Res Per, 4(6), 2016, e00269, doi: 10.1002/prp2.269
References
- Campbell PI (1982). Toxicity of some charged lipids used in liposome preparations. Cytobios 37: 21–26. [PubMed] [Google Scholar]
- Clark JM, Whitney RR, Olsen SJ, George RJ, Swerdel MR, Kunselman L, et al. (1991). Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob Agents Chemother 35: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill R, Evans D (1975). Pathogenesis of gangrene following intra‐arterial injection of drugs: a new hypothesis. Can Anaesth Soc J 22: 637–646. [DOI] [PubMed] [Google Scholar]
- Olsen SJ, Swerdel MR, Blue B, Clark JM, Bonner DP (1991). Tissue distribution of amphotericin B lipid complex in laboratory animals. J Pharm Pharmacol 43: 831–835. [DOI] [PubMed] [Google Scholar]
- Tolentino LF, Tsai SF, Witt MD, French SW (2004). Fatal fat embolism following amphotericin B lipid complex injection. Exp Mol Pathol 77: 246–248. [DOI] [PubMed] [Google Scholar]


