Abstract
Advanced electroencephalographic analysis techniques requiring high spatial resolution, including electrical source imaging and measures of network connectivity, are applicable to an expanding variety of questions in neuroscience. Performing these kinds of analyses in a rodent model requires higher electrode density than traditional screw electrodes can accomplish. While higher-density electroencephalographic montages for rodents exist, they are of limited availability to most researchers, are not robust enough for repeated experiments over an extended period of time, or are limited to use in anesthetized rodents.1-3 A proposed low-cost method for constructing a durable, high-count, transcranial electrode array, consisting of bilaterally implantable headpieces is investigated as a means to perform advanced electroencephalogram analyses in mice or rats.
Procedures for headpiece fabrication and surgical implantation necessary to produce high signal to noise, low-impedance electroencephalographic and electromyographic signals are presented. While the methodology is useful in both rats and mice, this manuscript focuses on the more challenging implementation for the smaller mouse skull. Freely moving mice are only tethered to cables via a common adapter during recording. One version of this electrode system that includes 26 electroencephalographic channels and 4 electromyographic channels is described below.
Keywords: Neuroscience, Issue 117, electroencephalography (EEG), electromyography (EMG), neuroscience, mouse, medicine, chronic implant, affordable, open source, high-density, seizure, sleep, anesthesia
Introduction
Neuronal activity can be recorded extracellularly with various levels of granularity from microscopic (individual action potentials) to mesoscopic (local field potentials) to macroscopic (electroencephalogram). These brainwave traces are classically analyzed in the frequency domain to characterize behavioral, neurophysiological, or electrophysiological states. This can be done with a single biopotential,4 but sparse density EEG recordings cannot resolve the spatial component of neuronal activity. Modern electroencephalogram analysis relies on multiple electrodes to produce detailed maps of spatiotemporal distribution of cortical activity in order to correlate that activity with specific psychological conditions and physiologic processes.5-7 Two of the more commonly used categories of analysis requiring high-density EEG montages are electrical source imaging and neural network connectivity measures.8-11
Electrical source imaging involves the localization of functionally active brain regions. Topographical mapping of the electrode array can visualize the current source density of the electrical activity within the brain during event related potentials (ERPs) and evoked potentials (EPs). Electrical source localization is commonly used in both seizure studies as well as in power distribution analyses.12-15 Since EEG has high temporal resolution, EEG studies permit real time evaluation of ERPs and EPs as well as temporally precise post hoc analysis.3,11,12
Associating cognitive states and functions with the interplay of oscillations seen on the electroencephalogram is the ultimate goal of the various measures of neural network connectivity. Numerous studies have shown synchronization and phase locking of oscillations among different brain regions are associated with specific states of arousal, attention, and action.6,13,14,16-19 Demonstrating such signal associations among brain regions requires high-density arrays that permit assessments of network connectivity.
Source localization and network analyses of EEG signals originated with studies in humans, but investigations into the neuronal basis for these signals necessarily involve animal models, as they require invasive techniques that are otherwise impossible in humans. In order to replicate these analyses in rodent models, a method for capturing high-density EEG signals in a rodent's brain is needed. While other groups have constructed high-density microelectrode arrays for use in mice, such approaches are of limited availability to researchers without access to nanofabrication facilities, are not robust enough for repeated experiments over an extended period of time, or are limited to use in anesthetized mice.1-3,7 A low cost alternative protocol for constructing chronic high-density, transcranial electrode array is demonstrated here.
The signal acquisition approach described here is not limited to EEG, but includes electromyographic (EMG) signals. Acquisition of EMG signals can be a complementary approach for defining behavior state and is particularly useful for sleep studies. This approach provides an intermediate between expensive, ultra-high-density intracranial grids, and the limited lead numbers possible with traditional screw electrodes that are insufficient for advanced analysis approaches. The headpiece design is easily constructed and affordable for high-throughput studies. Use of this acquisition system in conjunction with assorted genetic or pharmacologic manipulative techniques within rodent models can help uncover the mechanisms of cortical oscillation generation, behavioral divergences from true genotypic differences, source localization of ERPs and EPs, and large-scale network communication.
Protocol
The studies performed throughout this investigation were in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
1. Headpiece Design and Construction
Remove every eighth row of pins from the 2 x 50 pin brick of a 100 position receptacle connector with a pair of tweezers by pushing the receptacle portion of the pin through the plastic brick. Note: Pins facing downward will be the orientation that will be referenced for the rest of the protocol. (Take note of this specifically in 2.6).
Cover the pins with a very light coat of nail polish to insulate and let the nail polish dry completely.
Remove the nail polish from the tips of the pins with acetone and a small cloth.
Trim the excess plastic of the 2 x 7's using a razor blade or wire-cutting pliers. This will result in 2 x 7 bricks that are insulated along the length of the pins and exposed at the pin tip. These will eventually become the transcranial EEG electrodes. Two 2 x 7 bricks are necessary for a complete chronic electrode array (Figure 1A).
Cut two 1 x 2 pin bricks for EMG signal recording. Use the same process of removing unwanted pins with tweezers and cutting the excess plastic away to create the 1 x 2 bricks. Make sure these 1 x 2's have a smooth side to them from the original 100 Position Receptacle as this distance will become the standard pin distance for each headpiece so a single adapter will work for all headpieces (Figure 1A).
- Use 2-part epoxy to attach the 1 x 2 pin piece to the 2 x 7 pin piece (Figure 1D).
- As both sets of pins must be in the same orientation, epoxy the 1 x 2 on the lateral side of both halves of the headpiece with the smooth sides of the 1 x 2 and 2 x 7 contacting each other. Align the 1 x 2 pinholes and pins with the posterior-most 2 rows of pins on the 2 x 7. Note: The two halves of the headpiece are not epoxied together. This allows for flexibility within the two halves of the headpiece adapter for easier connection during habituation and during experimental days (Figure 1E).
- Let the headpiece halves cure overnight. Upon completion, the headpiece is bilaterally symmetric. Each half consists of a 2 x 7 pin brick with a laterally attached 2 x 1 pin brick that is in line with the posterior most 2 rows of the 2 x 7 pin brick.
- Prepare wires for EMG signal recording. Single stranded, 31 G perfluoroalkoxy-insulated silver wire is used for EMG signal recording (Figure 1D). However, multi-stranded or other metal wiring can be substituted if desired.
- To create thoracic EMG wires take a 3.0 cm long piece of perfluoroalkoxy-insulated silver wire and remove 1 cm of the plastic insulation from one end with a razor blade. Wrap the non-insulated wire around a pair of tweezers twice. Remove the wire from the tweezers and remove 25 mm of insulation on the non-looped end with a razor blade.
- To construct cervical EMG wires, repeat the process with a 1.5 cm segment of wire. Two cervical EMG wires and two thoracic EMG wires are needed for a complete headpiece.
Remove the lateral pin in the furthest anterior row of both headpieces, which corresponds to a stereotaxic coordinates of 3.3 mm anterior of Bregma and 2.3 mm lateral of Bregma, as there is no brain beneath this location as determined by a mouse brain atlas20 (Figure 2A).
- On both headpiece halves, cut the pins of the 1 x 2 brick to the plastic base of the headpiece with a pair of wire cutters (3.0 mm from the tip of the pin) and solder the cervical EMG wire to the anterior pin and the thoracic EMG to the posterior pin.
- Check that each pin is electrically isolated. Perform a continuity test with a digital multimeter by connecting the two leads of the voltmeter to different pins while in the continuity mode. Electrically isolated pins will not produce an audible beep with this multimeter test; however, electrically coupled pins will. Cover the soldered joints with nail polish and once dry, bend the EMG wires such that they are in parallel to the anterior/posterior axis with minimal lateral displacement.
- Trim pins to a relative length such that they match the surface profile of the brain.
- With the aide of a mouse brain atlas, record ventral distance to brain surface from Bregma for each pin coordinate.20 The pin whose ventral distance from Bregma is the largest will serve as the indicator for pin trimming. This pin will not be trimmed while all other pins will be cut with respect to this maximal ventral distance pin (Table 1). Note: Pins can be grinded down to size but it must be done carefully as the friction between pin and grinding wheel can cause the pins of the headpiece to bend. If a pin is bent, use tweezers to straighten it out. An alternative to grinding the pins down to length is to trim them with a pair of wire cutting pliers.
Cover all of the pin tips with a silver solution using a silver solution pen and let dry. This step lowers electrode impedances to ≤30 kΩ, which increases the signal to noise ratio and simultaneously eliminates the rough edges caused from pin trimming, therefore decreasing chances of tissue damage and accelerating recovery from surgery. A completed headpiece half weights approximately 0.5 g.
2. Adapter Construction and Channel Mapping
Cut the connector wires of a 36 Position Dual Row Male Nano-Miniature Connector to a uniform length of 2 or 3 cm using a razor blade. For each wire, strip off 2.5 mm of insulation from the end and tin the exposed metal for each wire. Make sure when tinning to have a single, thin strand of tinned wire for each nano adapter wire as this is crucial to isolate pins. Snip off the stripped insulation with wire-cutting pliers (Figure 1C).
Create a matching male/male connector to the headpiece using the Conn Strip Header 2 x 50. Cut two 2 x 7's and two 2 x 1's from a 2 x 50 brick. Remove unwanted pins from the 2 x 50 brick by breaking off one of the male pins, and then push the unbroken second half of the same piece off the connector with a pair of tweezers (Figure 1B). Note: One side of these pins will serve as adapter pins to plug into each headpiece, while the other half will be soldered to the tinned nano connector wires. Be sure to have the flat plastic edges of the 2 x 1 and 2 x 7 touching to guarantee proper mating of the male/male connector with the headpiece created in step 1.
Solder on one of the ground/reference wires from the nano connector to the desired ground/reference pin. Ground and reference wires are tied together on the RHD2132 amplifier chip. Use the single pin that is 0.60 mm anterior to Bregma and 1.00 mm lateral of Bregma as both reference and ground. (Left headpiece, medial pin of the third most anterior row; however, any other pin could be assigned if preferred, Figure 2.) Note that it is possible to separate ground and reference on the amplifier chip by removing the 0Ω resistor that ties ground and reference together if isolating the two is desired.
Solder the tinned nano connector wires to the same side of the male/male connectors as the ground/reference pin connection. Each wire maps to a specific channel, so channel setup can be completed at this time. Channel map diagrams for the amplifier headstages are found on the Open Ephys Wiki site (https://open-ephys.atlassian.net/wiki/display/OEW/Home). Solder the corresponding wire whose channel is known to the respective pin to achieve the desired mapping.
Cut off unused wires at the base of the nano connector with the wire cutting pliers.
Use a voltmeter to ensure that each pin is electrically isolated from all other pins. Once isolation is confirmed, apply a thin coat of nail polish around each soldering joint to further insulate each pin.
Using 2-part epoxy, reinforce the matching nano adapter to the bilateral 2 x 7 and 2 x 1 pins on the male-male bricks. Note: There will be two halves to this single adapter that match the pin arrangement of the headpiece halves created earlier. It is critical to make sure that the medial portion of each half of the adapter does not have excess epoxy overflowing the plastic edge of the male/male connector, as this will prevent both halves of the headpiece being plugged in simultaneously. Do not allow any epoxy to flow to the underside of the pin adapter portion of the adapter, as this would also prevent proper connections. Use the 2 headpiece halves as a mold for proper connector pin alignment.
Epoxy both halves of the adapter and epoxy the base of the nano connector to increase its durability. Be sure to cover all soldering joints with epoxy. Let the adapter cure overnight.
Confirmation of proper channel mapping can be performed using impedance measurements in the Open-Ephys graphical user interface (GUI). A completed adapter weighs approximately 1.3 g (Figure 1F).
3. Surgery
- Prepare a sterile surgical field.
- Wear sterile gloves and other personal protective equipment as required. Sterilize tools in an autoclave. Sterilize the stereotactic frame with a 1.0 mM chlorine dioxide solution. Spray the solution onto the frame and wait 5 min before rinsing with sterile water.
- To sterilize implantable headpiece parts, spray the components with a 1.0 mM chlorine dioxide solution, and wait 5 min before rinsing with sterile water. Place the now sterile implantable hardware into a sterile petri dish.
Obtain a pre-surgical weight for the mouse, then anesthetize the mouse in a 200 ml induction chamber using 1.5-2.0% isoflurane in 100% oxygen. Use a flow rate into the chamber of roughly 500 ml/min.
Confirm loss of righting reflex by rotating the induction chamber. Remove the mouse from the induction chamber and place into the nose cone on the stereotactic frame without completely securing the mouse's head with the ear bars. Continue to monitor for proper depth of anesthesia by toe pinch assessment while also assessing vital signs.
Maintain core body temperature at 37 °C with a closed-loop temperature controller, such as a rectal probe and heating pad system. Cover the eyes of the mouse with ophthalmic eye ointment before trimming off the fur on the top of the skull using curved scissors or clippers. Disinfect the head with betadine and allow the betadine to dry completely before proceeding.
Administer analgesics and antibiotics along with fluids intraperitoneally. For a 25 g mouse, 0.5 mg cefazolin, 0.125 mg meloxicam, 0.5 ml saline, and 2.5 μg buprenorphine q 4-6 hr prn.
Inject 250 µl 0.25% bupivacaine subcutaneously along midline on the head, and inject 100 µl 0.25% bupivacaine subcutaneously on both zygomatic arches of the mouse.
- Secure the mouse in the stereotaxic frame and expose the skull.
- Secure the head of the mouse with the stereotaxic ear bars to the stereotaxic frame. Ensure that the mouse is at a surgical plane of anesthesia by confirming the absence of the toe pinch reflex. Create a 1.5-2.0 cm incision along with a No. 11 disposable scalpel along midline of the skull. The incision will start from between the eyes and continue posteriorly to the occiput.
- Expose the skull by spreading the skin laterally with micro clamps. Reduce isoflurane concentration from 2.0% to a concentration that maintains a surgical plane of anesthesia, but do not reduce to lower than 1.0% isoflurane in 100% oxygen. Pre-operative analgesia reduces the amount of inhaled anesthetic needed to maintain a surgical depth of anesthesia, and can lead to faster recovery and improved survival outcomes.
- Level the skull and drill burr holes.
- Identify Bregma and zero the stereotactic coordinates at Bregma, which becomes the origin of the coordinate system. To level the skull in the medial/lateral axis, move a leveling probe attached to a stereotaxic manipulator arm 1.50 mm laterally in both directions from Bregma and confirm that the dorsal/ventral depth is less than 0.05 mm when the probe contacts the left and right sides of the skull. Note: The 10 µm resolution of the dorsal/ventral manipulator arm used in conjunction with a digital coordinate display simplifies leveling. Leveling the anterior/posterior axis about Bregma follows the same technique. The difference in the ventral distance for contacting Bregma and Lamda should also be less than 0.05 mm.
- Adjust the skull until leveling is complete in both directions so that the transverse plane is parallel to the ground. This allows for true stereotactic coordinates as seen in the mouse brain atlas.20
- With a 0.5 mm diameter micro drill bit within a stereotaxic drill, drill burr holes from 3.30 mm anterior to 4.50 mm posterior to Bregma in 1.30 mm increments at 1.00 mm laterally to the midline on both halves of the skull. For the 2.30 mm lateral columns of electrodes, drill burr holes from 2.00 mm anterior to Bregma to 4.50 mm posterior to Bregma in 1.30 mm increments on both sides of midline (Figure 2). The high accuracy and precision that is required for this drilling operation is simplified by the 10 µm resolution of a digital stereotaxic manipulator arm. Note: In order for the pins of the headpiece to properly be implanted, the skull of the mouse must be securely in place within the stereotaxic frame. If the skull moves during drilling, misalignment of the headpiece and burr holes may ensue.
- Implant the headpieces.
- With straight forceps, prepare EMG wire tunnels for the thoracic EMG wires. Burrow 2.5 cm between the skin and muscle in the back for both the left and right EMG wires. Insert the thoracic and cervical EMGs into the cavity created with the straight forceps first, and then maneuver the EEG brick with curved forceps such that the pins align with the previously drilled burr holes.
- Apply slight pressure onto the headpiece and wiggle the pins into the skull. Pin diameter is 0.46 mm. With insulating nail polish, the pins will fit tightly in the drilled burr holes. The headpiece will be stable once it is appropriately inserted. Adjust EMG wires to final positions. Repeat the same process for the headpiece on the other side.
- Secure the headpiece in place using dental cement.
- When both headpieces are set in place, mix 1:1 ratio of methyl methacrylate with its crosslinking compound. Apply the mixture such that it covers the exposed skull, nail-polished parts of the pin electrodes, and proximal portion of EMG wires, but does not cover the female receptacles of the headpiece.
- Be sure to not get cement on fur. Do not allow for ridges of cement to form that the mouse will be able to grab onto. Ensure sufficient time for the cement to dry, and then remove the mouse from the stereotactic frame. The total weight that the mouse will have to carry is from the 2 halves of the headpiece and the securing cement is approximately 1.2 g.
- Place the animal in a clean recovery area.
- Maintain core body temperature with a heating pad. Monitor the mouse until it regains all postural reflexes, signifying emergence from anesthesia. Individual housing is recommended for long-term recovery.
- Daily monitoring for a minimum of 3 days following surgery is recommended with interventional analgesia. Allow 10-14 days of recovery post-operatively before starting a tethered habituation period.
4. Habituate Animals to Tethering
Connect the adapter to the mouse using a mouse head restraint (Figure 1G-H). Hold onto opposite corners of the headpieces that are cemented into place with curved hemostats once the mouse is restrained and slowly insert the adapter pins into the implanted headpiece on both sides.
Connect the 32 channel amplifier to the adapter (Figure 1H). Be sure to align the logos on both the adapter and amplifier in a consistent orientation for both the adapter and the amplifier to prevent channel-mapping errors. Connect the amplifier to a RHD2000 standard serial peripheral interface (SPI) cable. This cable will connect to the acquisition system for signal recording.
Place the mouse within a chamber that has a cantilever arm installed on the chamber wall. Attach the SPI interface cable to the cantilever arm and adjust the tension in the cantilever arm to counteract the weight of the tethered cable. The mouse is able to move freely and is habituated for an hour a day the week before recording.
To disconnect the mouse, simply unplug the cable and adapter from the mouse while using a flat stainless steel micro spatula to aide in disconnecting the adapter from the mouse.
5. Signal Extraction System Setup/Signal Recording
Plug the constructed adapter into the headpiece of an implanted mouse. Connect a headstage amplifier to the adapter and attach a standard SPI interface cable to the amplifier and to the acquisition board. Have the SPI cable attach to a properly tensioned cantilever so that the additional weight on the mouse's head is minimized.
Place a local Faraday cage, created using conducting mesh or aluminum foil, around the headstage and ground the local Faraday cage.
Obtain electrode impedances before the beginning of each recording by selecting a 30 kS/sec sampling rate and measure impedances via the module in the GUI. An impedance value less than or equal to 10 kΩ for an individual pin is required for confirmation of proper electrode contact. Higher impedance values result in rejected data from that electrode.
For recording, create a signal chain of Rhythm FPGA, Bandpass Filter, and LFP viewer in the GUI. It is recommended to select a sampling rate of 1.00 kS/s, bandwidth of 0.1-7,500 Hz and deselect DSP. Set the Bandpass filter to 0.1-250 Hz and display the channels by opening the LFP viewer. 250 and 400 µV channel amplitudes with draw method selected best visualizes the data.
Begin recording using the GUI. Create a new folder for each recording and set the path for saving files to that folder. To begin a recording simply hit record. All 32 channels from the the connector are recorded by default, but unwanted channels can be unselected by clicking on the right side of the Rhythm FPGA module before the beginning of the recording.
Import data into Matlab for analysis. There are a multitude of open-source toolboxes that can be used to help in analysis.
Representative Results
Sample data recorded in a freely moving mouse implanted with a high-density EEG headpiece is shown in Figure 3. Individual EEG waveforms correspond to the channel-mapping scheme shown in Figure 2. Examples of cervical and thoracic EMG are also displayed in Figure 3. Note that the thoracic EMG recording also contains embedded electrical activity originating in the mouse's heart that becomes readily apparent when a differential signal between the two thoracic EMG wires (T) is computed. With this recording it is also possible to calculate the mouse's heart rate by measuring the time between electrocardiographic QRS spikes.23-24 Similarly, it is possible to measure the mouse's respiratory rate by calculating phasic variability of the QRS spike as the thoracic cavity expands and contracts with each breath.25 Hence, this setup permits for acquisition of murine polysomnography. Moreover, the setup enables cortical mapping of visual evoked potentials (Figure 4). When a 10 msec pulse of light is delivered only to the mouse's left eye, classic responses are recorded in the contralateral (but not ipsilateral) primary visual cortex that are followed by a delayed response in contralateral secondary visual cortex. The movie embedded in Figure 4 shows the time varying electrical potentials across the entire cortical surface along with graphs of activity in contralateral V1 and V2.
| AP | ||||||
| 3.3 | 0 | 0 | ||||
| 2 | 0.4 | 0.6 | 0.6 | 0.4 | ||
| 0.7 | 0.6 | 0.9 | 0.9 | 0.6 | ||
| -0.6 | 0.9 | 1 | 1 | 0.9 | ||
| -1.9 | 1 | 1.1 | 1.1 | 1 | ||
| -3.2 | 3 | 1 | 1 | 1 | 1 | 3 |
| -4.5 | 3 | 0.7 | 0.7 | 0.7 | 0.7 | 3 |
| ML | -2.3 | -1 | 1 | 2.3 |
Table 1: Pin Trimming Lengths. This figure shows the required trimming lengths, in mm, per pin for the headpiece. Lengths for pin trimming were acquired from a mouse brain atlas. After trimming pins, the headpiece matches the surface profile of the brain.20 EMG pins are completely cut off as the wires used to record EMG signal are soldered onto the pin stub.
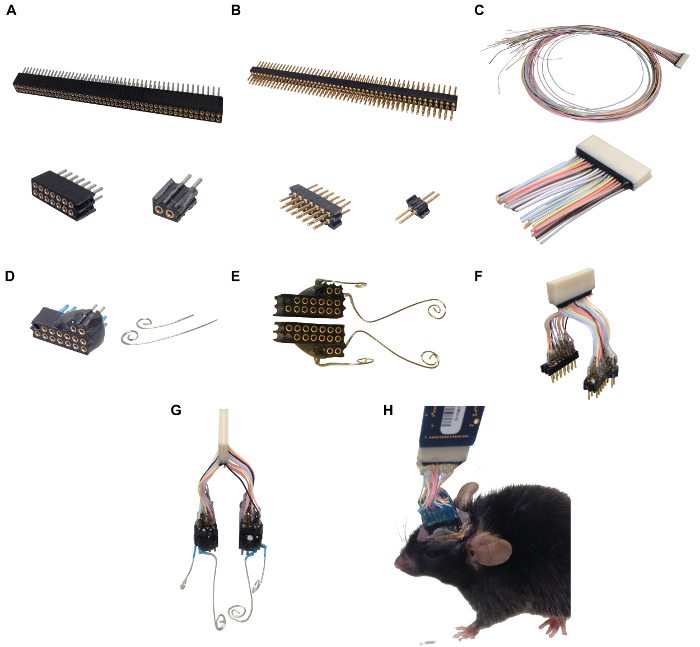 Figure 1:Headpiece Components, Intermediate Construction Steps, and Proper Connection for Recording. This figure shows the raw material used to create headpieces. Starting with a 100 pin receptacle connecter, smaller 2 x 7 and 2 x 1 components are created. Note that in the 2 x 1 component, the original edge of the 2 x 50 is intact, which permits consistent headpiece construction and allows for one adapter to connect to many implanted mice. Figure 1B and 1C present the raw materials necessary to create the adapter from the headpiece to the amplifier. 1B presents the headpiece end of the adapter that similarly is cut down to connect to the headpiece. Note that that 2 x 1 again has an original edge from the raw component, ensuring proper connection between the adapter and the headpiece. Figure 1C shows the end of the adapter that connects to the amplifier. Figure 1D illustrates the epoxied 2 x 7 and 2 x 1 components along with prepared EMG wires for signal recording. Figure 1E demonstrates a completed headpiece. Figure 1F displays a completed adapter. Figure 1G shows a proper connection between the headpieces and the adapter. Lastly, Figure 1H shows an implanted mouse with connected adapter and amplifier. The amplifier chip is connected to an interface cable that runs to the acquisition board (not shown). Please click here to view a larger version of this figure.
Figure 1:Headpiece Components, Intermediate Construction Steps, and Proper Connection for Recording. This figure shows the raw material used to create headpieces. Starting with a 100 pin receptacle connecter, smaller 2 x 7 and 2 x 1 components are created. Note that in the 2 x 1 component, the original edge of the 2 x 50 is intact, which permits consistent headpiece construction and allows for one adapter to connect to many implanted mice. Figure 1B and 1C present the raw materials necessary to create the adapter from the headpiece to the amplifier. 1B presents the headpiece end of the adapter that similarly is cut down to connect to the headpiece. Note that that 2 x 1 again has an original edge from the raw component, ensuring proper connection between the adapter and the headpiece. Figure 1C shows the end of the adapter that connects to the amplifier. Figure 1D illustrates the epoxied 2 x 7 and 2 x 1 components along with prepared EMG wires for signal recording. Figure 1E demonstrates a completed headpiece. Figure 1F displays a completed adapter. Figure 1G shows a proper connection between the headpieces and the adapter. Lastly, Figure 1H shows an implanted mouse with connected adapter and amplifier. The amplifier chip is connected to an interface cable that runs to the acquisition board (not shown). Please click here to view a larger version of this figure.
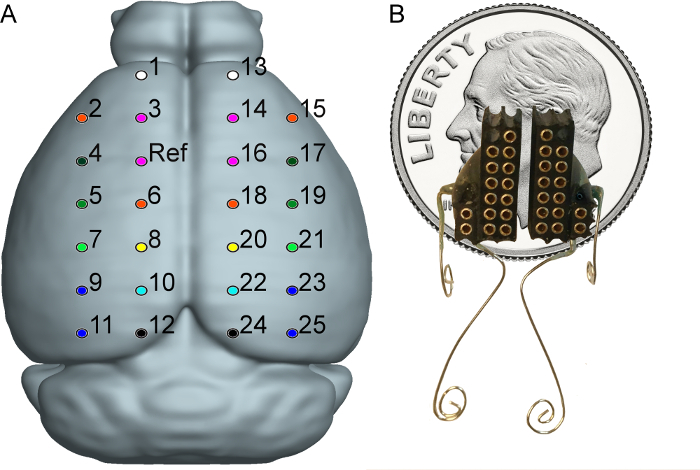 Figure 2: Electrode Montage and Fully Constructed Headpiece. This figure shows electrode placement with respect to mouse brain. Electrode locations are based on stereotaxic coordinates from Bregma. Coordinates for each electrode can be found in step 4.8 of the protocol. Electrode color corresponds to the underlying brain regions for each electrode. White = frontal association cortex (FrA), Orange = primary motor cortex (M1), Pink = secondary motor cortex (M2), Dark Green = primary somatosensory cortex, forelimb region (S1FL), Green = primary somatosensory cortex, dysgranular zone (S1DZ), Light Green = primary somatosensory cortex, barrel field (S1BF), Yellow = medial parietal association cortex (MPtA), Dark Blue = primary visual cortex (V1), Light Blue = secondary visual cortex, mediomedial area (V2MM), Black = retrosplenial dysgranular cortex (RSD).20 Common Reference/Ground is shown as well. This reference scheme minimizes respiratory artifact within the raw signal. Numbers associated with each individual electrode provide a channel map for the entire array. Image modified from Allen Mouse Brain Atlas.21,22
Figure 2B shows a fully constructed headpiece to scale with respect to a dime. Please click here to view a larger version of this figure.
Figure 2: Electrode Montage and Fully Constructed Headpiece. This figure shows electrode placement with respect to mouse brain. Electrode locations are based on stereotaxic coordinates from Bregma. Coordinates for each electrode can be found in step 4.8 of the protocol. Electrode color corresponds to the underlying brain regions for each electrode. White = frontal association cortex (FrA), Orange = primary motor cortex (M1), Pink = secondary motor cortex (M2), Dark Green = primary somatosensory cortex, forelimb region (S1FL), Green = primary somatosensory cortex, dysgranular zone (S1DZ), Light Green = primary somatosensory cortex, barrel field (S1BF), Yellow = medial parietal association cortex (MPtA), Dark Blue = primary visual cortex (V1), Light Blue = secondary visual cortex, mediomedial area (V2MM), Black = retrosplenial dysgranular cortex (RSD).20 Common Reference/Ground is shown as well. This reference scheme minimizes respiratory artifact within the raw signal. Numbers associated with each individual electrode provide a channel map for the entire array. Image modified from Allen Mouse Brain Atlas.21,22
Figure 2B shows a fully constructed headpiece to scale with respect to a dime. Please click here to view a larger version of this figure.
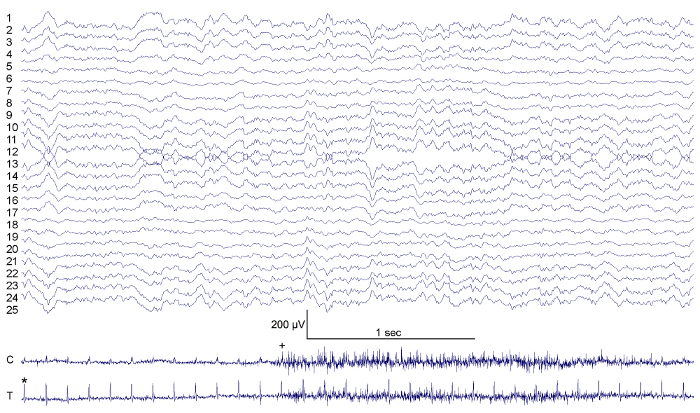 Figure 3:Sample EEG and EMG Traces from the Electrode Montage. Electrode waveforms correspond to the channel mapping shown in Figure 1A. Cervical EMG (C) provides the ability to determine nuchal muscle tone (+). EMG signals also contain cardiac QRS electrical impulses (*). Scale bars of 200 µV for trace amplitude and 1 sec for trace duration are shown. Please click here to view a larger version of this figure.
Figure 3:Sample EEG and EMG Traces from the Electrode Montage. Electrode waveforms correspond to the channel mapping shown in Figure 1A. Cervical EMG (C) provides the ability to determine nuchal muscle tone (+). EMG signals also contain cardiac QRS electrical impulses (*). Scale bars of 200 µV for trace amplitude and 1 sec for trace duration are shown. Please click here to view a larger version of this figure.
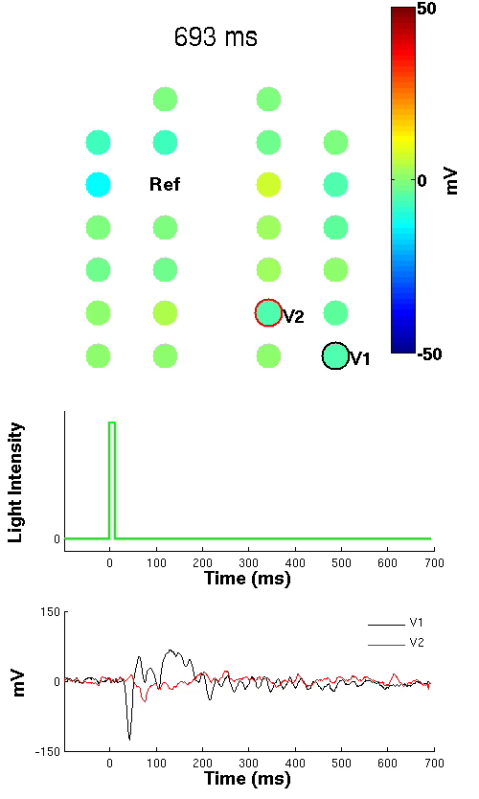 Figure 4: Spatial Distribution of Visual Evoked Potential. Spatial distribution of the evoked potential following application of a unilateral light flash administered only to the left eye. Upper diagram depicts the high-density EEG montage with each circle representing an electrode. Change in color over time corresponds to voltage changes over time for each respective electrode. At time = 0 msec, a 10 msec light pulse is delivered and represented in the middle figure. Bottom graphic illustrates mean evoked potential traces for contralateral V1 and V2 EEG electrodes (n = 108 EP trials). Light pulse occurs at 0 msec. Note that the corresponding evoked potential response is observed in contralateral V1 (black trace), followed by a longer latency evoked potential response in contralateral V2 (red trace). (Right click to download).
Figure 4: Spatial Distribution of Visual Evoked Potential. Spatial distribution of the evoked potential following application of a unilateral light flash administered only to the left eye. Upper diagram depicts the high-density EEG montage with each circle representing an electrode. Change in color over time corresponds to voltage changes over time for each respective electrode. At time = 0 msec, a 10 msec light pulse is delivered and represented in the middle figure. Bottom graphic illustrates mean evoked potential traces for contralateral V1 and V2 EEG electrodes (n = 108 EP trials). Light pulse occurs at 0 msec. Note that the corresponding evoked potential response is observed in contralateral V1 (black trace), followed by a longer latency evoked potential response in contralateral V2 (red trace). (Right click to download).
Discussion
The low-cost construction and surgical steps necessary in order to properly attain a 26 channel, high-density EEG montage in a mouse is described. Proper epidural electrode contact is critical in acquiring quality EEG signals in this system. Two steps within the protocol address this issue: pin trimming to match brain contour, and headpiece implantation prior to acrylic reinforcement. It is important not to cut a pin too short during the construction phase. When implanting the headpieces, it is imperative to check pin placement before the final acrylic reinforcement. One way to confirm proper electrode contact is through impedance testing. Ostensibly, impedances of 5-10 kΩ suggest proper epidural placement.26 Impedance measurements demonstrate the headpieces' durability, as electrode impedance values are stable within this 5-10 kΩ range for at least 4 months after implantation. The other essential step involves aligning the EMG pins with the two posterior-most rows of the 2 x 7 EEG brick. This is critical for adapter connection, as misaligned EMG and EEG pins will result in an inability to connect the adapter or bent adapter pins.
A major advantage of this acquisition system is the ease of modifying the shape of the electrode array in order to optimize varied experimental needs. Customized electrode arrangements that are optimally suited for specific experiments can be readily created. Customization for specific experiments could potentially combine EEG with cannula for directed drug delivery for combined pharmacological, electroencephalographic, and behavioral studies.27 Headpieces, adapters, and surgical procedures are easily tailored to a wide number of studies when following the methods described in the protocol above. A second major advantage of this acquisition system is its low cost. At present, this acquisition system can record 128 input channels on up to 4 separate cables, permitting simultaneous recordings from 4 mice or if desired, rats with higher density grids. Such expansion would only require extra cables and adapters.
This approach to high-density EEG acquisition addresses drawbacks of other high-density EEG acquisition methods in mice. The system described in this work is handily constructed with simple materials and uses open source hardware and software that is inexpensive and stable, allows for repeated measurements in the same animal over months, permits free movement during an experiment, and does not require mice to be anesthetized for recording. Limitations of this system are that it has only been validated to date in mice that weigh 20 g or more, and are older than 12 weeks. Smaller or younger mice may have difficulty with the headpiece implantation. A secondary limitation of this methodology is the inability to precisely control electrode depth after headpiece fabrication. However, this same limitation applies to traditional screw EEG electrodes since there is no way to precisely know the pre-mortem screw depth relative to the cortical surface. Troubleshooting for this method typically involves properly shielding interfering signal from the mouse when tethered in order to obtain noise-free signal.
High-density EEG arrays are essential for the complex spatiotemporal analyses of EEG data that are the new normal in modern EEG interpretation. While spatial distribution of a visual evoked potential is illustrated, data acquired using this system can be analyzed using electrical source imaging techniques and neuronal connectivity measures. A 60% to 70% reduction in contact area between these electrode pins compared to traditional screw contacts permits more precise signal localization, as shown in Figure 4. Employing high-density analytic techniques in genetically modified mice, following pharmacological intervention, or in animals with intrinsic pathology such as seizure disorders can help discern the mechanisms generating specific cortical oscillations, localize sources of ERPs and EPs, and reveal large-scale network properties. By better paralleling human systems, this approach will improve small animal models of human neurophysiology and neuropathology, providing easier translation of discoveries made in rodent models to scientific and clinical relevance in humans.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Foundation for Anesthesia Education and Research Mentored Research Training Grant (ARM), by the National Institutes of Health grants GM107117 (MBK) and GM088156 (MBK), and by the Department of Anesthesiology and Critical Care at the University of Pennsylvania, Perelman School of Medicine.
References
- Choi JH, Koch KP, Poppendieck W, Lee M, Shin H-S. High resolution electroencephalography in freely moving mic. J. Neurophysiol. 2010;104(3):1825–1834. doi: 10.1152/jn.00188.2010. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim D, Shin H, Sung H, Choi JH. High-density EEG Recordings of the Freely Moving Mice using Polyimide-based Microelectrode. J Vis Exp. 2011. pp. e2–e5. [DOI] [PMC free article] [PubMed]
- Megevand P, Quairiaux C, Lascano AM, Kiss JZ, Michel CM. A mouse model for studying large-scale neuronal networks using EEG mapping techniques. Neuroimage. 2008;42(2):591–602. doi: 10.1016/j.neuroimage.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Sabourin ME, Cutcomb SD, Crawford HJ, Pribram K. EEG correlates of hypnotic susceptibility and hypnotic trance: spectral analysis and coherence. Int J Psychophysiol. 1990;10(2):125–142. doi: 10.1016/0167-8760(90)90027-b. [DOI] [PubMed] [Google Scholar]
- Miller EK, Wilson MA. All My Circuits: Using Multiple Electrodes to Understand Functioning Neural Networks. Neuron. 2008;60(3):483–488. doi: 10.1016/j.neuron.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7(5):446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Kipke DR, et al. Advanced Neurotechnologies for Chronic Neural Interfaces: New Horizons and Clinical Opportunities. J Neurosci. 2008;28(46):11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Kayser C, Oeltermann A. In Vivo Measurement of Cortical Impedance Spectrum in Monkeys: Implications for Signal Propagation. Neuron. 2007;55(5):809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Michel CM, et al. Electric source imaging of human brain functions. Brain Res Rev. 2001;36(2-3):108–118. doi: 10.1016/s0165-0173(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65(1):37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Cook IA, O'Hara R, Uijtdehaage SHJ, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol. 1998;107(6):408–414. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Teplan M. Fundamentals of EEG measurement. Meas Sci Rev. 2002;2:1–11. [Google Scholar]
- Buzsáki G, Anastassiou Ca, Koch C. The origin of extracellular fields and currents- EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ. The Cognitive Correlates of Human Brain Oscillations. J Neurosci. 2006;26(6):1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak P. Neurophysiologic basis of the EEG. J Clin Neurophysiol. 2006;23(3):186–189. doi: 10.1097/01.wnp.0000220079.61973.6c. [DOI] [PubMed] [Google Scholar]
- Thut G. Modulating Brain Oscillations to Drive Brain Function. PLoS Biol. 2014;12(12):1–4. doi: 10.1371/journal.pbio.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal Oscillations in Cortical Networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Towards a neurobiological theory of consciousness. Semin Neurosci. 1990;2:263–275. [Google Scholar]
- Murakami S, Okada Y. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J Physiol. 2006;575(3):925–936. doi: 10.1113/jphysiol.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. New York: Elsevier; 2007. [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Allen Mouse Brain Atlas. Allen Institute for Brain Science; 2015. Available from: http://mouse.brain-map.org. [Google Scholar]
- Berger RD, Akselrodv S, Gordon D, Cohen RJ. An Efficient Algorithm for Spectral Analysis of Heart Rate Variability. IEEE Trans Biomed Eng. 1986;33(9):900–904. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- Pan J, Tompkins WJ. A Real-Time QRS Detection Algorithm. IEEE Trans Biomed Eng. 1985;32(3):230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- Moody GB, Mark RG, Zoccola A, Mantero S. Derivation of Respiratory Signals from Multi-lead ECGs. Comput Cardiol. 1985;12:113–116. [Google Scholar]
- Thongpang S, Richner TJ, Brodnick SK, et al. A Micro-Electrocorticography Platform and Deployment Strategies for Chronic BCI Applications. Clin EEG Neurosci. 2011;42(4):259–265. doi: 10.1177/155005941104200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird HEI, Hermansen JE, Huxtable RJ. An electrode-cannula unit for intracerebral electrical stimulation, EEG recording and drug administration in small animals. Pharmacolgy Biochem Behav. 1979;10(2):429–431. doi: 10.1016/0091-3057(79)90208-9. [DOI] [PubMed] [Google Scholar]


