Abstract
Percutaneous vascular interventions uniformly result in arterial denudation injuries that subsequently lead to thrombosis and restenosis. These complications can be attributed to impairments in re-endothelialization within the wound margins. Yet, the cellular and molecular mechanisms of re-endothelialization remain to be defined. While several animal models to study re-endothelialization after arterial denudation are available, few are performed in the mouse because of surgical limitations. This undermines the opportunity to exploit transgenic mouse lines and investigate the contribution of specific genes to the process of re-endothelialization. Here, we present a step-by-step protocol for creating a highly reproducible murine model of arterial denudation injury in the infrarenal abdominal aorta using external vascular clamping. Immunocytochemical staining of injured aortas for fibrinogen and β-catenin demonstrate the exposure of a pro-thrombotic surface and the border of intact endothelium, respectively. The method presented here has the advantages of speed, excellent overall survival rate, and relative technical ease, creating a uniquely practical tool for imposing arterial denudation injury in transgenic mouse models. Using this method, investigators may elucidate the mechanisms of re-endothelialization under normal or pathological conditions.
Keywords: Medicine, Issue 117, Blood vessels, endothelial cell, re-endothelialization, vascular injury, vasculature, mouse
Introduction
Thrombosis and restenosis are serious early and late complications in patients who undergo percutaneous vascular interventions, such as endovascular balloon angioplasty and stenting1,2. Several strategies have been employed to address these complications, particularly dual antiplatelet therapy and drug-eluting stents. However, little focus has been placed on the underlying cause of thrombosis and restenosis, namely the loss of endothelial cell coverage (denudation). Denudation injury is an inevitable consequence of interventional procedures due to mechanical trauma to the blood vessel wall. This mechanical trauma can result in damage and removal of the protective endothelial layer and exposure of the basement membrane and vascular smooth muscle to circulating blood3. The loss of endothelial cells in these areas creates a pro-thrombotic and pro-inflammatory environment that not only promotes platelet adhesion and subsequent thrombosis, but also stimulates migration and proliferation of vascular smooth muscle cells resulting in neointimal thickening and restenosis4. These complications, and their associated therapies, lead to significant morbidity, most notably recurrent ischemic disease and bleeding events that impact human health.
Re-endothelialization of the denuded injury from the wound margins is of paramount importance in preventing thrombosis and restenosis5. Autopsy results and animal models have effectively demonstrated reduced rates of thrombosis with coverage of stent struts6,7. Drug-eluting stents, devised to decrease rates of restenosis by inhibiting smooth muscle proliferation and neointimal hyperplasia, result in significant impairments in arterial re-endothelialization and increasing rates of late thrombosis3. Unfortunately, understanding the mechanisms for re-endothelialization has been a slow process, largely limited by the lack of appropriate animal models8.
Several animal models for understanding the roles of endothelial cells and vascular smooth muscle cells following arterial injury have been created7,9,10. The rat carotid artery balloon injury model is the best characterized and has been employed to study the effects of denudation injury at the gross, cellular and molecular level11. Nevertheless, a highly reproducible murine model of arterial denudation injury with excellent survival rates is missing and much needed to take advantage of multiple transgenic lines available to better elucidate vascular regeneration in multiple settings.
This manuscript presents a murine model of arterial denudation injury that is reproducible and simple to perform. The approach has shown minimal morbidity and mortality in several transgenic lines. Because of the broad number of genetically modified mouse lines, this model may be used to elucidate the molecular mechanisms underlying re-endothelialization after denudation injury.
Protocol
NOTE: This protocol has been approved by the Animal Research Committee at the University of California Los Angeles.
1. Preoperative Preparation and Anesthesia
Be sure to observe sterile technique throughout the procedure.
Sterilize all surgical supplies using a steam autoclave.
Turn on heated rodent surgical platform prior to anesthetic induction so that it may warm to appropriate temperature (37 °C) and place under stereomicroscope for visualization during surgical procedure.
- Place the mouse in the anesthesia induction chamber and induce with 4% isoflurane at a flow rate of 1 L/min.
- Carefully monitor the respiratory rate and footpad skin color of mouse during induction.
- After slowing of the respiratory rate, remove the mouse from the induction chamber and place on warmed pad for surgical preparation with face in separate nosecone and maintenance isoflurane concentration of 2.5%.
- Perform toe pinch to assess adequacy of anesthetic.
Apply ophthalmic ointment to the corneas and administer preoperative carprofen (5 mg/kg) subcutaneously.
Place mouse supine and use clippers to remove hair from abdomen.
Prepare the surgical area with three alternating scrubs of povidone iodine and 70% isopropyl alcohol applied with sterile gauze pads.
Place mouse supine on heated rodent surgical platform with face in nosecone and secure all extremities and carefully position mouse such that the abdomen is visible with the stereomicroscope.
Place sterile, adhesive dressings along each edge of the prepared surgical area.
Titrate isoflurane concentration to maintain adequate anesthesia during surgery while maintaining spontaneous respirations.
2. Infrarenal Aortic Clamping
Make a 3 cm incision down the midline of the abdomen using a scalpel, starting approximately 0.5 cm inferior to the xiphoid process.
Gently retract the skin with forceps and dissect skin away from the abdominal wall using fine scissors to cut the fine connective tissue.
- Apply 0.05-0.1 ml of 0.5% bupivacaine to the muscle wall and make a 2 cm incision into the abdominal wall to expose the abdominal organs.
- If bleeding occurs along abdominal wall, apply gentle pressure with a cotton-tipped applicator.
- Gently lift the intestines using saline-soaked cotton-tipped applicators. NOTE: Be careful to avoid blunt trauma to the jejunal and ileal arteries and place on a warm, sterile saline soaked gauze sponge outside the abdominal cavity.
- Cover the intestines with another warm, sterile saline soaked gauze sponge to avoid moisture loss.
Place a retractor to lateralize the rectum and expose the retroperitoneum.
Place small gauze pads as necessary for visualization of retroperitoneum keeping track of the number used.
- At the level of the inferior pole of the right kidney, use sharp dissecting forceps to make a retroperitonotomy lateral to the aorta. NOTE: Be careful not to injure the inferior vena cava or surrounding vessels.
- Bluntly dissect retroperitoneal tissue off the aortabeing careful not to perforate the inferior vena cava or surrounding vasculature.
Place the vascular clamp over the aorta for at least 1 min, or other specified time, and verify occlusion by visually observing lack of pulsatility in distal aorta.
- Remove the vascular clamp and verify hemostasis. Hemostasis is achieved and ensured when no active extravasation of blood is seen.
- Confirm extravasation by adding 0.5 ml saline into abdominal cavity and assessing if the saline becomes increasingly blood-tinged. If this is the case, apply gentle pressure using the saline-soaked applicator for 1 min to ensure hemostasis.
Remove any gauze pads placed to aid in visualization of retroperitoneum and replace intestines in situ into abdomen.
Irrigate the abdominal cavity using pre-warmed sterile saline.
3. Closure of Laparotomy and Skin
Close the abdominal wall muscle layer using a 5-0 braided, absorbable single running suture.
Close skin with 1-3 drops of polymer adhesive and then subsequently with wound clips once the adhesive is set.
4. Recovery and Postoperative Assessment
Transfer the mouse to a recovery cage on a heating pad with food and water on the floor of the cage.
- Closely monitor the mouse for signs of respiratory distress. Administer carprofen (5 mg/kg) daily for 48 hr postoperatively per institution guidelines.
- Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency.
- Return the mouse to a normal cage with food and water.
- Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
Assess wound on a daily basis for dehiscence and remove clips on postoperative day 14.
5. Aortic Dissection and Staining
Select mice for sacrifice based on the specific time point of interest following denudation injury.
Sacrifice mice via isoflurane inhalation and immediately inject with 5 mg methacholine chloride to cause vascular smooth muscle relaxation.
Upon confirmation of death by respiratory cessation, lack of corneal reflex and lack of movement, perfuse with 4% paraformaldehyde in phosphate buffered saline at a perfusion pressure of 100 mmHg via the left heart ventricle for 10 min.
Use a dissecting stereomicroscope to carefully separate the intact abdominal aorta from the surrounding tissues.
Transect the aorta rostral to the renal arteries and caudal to the iliac bifurcation and opened via a longitudinal incision along the dorsal surface.
Pin the aorta flat on a 35 mm silicone coated dish with the luminal side up for fixation for no less than 2 hr but no more than 12 hr. Subsequently, the tissue can be either embedded for sectioning or used in en face whole-mount immunocytochemistry.
Mount stained aortas with luminal side facing the coverslip on glass slides for confocal microscopy.
Representative Results
Eighty-five mice have undergone the survival surgical technique described in this report for infrarenal abdominal aortic clamping. The overall survival rate was 85.9%. Operative complications included intestinal bleeding and great vessel perforation, resulting in 5.9% and 3.5% mortality respectively (Table 1). After recovery from anesthesia, mice walk normally and show no signs of ischemic injury to the inferior limbs. No weight loss or lack of appetite were noted.
Figures 1A and 1B show images of the infrarenal abdominal aorta following laparotomy and retroperitoneal dissection under the stereomicroscope without (A) and with (B) aortic clamping. As shown, it is vital to clamp the aortic width in its entirety to ensure denudation along the width of the aorta. Our protocol found that using sharp bend Schwartz Micro Serrefines with a jaw width of 1.75 mm and 'strong' clamp press produced the largest continuous wound across the entire width of the aorta with minimal clamp time. However, other clamps were tested at several occlusion time intervals with variable injury sizes (Figure 1C). The remainder of the results presented were produced using the above mentioned clamp.
Histological evaluation of the aorta demonstrates complete denudation of the endothelial lining with moderate damage to the underlying smooth muscle cell layer as demonstrated by H&E staining. Denudation of the endothelium occurs at both 10 sec and 10 min, although to different degrees (Figure 2). To determine the optimal aortic clamping time interval required for complete arterial denudation, mice underwent clamping for 10 sec, 1 min and 10 min and were immediately sacrificed for analysis as described in the above protocol. The area of denudation increased with increasing clamp timings (Figure 3). At 10 sec, an incomplete and patchy denudation injury of approximately 0.75 mm2 was identified by patchy fibrinogen staining, where fibrinogen serves as a marker for denudation injury. Ten minutes of aortic clamping produced a completely denuded endothelial area of approximately 1.2 mm2, however this amount of time of clamping was considered high risk for limb ischemia and reperfusion injury. As such, we deemed 1 min of aortic clamping sufficient, which produced a 0.88 mm2 area of denudation on average, to result in near complete arterial denudation injury without evidence of ischemic injury.
The extent of denudation injury with 1 min of aortic clamping is extremely reproducible, producing a 600 µm diameter and 0.88 mm2 area of denudation (Figures 3 and 4). The intensity of fibrinogen staining is sufficient to identify the original injury at later time points. Figure 4 shows fibrinogen staining of three mice that underwent 1 min of aortic clamping 24 hr prior to sacrifice, creating a highly reproducible injury. Measurement of the fibrinogen staining 24 hr after injury of six aortas is approximately 0.81 mm2 (Figure 5A), statistically similar to the 0.88 mm2 injury noted immediately after injury (p = 0.14). Compared to uninjured endothelium, the wound margin 24 hr after injury shows migrating endothelial cells with overlying fibrinogen staining, marking re-endothelialization into the denudation injury (Figure 5B). On average, complete re-endothelialization of the 0.88 mm2 denudation injury took approximately 3 days. However, this approach can be further adapted to produce even larger denudation injuries. Clamping multiple times, each for 1 min, from rostral to caudal along the infrarenal aorta produced a 3.7 mm2 denudation injury. Although this approach also damages the tunica media, we have never observed dissection of the abdominal aorta as a result of the clamp injury.
This protocol demonstrates that infrarenal abdominal aortic clamping can be safely performed in a murine model for the purposes of creating an arterial denudation injury and measuring endothelial regeneration.
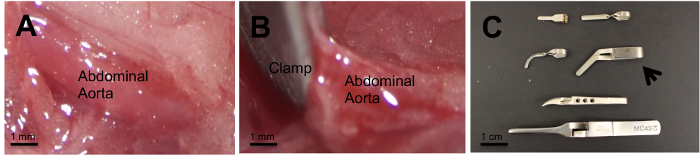 Figure 1: Isolated Infrarenal Abdominal Aorta. Isolated infrarenal abdominal aorta is shown without (A) and with (B) a vascular clamp. Several clamps of various lengths and clamp press strength were used to select the one that offers consistent injury (C). The Schwartz Micro Serrefine (arrow) produced the largest continuous wound. Please click here to view a larger version of this figure.
Figure 1: Isolated Infrarenal Abdominal Aorta. Isolated infrarenal abdominal aorta is shown without (A) and with (B) a vascular clamp. Several clamps of various lengths and clamp press strength were used to select the one that offers consistent injury (C). The Schwartz Micro Serrefine (arrow) produced the largest continuous wound. Please click here to view a larger version of this figure.
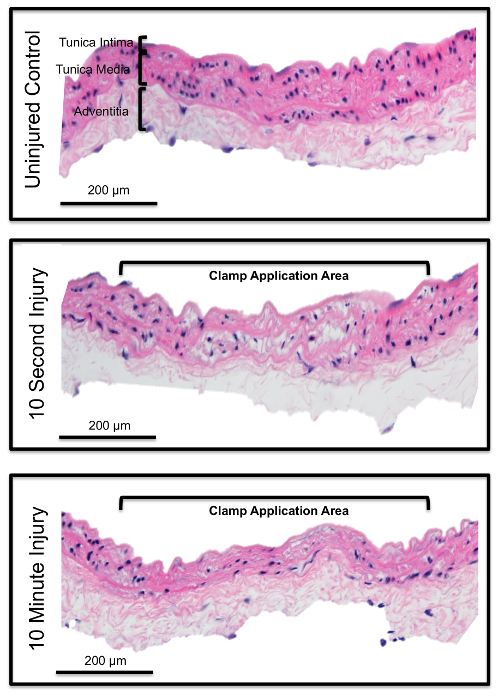 Figure 2: Efficiency of Endothelial Denudation. H&E staining of an infrarenal abdominal aortic section in a non-injured mouse (A) and after 10 sec (B) and 10 min of aortic clamping (C). Vascular clamping completely denudes the endothelial layer, as can be verified by absence of nuclei staining. Also loss of smooth muscle cell nuclei shows damage to the tunica media. Please click here to view a larger version of this figure.
Figure 2: Efficiency of Endothelial Denudation. H&E staining of an infrarenal abdominal aortic section in a non-injured mouse (A) and after 10 sec (B) and 10 min of aortic clamping (C). Vascular clamping completely denudes the endothelial layer, as can be verified by absence of nuclei staining. Also loss of smooth muscle cell nuclei shows damage to the tunica media. Please click here to view a larger version of this figure.
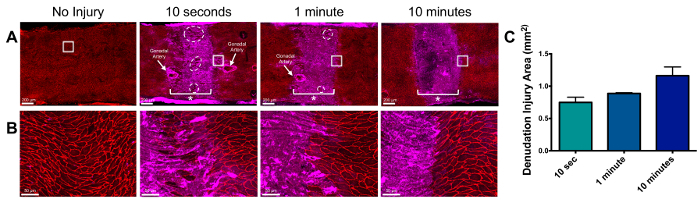 Figure 3: Relationship of Clamp Time to Wound Length. Infrarenal abdominal aortic clamping was performed for 10 sec, 1 min and 10 min. Immediately following injury, immunocytochemistry was performed en face for a sham surgery and these time points as shown (A) and at higher resolution (B). Fibrinogen (in purple) identifies the area of injury. β-catenin (in red) identifies endothelial borders. The asterisk (*) marks the clamp application area. Absence of β-catenin denotes lack of endothelial cells in the area of injury. (C) uses fibrinogen staining as a marker of denudation injury area to show that increasing clamping time increases denudation area (n= 2 for each time point). Error bars represent standard deviation. Please click here to view a larger version of this figure.
Figure 3: Relationship of Clamp Time to Wound Length. Infrarenal abdominal aortic clamping was performed for 10 sec, 1 min and 10 min. Immediately following injury, immunocytochemistry was performed en face for a sham surgery and these time points as shown (A) and at higher resolution (B). Fibrinogen (in purple) identifies the area of injury. β-catenin (in red) identifies endothelial borders. The asterisk (*) marks the clamp application area. Absence of β-catenin denotes lack of endothelial cells in the area of injury. (C) uses fibrinogen staining as a marker of denudation injury area to show that increasing clamping time increases denudation area (n= 2 for each time point). Error bars represent standard deviation. Please click here to view a larger version of this figure.
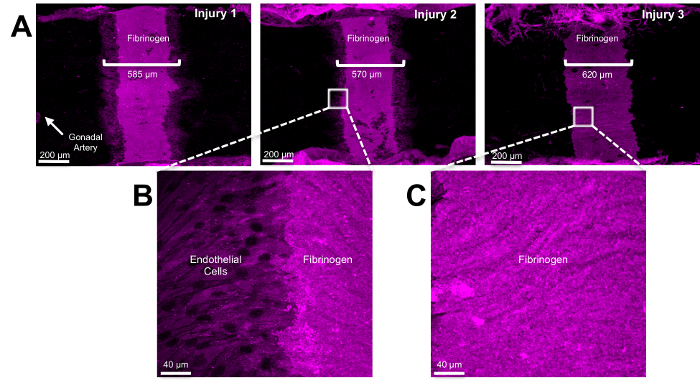 Figure 4: Identification of the Injury Area. Twenty-four hours following aortic clamping for 1 minute, the aorta was dissected and cut to expose the tunica intima. After fixation in 4% paraformaldehyde, immunocytochemistry was performed en face. Fibrinogen (in purple) identifies the area of injury(A). Higher magnification of the wound margin (B) and within the wound (C) are shown. Please click here to view a larger version of this figure.
Figure 4: Identification of the Injury Area. Twenty-four hours following aortic clamping for 1 minute, the aorta was dissected and cut to expose the tunica intima. After fixation in 4% paraformaldehyde, immunocytochemistry was performed en face. Fibrinogen (in purple) identifies the area of injury(A). Higher magnification of the wound margin (B) and within the wound (C) are shown. Please click here to view a larger version of this figure.
 Figure 5: Reproducibility of Denudation Injury and Identification of Re-endothelialization. Twenty-four hr following injury, the aorta was dissected as stated and underwent immunocytochemistry en face. Using fibrinogen as a marker for denudation injury, a highly reproducible injury is observed in (A) with a mean denudation area of 0.81 mm2 (n = 6). Higher resolution images show that the wound margin exhibits fibrinogen staining and migrating endothelial cells (arrows, purple staining) (B) compared to uninjured endothelium (C). Error bars represent standard deviation. Please click here to view a larger version of this figure.
Figure 5: Reproducibility of Denudation Injury and Identification of Re-endothelialization. Twenty-four hr following injury, the aorta was dissected as stated and underwent immunocytochemistry en face. Using fibrinogen as a marker for denudation injury, a highly reproducible injury is observed in (A) with a mean denudation area of 0.81 mm2 (n = 6). Higher resolution images show that the wound margin exhibits fibrinogen staining and migrating endothelial cells (arrows, purple staining) (B) compared to uninjured endothelium (C). Error bars represent standard deviation. Please click here to view a larger version of this figure.
 Table 1: Operative and Postoperative Mortality. A total of 85 mice underwent survival infrafrenal abdominal aortic clamping with a survival rate of 85.9%. Eight mice (9.4%) did not survive surgery due to intestinal hemorrhage or great vessel perforation. Four mice died postoperatively.
Table 1: Operative and Postoperative Mortality. A total of 85 mice underwent survival infrafrenal abdominal aortic clamping with a survival rate of 85.9%. Eight mice (9.4%) did not survive surgery due to intestinal hemorrhage or great vessel perforation. Four mice died postoperatively.
Discussion
Arterial denudation injury due to percutaneous interventions, such as balloon angioplasty and vascular stenting, result in early and late vascular thrombosis and restenosis and contributes to recurrent ischemic events3,12. Interestingly, surgical vascular clamping has also been implicated as a cause of arterial denudation, expanding the scope of the problem to patients undergoing any vascular procedure, whether percutaneous or open13. While impaired re-endothelialization is a fairly recognized cause of thrombosis and restenosis, the molecular mechanisms surrounding re-endothelialization have been difficult to elucidate. Animal models have become a necessity to gain further understanding of re-endothelialization following arterial denudation injury.
Existing animal models to study the cellular and molecular effects of arterial denudation injury include those in swine, rabbit and rodent7,9-11. Rodent models frequently used include the rat carotid artery balloon injury model, the mouse wire injury model, and rat and mouse ligation models14,15. While the rat carotid artery balloon injury model is the best characterized and most commonly used, the carotid injury procedures described by Lindner in mice have broadened the possibilities of using transgenic strains16,17. Genetically modified mouse models decreased the incidence of potential off-target effects and specificity issues associated with pharmacological inhibitors, and may allow for tissue-specific and conditional ablation of specific elements of interest. Nonetheless, carotid injury in the mouse is extremely challenging and difficult to perform.
The murine arterial denudation model of the infrarenal abdominal aorta described here is relatively easy to apply and enables studies of re-endothelialization of a large caliber artery in vivo. Surgeries exhibited an acceptable survival rate of 85.9%. Wound length is dependent on the clamp jaw dimension, strength of the clamp press, and length of time the vascular clamp is occluding the vessel. This model uses a 10 x 1.75 mm jaw dimension and 'strong' clamp press for 1 min to produce a 0.88 mm2 denudation injury that is highly reproducible. Variability in wound length was observed with different jaw dimensions and length of vessel occlusion. In addition, the area of denudation can be expanded by serially clamping the infrarenal abdominal aorta from rostral to caudal. As such, this model offers versatility in denudation injury size that may be manipulated to fit one's study aims.
To assess extent of injury and endothelial closure rates following injury, immunocytochemistry was performed at various time points with antibodies raised against β-catenin to identify endothelial cell junctions or ERG to identify endothelial cell nuclei and fibrinogen to identify the injured area. As re-endothelialization occurred, fibrinogen staining was used to identify the original injury in comparison to the extent of re-endothelialization. While the strong staining of fibrinogen is significantly attenuated after regrowth of the endothelium, there remains sufficient intensity to identify the original injury even after at least 4 days post-surgery.
This model is not without limitations. As with any animal model, murine surgery requires good surgical technique and entails a learning curve. Mice can quickly succumb to vessel perforation if dissection is not performed with care, which is sometimes impossible to control. The most common causes of operative death included intestinal hemorrhage and great vessel perforation, at 5.9% and 3.5%, respectively. However, with careful dissection, hemorrhage is rare and blood loss can usually be kept to below 0.1 ml in our experience. In addition, adequate dissection of the aorta and proper clamp placement is integral to a reproducible injury. While examining distal aortic pulsatility may be used to confirm complete aortic occlusion, removing all retroperitoneal tissue adherent to the aorta is vital for obtaining a clean, uninterrupted wound. As mentioned previously, damage to the tunica media is noted with this procedure of aortic clamping. However, medial smooth muscle death is a well-known consequence of prolonged or chronic vascular expansion due to stent implantation, and results in subsequent neointimal hyperplasia18. Medial damage in our model does not appear to have pathologic sequelae, as we have no postoperative death and these mice have survived up to 2 months postoperatively.
We present a versatile murine model of arterial denudation injury of the infrarenal abdominal aorta that is reproducible with good survival rates. This model fills a need for studying arterial injury with a multitude of transgenic mouse models. Adoption of this murine model may be used to elucidate the molecular mechanisms of re-endothelialization under normal and pathologic conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA Training Program to ASS and AIM, Philip J. Whitcome Fellowship to AIM, and National Institutes of Health (HL130290) to MLIA.
References
- Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv. 2011;4:195–205. doi: 10.1161/CIRCINTERVENTIONS.110.959882. [DOI] [PubMed] [Google Scholar]
- Tada T, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. 2013;6:1267–1274. doi: 10.1016/j.jcin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Otsuka F, et al. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- Kipshidze N, et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44:733–739. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Finn AV, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- Joner M, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Joner M, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- McDonald AI, Iruela-Arispe ML. Healing arterial ulcers: Endothelial lining regeneration upon vascular denudation injury. Vascul Pharmacol. 2015;72:9–15. doi: 10.1016/j.vph.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle J, Tina Au YP, Clowes AW, Reidy MA. Intimal Lesion Formation in Rat Carotid Arteries after Endothelial Denudation in Absence of Medial Injury. Arteriosclerosis. 1990;10:1082–1087. doi: 10.1161/01.atv.10.6.1082. [DOI] [PubMed] [Google Scholar]
- Granada JF, et al. Vascular response to zotarolimus-coated balloons in injured superficial femoral arteries of the familial hypercholesterolemic Swine. Circ Cardiovasc Interv. 2011;4:447–455. doi: 10.1161/CIRCINTERVENTIONS.110.960260. [DOI] [PubMed] [Google Scholar]
- Tulis DA. Rat carotid artery balloon injury model. Methods Mol Med. 2007;139:1–30. doi: 10.1007/978-1-59745-571-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet (London, England) 2008;371:2134–2143. doi: 10.1016/S0140-6736(08)60922-8. [DOI] [PubMed] [Google Scholar]
- Gucu A, et al. Effects of temporary vascular occluder poloxamer 407 Gel on the endothelium. J Cardiothorac Surg. 2013;8 doi: 10.1186/1749-8090-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AW, Tulis DA. Experimental Rat and Mouse Carotid Artery Surgery: Injury & Remodeling Studies. ISRN Minim Invasive Surg. 2013;2013 doi: 10.1155/2013/167407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam D, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V, Fingler J, Reidy MA. Mouse Model of Arterial Injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- Hehrlein C, Weinschenk I, Metz J. Long period of balloon inflation and the implantation of stents potentiate smooth muscle cell death. Possible role of chronic vascular injury in restenosis. Int J Cardiovasc Intervent. 1999;2:21–26. doi: 10.1080/acc.2.1.21.26. [DOI] [PubMed] [Google Scholar]


