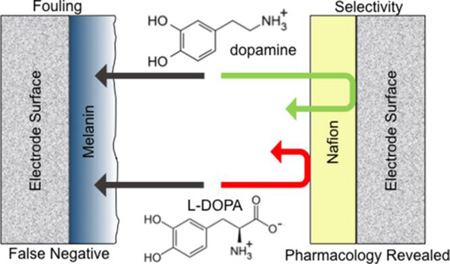
Abstract
L-DOPA has been the gold standard for symptomatic treatment of Parkinson’s disease. However, its efficacy wanes over time as motor complications develop. Very little is known about how L-DOPA therapy affects the dynamics of fluctuating dopamine concentrations in the striatum on a rapid timescale (seconds). Electrochemical studies investigating the effects of L-DOPA treatment on electrically evoked dopamine release have reported conflicting results with significant variability. We hypothesize that the uncertainty in the electrochemical data is largely due to electrode fouling caused by polymerization of L-DOPA and endogenous catecholamines on the electrode surface. Thus, we have systematically optimized the procedure for fabricating cylindrical, Nafion-coated, carbon-fiber microelectrodes. This has enabled rapid and reliable detection of L-DOPA’s effects on striatal dopamine signaling in intact rat brain using fast-scan cyclic voltammetry. An acute dose of 5 mg/kg L-DOPA had no significant effect on dopamine dynamics, demonstrating the highly efficient regulatory mechanisms at work in the intact brain. In contrast, administration of 200 mg/kg L-DOPA significantly increased the amplitude of evoked dopamine release by ~200%. Overall, this work describes a reliable tool that allows a better measure of L-DOPA augmented dopamine release in vivo, measured using fast-scan cyclic voltammetry. It provides a methodology that improves the stability and performance of the carbon fiber microelectrode when studying the molecular mechanisms underlying L-DOPA therapy, and also promises to benefit a wide variety of studies because Nafion is so commonly used in electroanalytical chemistry.
Keywords: fast-scan cyclic voltammetry, in vivo, electrode fouling, Parkinson’s disease, ascorbic acid
Graphical Abstract
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder that affects more than a million people in the United States 1. It is characterized by motor deficits including bradykinesia, rigidity, and resting tremor, resulting from a progressive loss of nigrostriatal dopamine (DA) neurons 2, 3. DA does not readily cross the blood-brain barrier, thus its use for treating the symptoms of PD is precluded 4. 3, 4-dihydroxyphenyl-L-alanine (L-DOPA), the metabolic precursor to DA, has routinely been used for symptomatic treatment of PD since the late 1960s 3, 5, 6. However, the efficacy of prolonged L-DOPA treatment wanes over time and patients develop serious motor complications 3, 7, 8. Despite common use in the therapeutic management of PD, remarkably little is known about how L-DOPA replacement therapy alters the dynamics of pulsatile DA fluctuations that occur on a fast (seconds) timescale.
The vast majority of studies that have investigated these questions have analyzed dialysate collected in the striatum 9–12. Microdialysis is a diffusion-based sampling method that is well suited to examine steady state or slowly changing levels of analytes in the extracellular fluid 13. Thus, these studies have significantly advanced our knowledge on how L-DOPA serves to gradually increase striatal DA levels 9–12. However, quantification of the effects of L-DOPA on rapid DA dynamics is also important, because patients with Parkinson’s disease are impaired in cognitive tasks that require learning from positive (and negative) feedback14, 15, and rapid DA signaling in both the sensorimotor and limbic corticostriatal circuits has been shown to play a key role in reinforcement learning processes16–20. Over the past twenty years, electrochemical techniques have proven to be particularly useful for monitoring rapid changes in DA concentration resulting from discrete neurochemical events, and voltammetric studies have provided fundamental information to describe how rapid DA fluctuations underlie discrete aspects of motivated behavior in animal subjects. For example, phasic dopamine release in the nucleus accumbens core has been shown to correlate with the presentation of Pavlovian reward-predictive cues and to precede the initiation of reward-seeking actions19. However, few electrochemical studies have investigated the effects of L-DOPA administration on phasic DA fluctuations in the dorsal striatum (but see Phillips et al.21), and measurements of the effects of L-DOPA on electrically evoked DA release have reported conflicting results with significant variability22–25. Clarifying this question is critical to developing improved therapies for the treatment of PD.
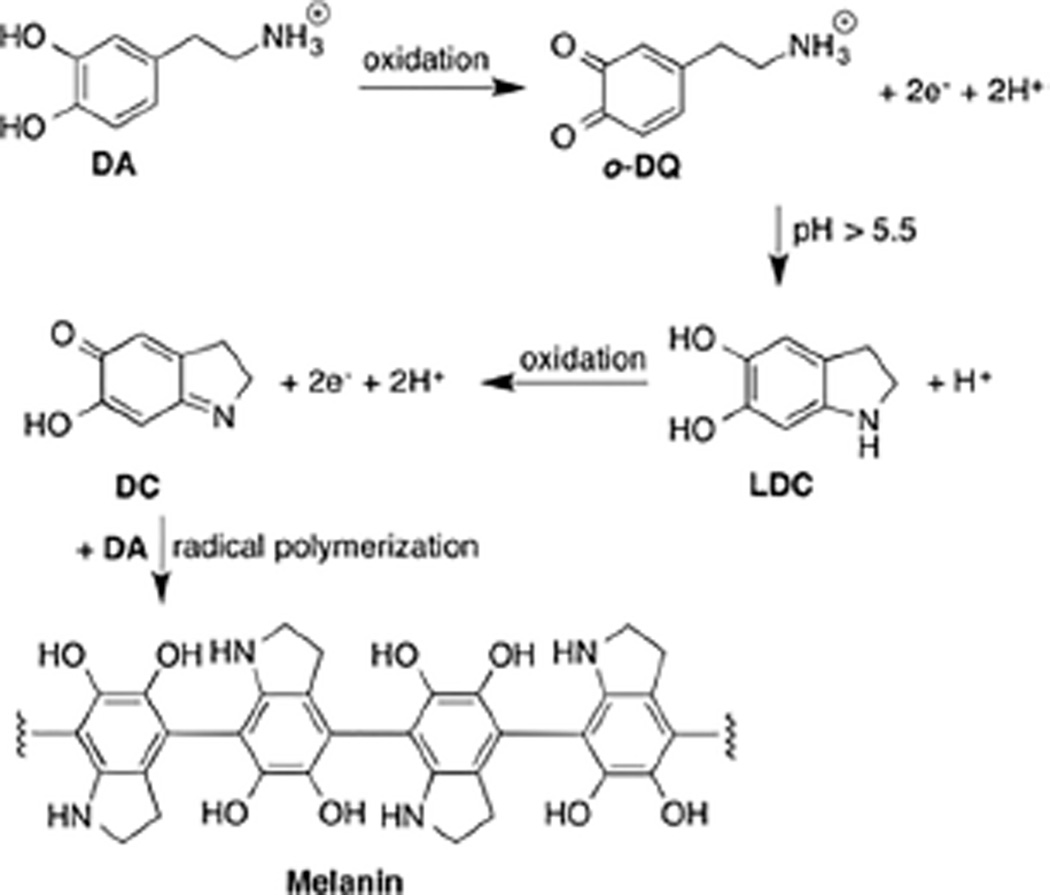
We hypothesize that much of the uncertainty in the electrochemical data is due to complications associated with the chemical nature of L-DOPA and DA. Both species self-polymerize in a mechanism that likely involves oxidation of the catechol to a quinone 26. Near neutral pH, the amine group inherent to dopamine-o-quinone (o-DQ) can serve as a nucleophile, initiating an intramolecular cyclization to generate leucodopachrome (LDC) 27. Further oxidation generates dopachrome (DC), which may polymerize to melanin by a free radical polymerization 28 (Scheme 1). The oxidative cross-linking generates robust water resistant bonds that enable the formation of thin, surface-adherent films on a wide range of materials, including the surface of electrodes 29–31. This film reduces the active surface area of the sensor and attenuates sensitivity 32–34.
Scheme 1.
DA polymerization pathway.
Nafion, a perfluorinated ion-exchange polymer, has been extensively used on the surface of sensors to repel interfering and adsorbing species 35–37. There are numerous protocols for creating a Nafion membrane at the electrode surface 35, 38–46; however, a reliable means for consistent generation of a simple Nafion membrane on cylindrical carbon-fiber microelectrodes has not yet been achieved (but see Heien et al.46 for description of a robust Nafion/polyethylenedioxythiophene composite membrane). Electrochemical data collected using Nafion-coated electrodes can be highly variable, presumably due to inconsistent adhesion of the Nafion (both hydrophobic and hydrophilic regions) with the electrode surface 45. Reliable generation of a membrane is confounded by the fact that the morphology of Nafion is very sensitive to the nature of the solvent in which it is dissolved 43. The problem is exacerbated at carbon-fiber microelectrodes, as electrochemical conditioning changes the chemical functionalities inherent to the carbon surface 47, even etching the surface when electrodes are rapidly scanned to anodic limits of ~1.3 V 48. By refining the parameters by which a Nafion coating is applied to the electrode, we have advanced a technique for generating a carbon fiber microelectrode that is less sensitive to fouling by polymerization of L-DOPA or dopamine at the surface. We use fast-scan cyclic voltammetry (FSCV) to demonstrate that generation of a Nafion membrane after electrochemical conditioning reliably produces a uniform film on the sensor surface that preserves a rapid response to DA while preventing fouling. We then use this approach to assess the effects of various concentrations of systemic L-DOPA administration on DA dynamics measured in rat striatum with sub-second temporal resolution. In addition to providing chemical information that is highly relevant to the most common therapy used in the treatment of PD, this work serves to benefit a wide variety of studies plagued by electrode fouling because Nafion is so commonly used in electroanalytical chemistry.
EXPERIMENTAL SECTION
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used as received, unless otherwise noted. Solutions were prepared using doubly distilled deionized water (Milli-Q Millipore, Billerica, MA). In vitro characterization of electrode response to DA and ascorbic acid (AA) was conducted in phosphate buffered saline (PBS:140 mM NaCl, 3 mM KCl, 10 mM NaH2PO4) at pH 7.40. Calibration of electrodes was accomplished in Tris buffer (15 mM Trisma HCl, 3.25 mM KCl, 1.2 mM CaCl2, 1.2 MgCl2, 2.0 mM Na2SO4, 1.25 mM NaH2PO4, and 145 nM NaCl) at pH 7.40.
Carbon-fiber Microelectrode Fabrication
Carbon-fiber microelectrodes were fabricated as described previously 49. Briefly, a single T-650 carbon fiber (7µm diameter, Cytec Industries, West Patterson, NJ) was aspirated into a glass capillary tube (0.60 mm external diameter and 0.40 mm internal diameter, A-M Systems, Carlsburg, WA) and heat pulled with a micropipette puller (Narishige, Tokyo, Japan) to taper the glass to form two sealed microelectrodes. The carbon fiber extending beyond the glass seal was cut to approximately 100 µm under an optical microscope. A stainless steel lead with conductive silver paint (GC Electronics, Rockford, IL) was inserted into the capillary for electrical contact.
Data Acquisition
All in vitro data were collected in a custom-built flow injection apparatus housed within a Faraday cage. A syringe pump (New Era Pump Systems, Inc., Wantagh, NY) supplied a continuous buffer flow of 1 mL/min across both the working and reference electrode. Two-second bolus injections of analyte were accomplished using a six-port HPLC valve and air actuator controlled by a digital valve interface (Valco Instruments Co., Inc., Houston, TX). All electrodes were conditioned prior to data collection by applying a triangular cyclic waveform (−0.4 V to +1.4 V versus Ag/AgCl) with a resting potential of −0.4 V at a scan rate of 400 V· s−1 and at a frequency of 60 Hz for ~15 minutes, followed by another 15 minutes of conditioning at 10 Hz. In some instances, this conditioning occurred after Nafion electrodeposition. Electrochemical measurements used the same waveform applied at a frequency of 10 Hz, as described previously 50. Commercially available TH-1 software (ESA, Chelmsford, MA) was used with a DAC/ADC card (6251, National Instruments, Austin, TX), for waveform generation and data collection. A second National Instruments card (6711) was used for synchronization of waveform, data collection, and electrical stimulation. Signal processing (background subtraction, signal averaging, and digital filtering) was software-controlled.
Nafion-Coating Protocols
Prior to use, all bare carbon-fiber microelectrodes were soaked in filtered isopropyl alcohol purified with Norit A® activated carbon (MP Biomedicals, LLC, Solon, OH) for at least 30 min to remove surface impurities 51. Two different protocols were used to create the Nafion membrane: dip coating and electrodeposition. For the dip-coating procedure, the tip of the microelectrode was immersed in the Nafion solution (DE520, Ion Power, DE) for 5 min, allowed to air dry for 10–15 s, and then dried in an oven for 10 min at 70°C. For electrodeposition, the carbon-fiber microelectrode tip was lowered into the Nafion solution and a continuous potential (+0.5, +1.0, or +1.5 V vs. Ag/AgCl) was applied for 30, 60, or 90 s with a DC power supply (3B Scientific, Tucker, GA) to generate the Nafion layer. The electrodes were then allowed to air dry for 10–15 s, and subsequently dried in an oven for 10 min at 70°C. All electrodes were stored at room temperature. Prior to Nafion coating, some carbon-fiber microelectrodes were electrochemically conditioned with a triangular waveform (−0.4 V to 1.4 V vs. Ag/AgCl, 400 V/s) applied at a frequency of 60 Hz for 10 min, and subsequently at a frequency of 10 Hz for an additional 5 min.
Surgery
Surgical procedures were performed as described previously 52. Briefly, adult male Sprague-Dawley rats weighing 250–300 g were purchased from Charles River Laboratories (Wilmington, MA). Rats were deeply anaesthetized with an intraperitoneal (i.p.) injection of sodium urethane (3 g/kg) and positioned in a stereotaxic frame (Kopf Instrumentation; Tujunga, CA). A heating pad (Harvard Apparatus, Holliston, MA) was used to maintain body temperature at 37 °C. Holes for electrodes were drilled in the skull according to coordinates from the brain atlas of Paxinos and Watson 53. Working electrodes were placed in caudate-putamen (CPu, +1.2 mm anterior-posterior and + 2.0 mm medial-lateral relative to bregma, −4.5 mm dorsal-ventral from the skull surface). The Ag/AgCl reference electrode was placed contralateral to the working electrode. The bipolar stimulating electrode (Plastics One, Roanoke, VA) was placed above the medial forebrain bundle (MFB, −4.6 mm anterior-posterior and + 1.3 mm medial-lateral to bregma, - 8.5 mm dorsal-ventral from the skull surface). Biphasic stimulation pulses (60 Hz, 24 pulses, 150 µA, 2 ms per phase) were delivered to the MFB every 5 min to evoke DA release in the terminal region of the CPu. The working electrode was cycled as it was lowered into tissue, and the positions of the working and stimulating electrodes were optimized to maximize electrically evoked DA signaling. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the North Carolina State University.
In-Vivo Experimental Design
Following electrode implantation, carbon-fiber microelectrodes were cycled at 10 Hz for 15 min to stabilize the background current. L-DOPA methyl-ester and benserazide-hydrochloride, a peripheral DOPA decarboxylase inhibitor 54, were dissolved together in physiological saline. After baseline data collection, each animal received an acute treatment with L-DOPA methyl-ester/benserazide cocktail at a clinically relevant dose (6 mg/kg + 10 mg/kg benserazide, i.p.)25, 55, followed by a higher dose (250 mg/kg + 400 mg/kg benserazide, i.p.). These doses of L-DOPA methyl-ester are equivalent to 5 mg/kg and 200 mg/kg of L-DOPA, respectively 25. Data was collected for one hour after each drug administration.
Statistics
All data are presented as the mean ± standard error of the mean (SEM), unless otherwise noted. Statistical and graphical analyses were carried out using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA) or SPSS (IBM SPSS Statistics Software v. 17.0, Armonk, NY). The Student’s t-test was used to compare two groups. One-way analysis of variance (ANOVA) with paired samples t-test was used for post hoc determination of statistical differences between three or more groups across a single independent variable. When two classes of variables were compared, two-way ANOVA was applied with independent t-tests. Significance was designated at p<0.05.
RESULTS AND DISCUSSION
The Effects of L-DOPA on Electrically Evoked Dopamine Release
DA is normally synthesized from tyrosine in a two-step process. First, a hydroxyl group is attached to tyrosine by the enzyme tyrosine hydroxylase (TH), using oxygen, tetrahydrobiopterin, and Fe2+ as cofactors to produce L-DOPA56. L-DOPA is then efficiently converted to DA by the enzyme L-amino acid decarboxylase (AADC), using pyridoxal phosphate as a cofactor. There are several physiological mechanisms by which exogenous L-DOPA can affect presynaptic DA release (for review see 57). Studies using single cells have demonstrated that L-DOPA is effectively loaded into the neuronal cytosol by the L-amino acid transporter 58. In primary cultures of murine substantia nigra neurons, L-DOPA treatment increases DA levels in the cytosol by > 100-fold 59, 60. The DA is loaded into vesicles by the vesicular monoamine transporter (VMAT2). Amperometric recordings from these cells have demonstrated that the number of DA molecules released in single synaptic vesicle fusion events (termed quantal size) is increased from ~3,000 to ~10,000 DA molecules (~300%) in only 30 minutes 61, 62. A similar increase in quantal size occurs in PC12 cells, and electron micrographs have shown that L-DOPA treatment also serves to increase the volume of individual dense core vesicles in these cells, in order to accommodate the additional DA63, 64. However, in healthy brain tissue L-DOPA treatment could decrease evoked dopamine release by D2-mediated autoinhibition of DA release 65. Additionally, the principal enzymes involved in DA synthesis, TH and AADC, are both regulated by DA autoreceptor-mediated second-messenger systems, such that DA synthesis is decreased when extracellular DA concentrations are increased 66–68.
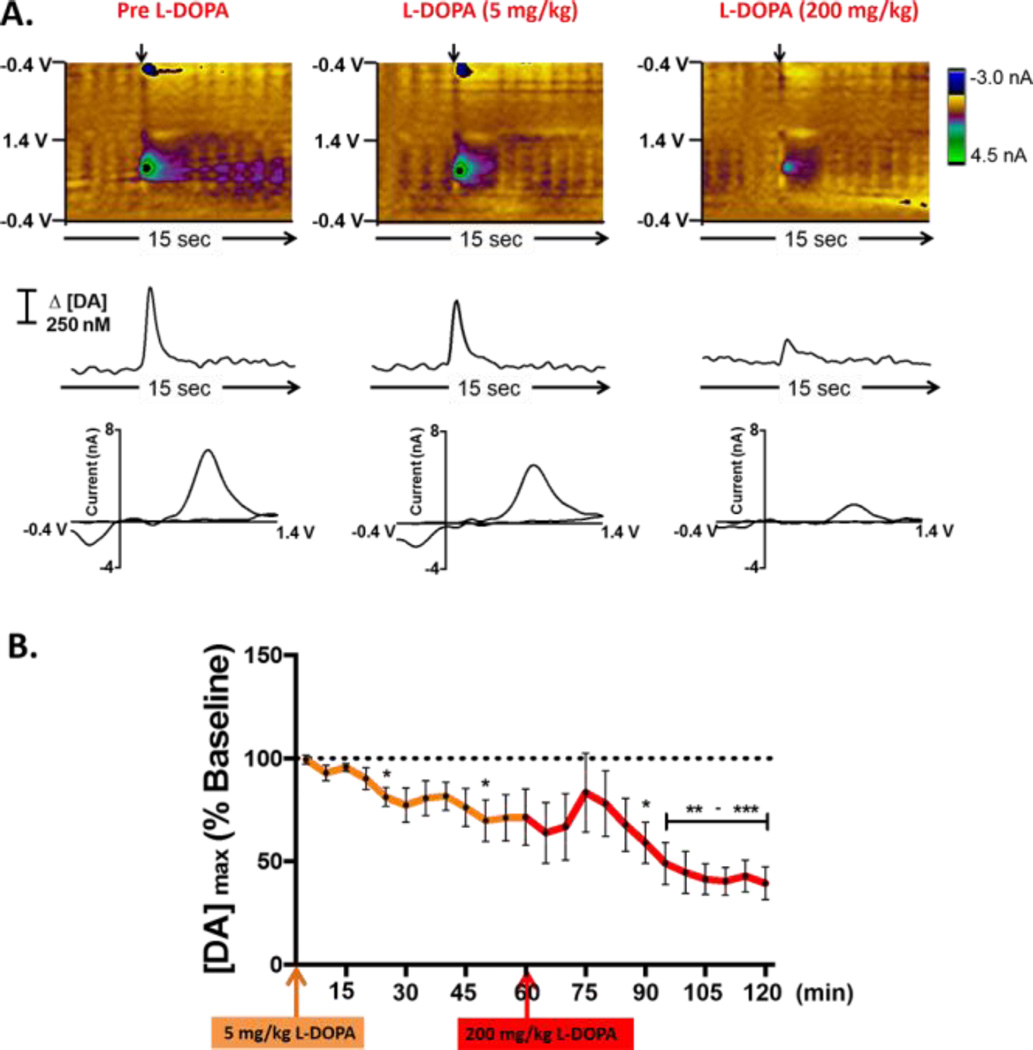
Initial experiments assessed the effects of L-DOPA administration on real-time striatal DA dynamics using bare carbon-fiber microelectrodes in intact Sprague-Dawley rats. Representative color plots, each containing 150 background-subtracted voltammograms, are shown in Figure 1A. These plots provide a representation of all changes in current collected across the entire potential window, enabling discrimination of specific electroactive species as they fluctuate over time 69. The left column shows DA dynamics elicited in response to electrical stimulation of the MFB (arrow) prior to any pharmacological manipulation. A current versus time trace extracted at the oxidation potential of DA (~0.6 V), and converted to concentration following post-calibration of the electrode, is shown in the middle of each panel. A cyclic voltammogram that serves to identify DA is also shown (lower panel). In this representative example, electrical stimulation elicited the release of 595 nM DA in the vicinity of the carbon-fiber recording electrode positioned in the dorsal striatum. Electrically-evoked DA release appeared to decrease to 482 nM after administration of L-DOPA (5 mg/kg, i.p. middle) , and to decrease even further (to 292 nM) after a higher dose (200 mg/kg i.p. right) was administered. By contrast, administration of an identical volume of saline (vehicle control) did not have any significant effect on the evoked DA signal (Figure S1). Figure 1B summarizes the entire data set (n = 5 animals) by plotting the concentration of electrically evoked DA release measured after administration of L-DOPA as a percentage of the baseline DA concentration. Twenty-five minutes after L-DOPA (5 mg/kg, orange arrow), electrically evoked DA release was significantly decreased from 529 ± 19 nM to 428 ± 24 nM (* p<0.05). This DA signal was further decreased to 362 ± 54 nM after 50 min. Subsequent administration of L-DOPA at a higher dose (200 mg/kg, red arrow) further decreased the amplitude of electrically evoked DA to ~45% of baseline (207 ± 33 nM, *** p< 0.001). However, it is important to note that large increases in extracellular L-DOPA are observed in the brain after systemic L-DOPA administration using microdialysis 70–72, and polymerization of L-DOPA could foul the electrode, counfounding interpretation of the results.
Figure 1.
L-DOPA administration affects electrically evoked DA release recorded at bare carbon-fiber microelectrodes. (A) Representative data collected at a bare carbon-fiber microelectrode before L-DOPA administration (left column), 20 min after administration of L-DOPA (5 mg/kg, i.p., middle), and 50 min after administration of a higher dose (200 mg/kg, i.p., right). Top: Color plots depicting all changes in current (false color) collected over a 15-s window (x-axis) across all potentials (y-axis). Time of electrical stimulation is indicated by the black arrow. Middle: Current vs. time traces extracted at the oxidation potential of DA (+0.6V). Bottom: Cyclic voltammograms identifying DA. (B) Across the entire data set, there is a significant decrease in electrically evoked DA release upon acute L-DOPA treatment (n = 5, F(23,92) = 9.05, ****p<0.0001, one-way repeated measures ANOVA). Post-hoc analysis with a paired samples t-test demonstrated a significant decrease in dopamine release at specific time points (n = 5, *p < 0.05, **p<0.01, ***p<0.001).
Microelectrode Fouling by L-DOPA
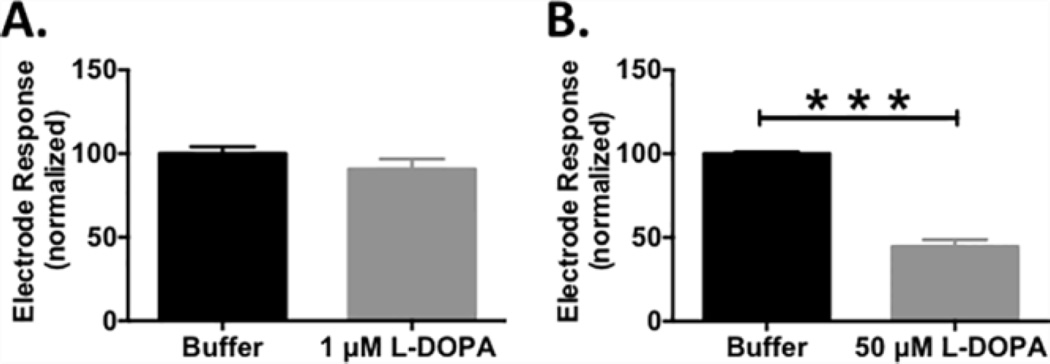
Background subtracted FSCV is a differential measurement that measures rapid changes in electroactive analytes at the electrode surface. As such, basal levels of DA and L-DOPA in brain tissue are not detected, even if high concentrations of these analytes are present. However, the catechol moiety inherent to both DA and L-DOPA is easily oxidized to the reactive o-quinone by the potentials used in this study 13, 73, 74, and both molecules can also be enzymatically oxidized in the brain. As described above, the oxidized form can then immediately undergo a series of complex chemical polymerization reactions to produce melanin75–79. To investigate if the decrease in electrically evoked DA release measured at the bare carbon-fiber electrodes following L-DOPA administration (Figure 1) could be attributed to fouling, electrode sensitivity to 1 µM DA was assessed in vitro. Sensitivity was not compromised following exposure to 1 µM L-DOPA (Figure 2A); however, exposure to 50 µM L-DOPA significantly attenuated sensitivity to DA (Figure 2B, n = 6, *** p < 0.001). Indeed, Hefti et al. have reported a peak value of ~100 µM L-DOPA (20 µg/g) in the rat striatum after administration of a very high dose of L-DOPA (500 mg/kg) 80. Thus, the decrease in evoked DA detected at bare carbon-fiber microelectrodes after a 200 mg/kg L-DOPA treatment suggests fouling of the electrode surface. If not taken into account, this significantly skews interpretation and quantification of in vivo data.
Figure 2.
L-DOPA fouls bare carbon-fiber microelectrodes in vitro. Normalized electrode response to 1 µM DA in the absence (black) and presence (grey) of (A) 1 µM or (B) 50 µM L-DOPA in the running buffer of the flow-injection apparatus. Sensitivity was significantly attenuated in the presence of 50 µM L-DOPA (n = 6, paired t- test, *** p<0.001).
Systematic Characterization of Nafion-Coating Procedure
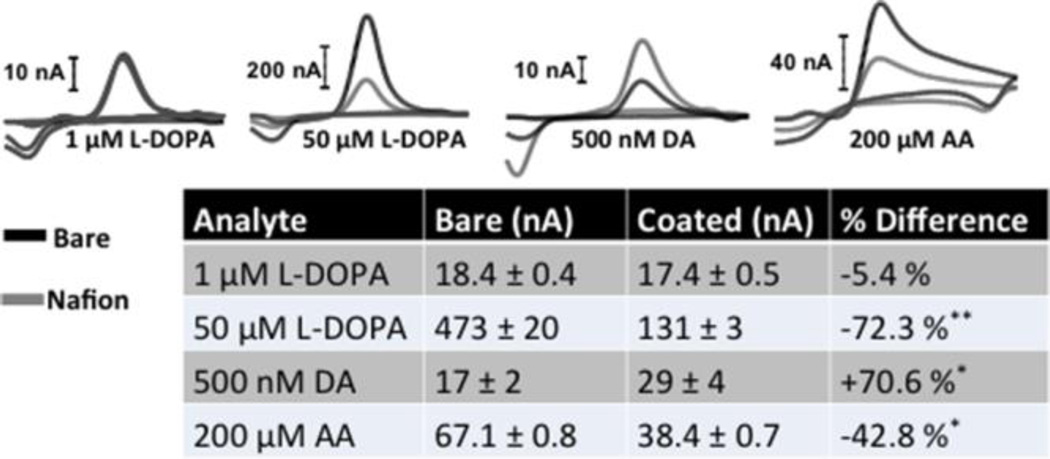
Nafion is a cation-exchange polymer. Thus, when coated on the carbon-fiber electrode surface it serves to increase sensitivity to positively charged species, such as DA at physiological pH 38–41, 43, 44. It also decreases sensitivity to several negatively-charged species, including AA, which is ubiquitous in the brain 38. L-DOPA is a zwitterion at physiological pH. It is electrochemically active, with a voltammogram that looks identical to that for DA. Nafion is not effective at excluding L-DOPA when relatively low concentrations (1 µM) are investigated. However, Nafion is quite effective at excluding higher concentrations of L-DOPA (50 µM). These trends are demonstrated by the representative cyclic voltammograms presented in the upper panel of Figure 3, which were collected in vitro using bare and Nafion-coated carbon-fiber microelectrodes (Nafion electrodeposited at 1.0 V for 90 s onto electrochemically pretreated electrodes). The entire data set is summarized in the Table.
Figure 3.
The presence of a Nafion membrane significantly affects sensitivity to L-DOPA, DA, and AA. Upper panel shows representative cyclic voltammograms for bare (black) and Nafion-coated (gray) electrodes for L-DOPA (1 µM), DA (500 nM), and AA (200 µM). Lower panel: A summary of the entire data set. The values are averages ± SEM. (n = 3–9 electrodes per group, unpaired t-test, **p <0.01, *p <0.05).
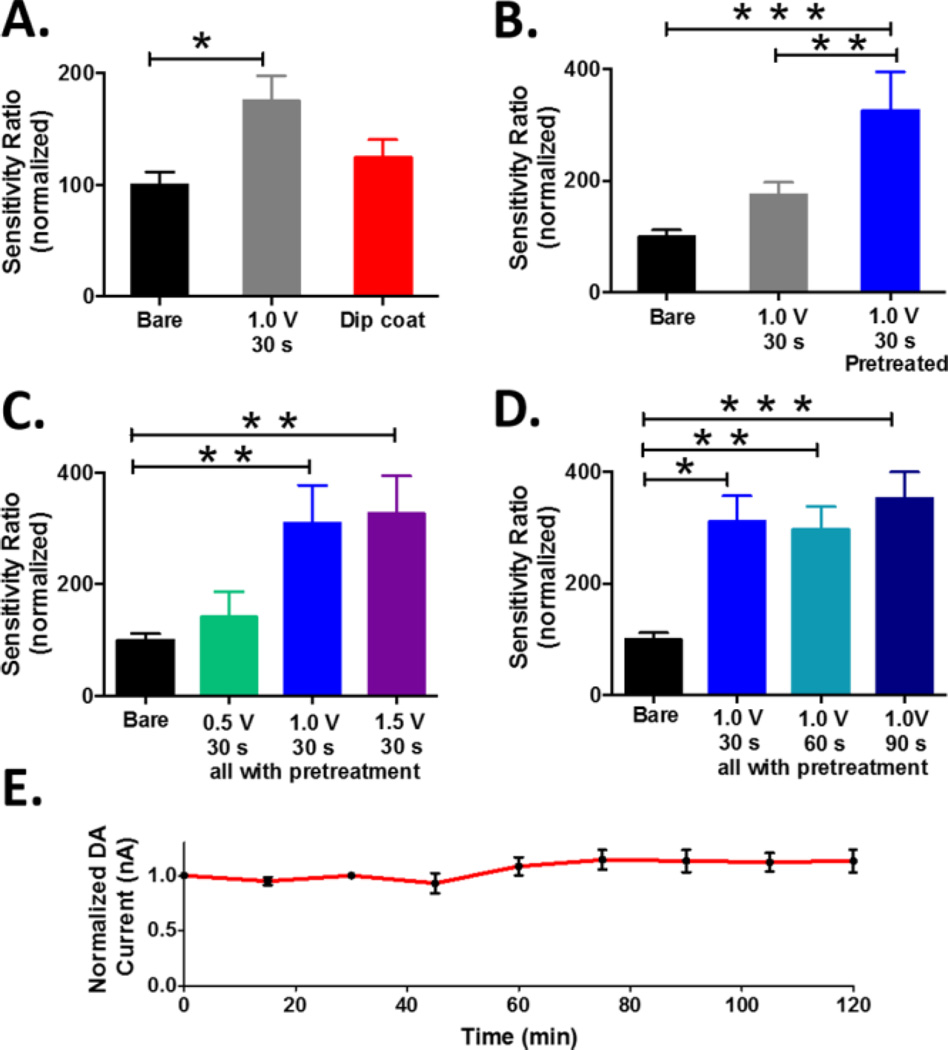
The presence of a Nafion layer can be confirmed by reporting a sensitivity ratio for DA (the quotient of the sensitivities measured before and after coating). A large deviation from unity indicates the presence of a robust membrane. Figure 4 presents normalized sensitivity ratios for DA. Electrodes were prepared using a variety of Nafion deposition procedures. The most straightforward protocol is dip coating. With this approach, the electrode surface is covered with Nafion by dipping the electrode in the polymer solution and evaporating the solvent 37, 38, 43, 45, 81, 82. Alternatively, Nafion can be electrodeposited onto the carbon surface by application of a potential sufficient to generate an anodic current 39–41. The data demonstrate that coating cylindrical carbon-fiber electrodes by way of electrodeposition is more effective than a simple dip coating procedure. Thus, all subsequent studies presented herein utilized electrodeposition to generate a Nafion membrane.
Figure 4.
Systematic characterization of Nafion coating procedures. (A) Dip coating versus electrodeposition (F(2,40)=4.76, * p<0.05), (B) Electrochemical pretreatment before (blue) vs. after (gray) Nafion electrodeposition (F(2,36)=12.20, **p<0.01, ***p<0.001), (C) Electrodeposition potentials (F(3,33)=7.78, **p<0.01), (D) Electrodeposition times (F(3,51)=8.30, *p<0.05, **p<0.01, ***p<0.001), (E) Stability of coated electrodes. In panels A-D, data are presented as normalized DA sensitivity ratio (n = 6–17 electrodes per protocol, one way ANOVA with Tukey’s post-hoc test, * p<0.05; ** p< 0.01; *** p<0.001).
It is well established that electrochemical pretreatment of the carbon fiber surface can enhance electron transfer kinetics and significantly improve electrochemical performance by shifting the surface chemistry of the sensor to that which is present during use 47, 83–87. Thus, carbon-fiber microelectrodes are commonly conditioned immediately prior to data collection49. It has also more recently been established, using various spectroscopic techniques including X-ray photoelectron spectroscopy 88, thermal desorption mass spectrometry 89, enzyme-immobilized fluorescence microscopy 90, 91, optical spectroscopy 92, and Raman spectroscopy 47, that application of potentials greater than ~1.3V can chemically alter, or even etch, the electrode surface 47, 48, 87. Thus, we hypothesized that electrochemical conditioning with a commonly used waveform (triangular, ranging from −0.4 V to 1.4 V vs. Ag/AgCl) before membrane deposition would improve adhesion of Nafion to the electrode surface. We quantitatively compared the performance of carbon-fiber microelectrodes that were conditioned before and after Nafion coating with microelectrodes that were electrochemically pre-treated and left bare. The data unequivocally demonstrate that Nafion electrodeposition (by application of 1.0 V for 30 sec) was most effective when electrodes were conditioned prior to the deposition procedure (Figure 4B). Based on this, all subsequent protocols included electrochemical pretreatment of bare carbon-fiber microelectrodes prior to electrodeposition of Nafion.
Next, the potentials employed to electrodeposit the Nafion membrane were systematically investigated. Three potentials were selected: +0.5, +1.0 and +1.5 V vs. Ag/AgCl, as these have all been previously reported in the literature 41, 44, 93, 94. The results indicate that +1.0 and +1.5 V were more effective than +0.5 V in generating a reliable membrane (Figure 4C). However, as described above, the application of positive potentials can modify the surface of the carbon fiber, making it highly adsorptive and potentially slowing electrode response time, convoluting electrochemical performance 85, 87. Thus, 1.0 V (vs. Ag/AgCl) was selected for the electrodeposition of Nafion.
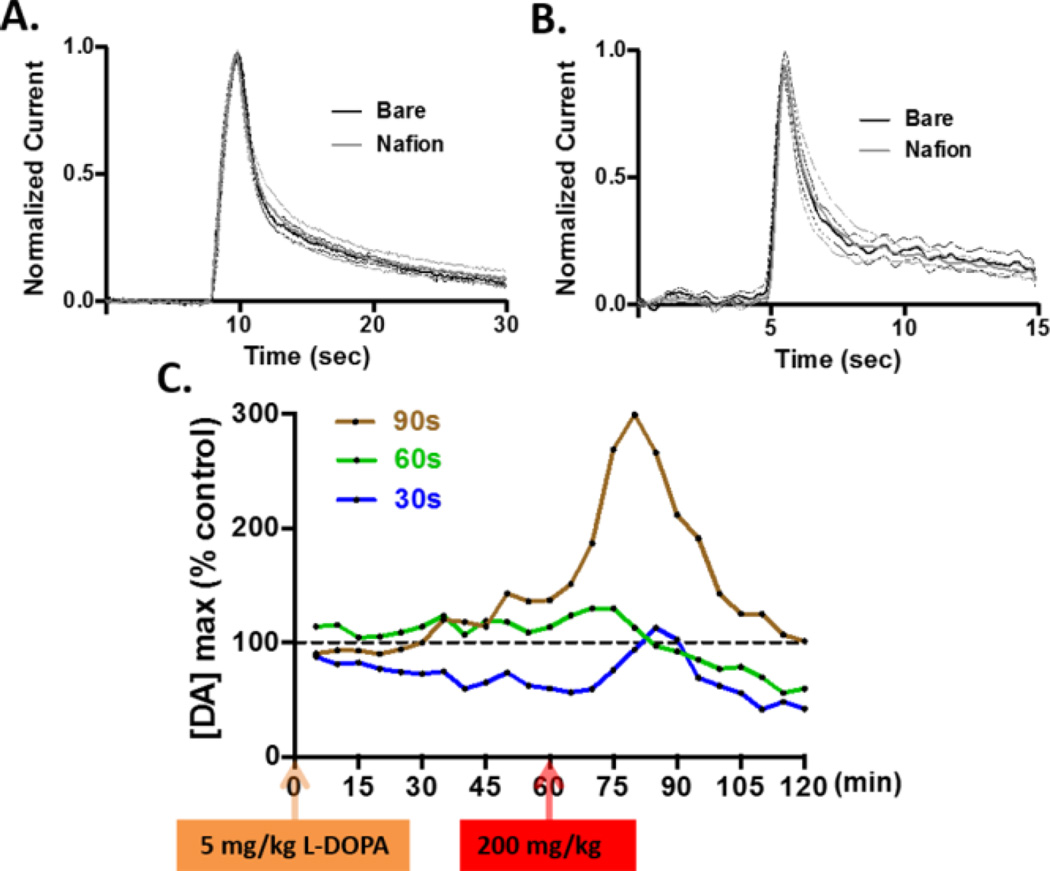
It is important to note that the Nafion membrane itself presents a diffusion barrier that can reduce electrode response time. To maintain the rapid temporal response required for detection of neurotransmitter fluctuations in vivo, a thin Nafion layer is required. Indeed, previous studies have demonstrated that Nafion membranes can be generated such that electrode response times are not significantly different from uncoated microelectrodes 35, 37. Figure 4 shows that with our approach, an electrodeposition time of 90 s produced the best membrane performance in terms of sensitivity (Figure 4D) and stability (Figure 4E). With this approach, electrode performance was stable for at least two hours, presumably because the structural integrity of the Nafion coating (a perfluorosulfonated polymer) is derived from strong interactions between Nafion chains, rather than interactions between the Nafion and the carbon surface. This membrane did not significantly affect the sub-second response time of the electrode to a step change in the concentration of DA in a flow injection system (Figure 5A), or to the stimulated secretion of DA in an intact brain (Figure 5B). Finally, Nafion-coated electrodes prepared using different electrodeposition times were tested in vivo. Figure 5C shows representative data collected in the striatum after administration of 5 mg/kg (orange arrow) and 200 mg/kg (red arrow) L-DOPA. These data suggest that the Nafion-coated electrodes prepared using electrodeposition times of 30 s (blue) and 60 s (green) did not resist L-DOPA induced fouling as efficiently as the Nafion-coated electrode prepared using an electrodeposition time of 90 s (brown), which was able to detect a robust increase in electrically-evoked DA release following L-DOPA treatment.
Figure 5.
DA concentrations recorded at Nafion coated electrodes. The optimized Nafion membrane (90 s electrodeposition time) did not affect the sub-second response time of the electrode when it was used (A) to record bolus injections of 500 nM DA in a flow cell, or (B) to record electrically evoked DA release in rat striatum. In both (A) and (B), the mean current (solid) ± SEM (dashed) is plotted for n = 6 electrodes. (C) Electrical stimulation was used to evoke striatal DA release every 5 minutes after L-DOPA administration. The x-axis shows the 2- h window of data collection. The y-axis represents the normalized amplitude of electrically evoked DA release collected using electrodes that were electrodeposited with Nafion for 30 (blue), 60 (green), or 90 (brown) seconds. Arrows indicate the time at which L-DOPA was administrated (orange: 5 mg/kg, red: 200 mg/kg ).
The Effects of L-DOPA on Striatal DA Dynamics
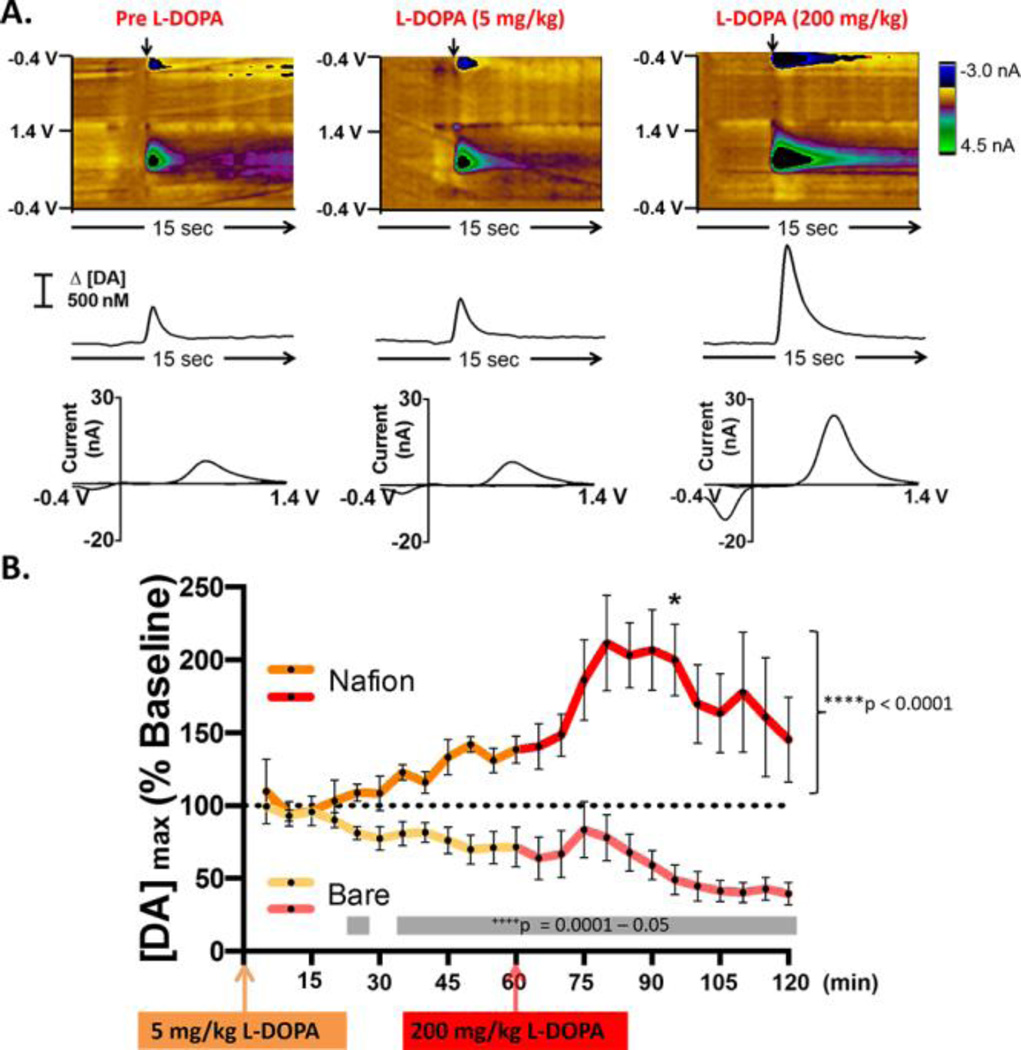
The optimized Nafion deposition procedure (electrodeposition using 1.0 V for 90 s) was used to investigate the effects of L-DOPA administration on electrically evoked striatal DA release. Representative color plots with corresponding DA concentration traces and cyclic voltammograms are shown in Figure 6. These data demonstrate that DA release increased across the entire data set (****p<0.0001, one-way repeated measures ANOVA). Post-hoc analysis with a paired samples t-test demonstrated that a clinically relevant dose of 5 mg/kg had no significant effect on electrically-evoked DA release as compared to baseline; however, L-DOPA administered at dose of 200 mg/kg significantly increased DA release (~200%), 25 min after drug administration (*p < 0.05). These data suggest that the regulatory mechanisms of intact animals are capable of controlling extracellular DA very efficiently in response to acute administration of a clinically relevant dose of L-DOPA (5 mg/kg). However, administration of a higher dose of L-DOPA (200 mg/kg) enhances DA overflow. This finding is consistent with previous studies using microdialysis, which report that striatal DA was increased by ~200% in intact animals following a 200 mg/kg or higher dose of L-DOPA 10, 24, 95. Importantly, the data collected using the Nafion-coated electrodes significantly contrast with the data collected using bare electrodes (Figure 1 and re-plotted here in light orange and red). The differences between these data sets (gray bar, ++++p = 0.0001–0.05, repeated measures two-way ANOVA) confirm that a robust Nafion membrane is necessary to quantitatively report the effects of L-DOPA treatment on DA dynamics when using carbon-fiber microelectrodes and high concentrations of L-DOPA.
Figure 6.
The optimized Nafion membrane reveals that L-DOPA administration increases electrically evoked DA release. (A) Representative data collected at a Nafion-coated carbon-fiber microelectrode in an intact animal before L-DOPA administration (left column), 20 min after administration of L-DOPA (5mg/kg i.p., middle), and 20 min after administration of a higher dose of L-DOPA (200 mg/kg i.p., right). (B) When using Nafion-coated electrodes for the measurements, L-DOPA administration increased the amplitude of electrically evoked DA release overall (n = 4, bright orange and red trace, F(23,69) = 2.880, ****p < 0.00001, one-way repeated measures ANOVA). Post-hoc analysis with a paired samples t-test demonstrated a significant increase 25 minutes after administration of 200 mg/kg L-DOPA (*p < 0.05). Data collected using bare electrodes are also shown (n = 5, light orange and red trace) to enable direct statistical comparison of electrode performance in vivo. The differences between the two data sets are significant at several time points (gray boxes, repeated measures two-way ANOVA, all main and interaction effects, F(1–23, 7–161) = 2.196 – 54.432, ++++p = 0.00001–0.05).
CONCLUSIONS
Overall, the data indicate that when systemically treating with high doses of L-DOPA, a carbon-fiber microelectrode implanted in the brain is easily fouled, resulting in decreased sensitivity to DA. This is likely due to the polymerization of catecholamines on the electrode surface. By refining the parameters by which a Nafion coating is applied to the electrode, we have advanced a technique for generating a carbon fiber microelectrode that maintains a rapid electrode response time and is less sensitive to fouling. The improved performance of these electrodes allows a better measure of L-DOPA augmented DA release in vivo when using FSCV. With this approach, an acute dose of 5 mg/kg L-DOPA had little effect on DA release in healthy striatal tissue, consistent with several reports in the literature that have used microdialysis measurements 10, 96 or mathematical modeling 57 to demonstrate that DA terminals play a crucial role in the clearance of extracellular DA formed from exogenous L-DOPA in healthy striatum. In contrast, administration of 200 mg/kg L-DOPA was capable of significantly increasing evoked DA release.
Supplementary Material
Acknowledgments
We thank Leyda Lugo and Vincent Toups for their help in determination of Nafion membrane thickness, James Roberts for assistance in manuscript preparation, Xiaohu Xie for assistance with statistical data analysis, and Leyda Lugo and Robert Grossfeld for critique of this manuscript and thoughtful discussion. This work was supported by the U.S. National Institutes of Neurological Disorders and Stroke (1R01NS076772-01to L.A.S.).
Footnotes
ASSOCIATED CONTENT
Supplemental Figure S1. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Sutachan JJ, Casas Z, Albarracin SL, Stab BR, 2nd, Samudio I, Gonzalez J, Morales L, Barreto GE. Nutr. Neurosci. 2012;15(3):120–126. doi: 10.1179/1476830511Y.0000000033. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J. J. Neurol. Neurosurg. Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. J. Neural Transm. Suppl. 2006;(71):1–15. doi: 10.1007/978-3-211-33328-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Bear MF, Connors BW, Paradiso MA. Neuroscience : Exploring the Brain. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 5.Cotzias GC, Van Woert MH, Schiffer LM. N Engl J Med. 1967;276(7):374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- 6.Hornykiewicz O. Amino Acids. 2002;23(1–3):65–70. doi: 10.1007/s00726-001-0111-9. [DOI] [PubMed] [Google Scholar]
- 7.Ahlskog JE, Muenter MD. Movement Disorders. 2001;16(3):448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 8.Nutt JG. Dyskinesia Induced by Levodopa and Dopamine Agonists in Patients with Parkinson’s Disease, in Drug-Induced Movement Disorders. Mount Kisko: Futura Publishing Co. Inc; 1992. [Google Scholar]
- 9.Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. J. Neurochem. 2010;112(6):1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- 10.Abercrombie ED, Bonatz AE, Zigmond MJ. Brain Res. 1990;525(1):36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. J. Neurosci. 1994;14(5 Pt 1):2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang WQ, Tilson HA, Nanry KP, Hudson PM, Hong JS, Stachowiak MK. Brain Res. 1988;461(2):335–342. doi: 10.1016/0006-8993(88)90264-8. [DOI] [PubMed] [Google Scholar]
- 13.Robinson DL, Hermans A, Seipel AT, Wightman RM. Chem. Rev. 2008;108(7):2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank MJ, Seeberger LC, O’Reilly RC. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 15.Frank MJ. J. Cogn. Neurosci. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- 16.Smith KS, Graybiel AM. J. Neurophysiol. 2016;115(3):1487–1498. doi: 10.1152/jn.00925.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 19.Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Biol. Psychiatry. 2013;73(8):747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Biol. Psychiatry. 2015;77(10):903–911. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Nat. Neurosci. 2014;17(5):704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May LJ, Kuhr WG, Wightman RM. J. Neurochem. 1988;51(4):1060–1069. doi: 10.1111/j.1471-4159.1988.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 23.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. J. Neurosci. 1994;14(10):6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez M, Morales I, Gonzalez-Mora JL, Gomez I, Sabate M, Dopico JG, Rodriguez-Oroz MC, Obeso JA. Synapse. 2007;61(2):61–71. doi: 10.1002/syn.20342. [DOI] [PubMed] [Google Scholar]
- 25.Lundblad M, af Bjerken S, Cenci MA, Pomerleau F, Gerhardt GA, Stromberg I. J. Neurochem. 2009;108(4):998–1008. doi: 10.1111/j.1471-4159.2008.05848.x. [DOI] [PubMed] [Google Scholar]
- 26.Yu ME, Hwang JY, Deming TJ. Journal of the American Chemical Society. 1999;121(24):5825–5826. [Google Scholar]
- 27.Tse DC, McCreery RL, Adams RN. J Med Chem. 1976;19(1):37–40. doi: 10.1021/jm00223a008. [DOI] [PubMed] [Google Scholar]
- 28.Harreither W, Trouillon R, Poulin P, Neri W, Ewing AG, Safina G. Anal Chem. 2013;85(15):7447–7453. doi: 10.1021/ac401399s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azari S, Zou LD. Journal of Membrane Science. 2012;401:68–75. [Google Scholar]
- 31.Xi ZY, Xu YY, Zhu LP, Wang Y, Zhu BK. Journal of Membrane Science. 2009;327(1–2):244–253. [Google Scholar]
- 32.Koile RC, Johnson DC. Analytical Chemistry. 1979;51(6):741–744. [Google Scholar]
- 33.Adams RNMC. Electrochemical Detection Methods for Monoamine Measurements in Vitro and in Vivo. Plenum Press; 1982. [Google Scholar]
- 34.Stamford JA. J Neurosci Methods. 1986;17(1):1–29. doi: 10.1016/0165-0270(86)90031-2. [DOI] [PubMed] [Google Scholar]
- 35.Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Anal. Chem. 1996;68(18):3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- 36.Burmeister JJ, Pomerleau F, Huettl P, Gash CR, Werner CE, Bruno JP, Gerhardt GA. Biosens Bioelectron. 2008;23(9):1382–1389. doi: 10.1016/j.bios.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Kristensen EW, Kuhr WG, Wightman RM. Anal. Chem. 1987;59(14):1752–1757. doi: 10.1021/ac00141a003. [DOI] [PubMed] [Google Scholar]
- 38.Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. Brain Res. 1984;290(2):390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- 39.Rice ME, Oke AF, Bradberry CW, Adams RN. Brain Res. 1985;340(1):151–155. doi: 10.1016/0006-8993(85)90785-1. [DOI] [PubMed] [Google Scholar]
- 40.Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Anal. Chem. 2011;83(17):6658–6666. doi: 10.1021/ac2011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Anal. Chem. 2009;81(22):9462–9471. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valdes TI, Moussy F. Diabetes Technol Ther. 2000;2(3):367–376. doi: 10.1089/15209150050194233. [DOI] [PubMed] [Google Scholar]
- 43.Mauritz KA, Moore RB. Chem Rev. 2004;104(10):4535–4585. doi: 10.1021/cr0207123. [DOI] [PubMed] [Google Scholar]
- 44.Capella P, Ghasemzadeh B, Mitchell K, Adams RN. Electroanalysis. 1990;2(3):175–182. [Google Scholar]
- 45.Ross AE, Venton BJ. Analyst. 2012;137(13):3045–3051. doi: 10.1039/c2an35297d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F, Heien ML. Anal. Chem. 2015;87(5):2600–2607. doi: 10.1021/ac502165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts JG, Moody BP, McCarty GS, Sombers LA. Langmuir. 2010;26:9116–9122. doi: 10.1021/la9048924. [DOI] [PubMed] [Google Scholar]
- 48.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM. Anal. Chem. 2010;82(5):2020–2028. doi: 10.1021/ac902753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts JG, Lugo-Morales LZ, Loziuk PL, Sombers LA. Methods Mol. Biol. 2013;964:275–294. doi: 10.1007/978-1-62703-251-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, Sombers LA. Anal. Chem. 2010;82(12):5205–5210. doi: 10.1021/ac100536s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal. Chem. 2000;72(24):5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 52.Dragun AE, Quillo AR, Riley EC, Roberts TL, Hunter AM, Rai SN, Callender GG, Jain D, McMasters KM, Spanos WJ. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(3):e123–e128. doi: 10.1016/j.ijrobp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos GWC. The Rat Brain in Stereotaxic Coordinates. 2nd. Orlando, FL: Academic Press; 1986. [Google Scholar]
- 54.Silva MA, Mattern C, Hacker R, Tomaz C, Huston JP, Schwarting RK. Synapse. 1997;27(4):294–302. doi: 10.1002/(SICI)1098-2396(199712)27:4<294::AID-SYN3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Lindgren HS, Rylander D, Ohlin KE, Lundblad M, Cenci MA. Behav. Brain Res. 2007;177(1):150–159. doi: 10.1016/j.bbr.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 56.Ramsey AJ, Fitzpatrick PF. Biochemistry. 2000;39(4):773–778. doi: 10.1021/bi991901r. [DOI] [PubMed] [Google Scholar]
- 57.Mosharov EV, Borgkvist A, Sulzer D. Mov. Disord. 2015;30(1):45–53. doi: 10.1002/mds.26103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vieira-Coelho MA, Soares-Da-Silva P. Am. J. Physiol. 1998;275(1 Pt 1):C104–C112. doi: 10.1152/ajpcell.1998.275.1.C104. [DOI] [PubMed] [Google Scholar]
- 59.Mosharov EV, Staal RG, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen KE, Moore CM, Troyer MD, Edwards RH, Przedborski S, Sulzer D. J. Neurosci. 2006;26(36):9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pothos EN, Davila V, Sulzer D. J. Neurosci. 1998;18(11):4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staal RG, Mosharov EV, Sulzer D. Nat. Neurosci. 2004;7(4):341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- 63.Sombers LA, Maxson MM, Ewing AG. J. Neurochem. 2005;93(5):1122–1131. doi: 10.1111/j.1471-4159.2005.03087.x. [DOI] [PubMed] [Google Scholar]
- 64.Colliver TL, Pyott SJ, Achalabun M, Ewing AG. J. Neurosci. 2000;20(14):5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmitz Y, Schmauss C, Sulzer D. J. Neurosci. 2002;22(18):8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hadjiconstantinou M, Neff NH. CNS Neurosci Ther. 2008;14(4):340–351. doi: 10.1111/j.1755-5949.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf ME, Roth RH. Ann. N. Y. Acad. Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- 68.Lindgren N, Xu ZQ, Herrera-Marschitz M, Haycock J, Hokfelt T, Fisone G. Eur. J. Neurosci. 2001;13(4):773–780. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- 69.Michael D, Travis ER, Wightman RM. Anal Chem. 1998;70(17):586A–592A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
- 70.Golembiowska K, Dziubina A. Brain Research. 2004;998(2):208–217. doi: 10.1016/j.brainres.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 71.Marburger A, Sohr R, Reum T, Morgenstern R. Journal of Neuroscience Methods. 2000;102(2):127–132. doi: 10.1016/s0165-0270(00)00283-1. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Qu FR, Nakatsuka A, Nomura T, Nagai M, Nomoto M. Brain Res. 2003;993(1–2):54–58. doi: 10.1016/j.brainres.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 73.Eslami M, Zare HR, Namazian M. J Phys Chem B. 2012;116(41):12552–12557. doi: 10.1021/jp3054229. [DOI] [PubMed] [Google Scholar]
- 74.Aslanoglu M, Kutluay A, Goktas S, Karabulut S. Journal of Chemical Sciences. 2009;121(2):209–215. [Google Scholar]
- 75.Cabanes J, Garciacanovas F, Lozano JA, Garciacarmona F. Biochimica Et Biophysica Acta. 1987;923(2):187–195. doi: 10.1016/0304-4165(87)90003-1. [DOI] [PubMed] [Google Scholar]
- 76.Robinson GM, Smyth MR. Analyst. 1997;122(8):797–802. [Google Scholar]
- 77.Prota G. Melanins and Melanogenesis. New York: Academic Press; 1992. [Google Scholar]
- 78.Graham DG. Molecular Pharmacology. 1978;14(4):633–643. [PubMed] [Google Scholar]
- 79.Nicolai M, Goncalves G, Natalio F, Humanes M. Journal of Inorganic Biochemistry. 2011;105(6):887–893. doi: 10.1016/j.jinorgbio.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 80.Hefti F, Melamed E, Wurtman RJ. J. Pharmacol. Exp. Ther. 1981;217(1):189–197. [PubMed] [Google Scholar]
- 81.Jackson BP, Dietz SM, Wightman RM. Anal. Chem. 1995;67(6):1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 82.Pihel K, Walker QD, Wightman RM. Anal. Chem. 1996;68(13):2084–2089. doi: 10.1021/ac960153y. [DOI] [PubMed] [Google Scholar]
- 83.Engstrom RC. Analytical Chemistry. 1982;54(13):2310–2314. [Google Scholar]
- 84.Engstrom RC, Strasser VA. Anal. Chem. 1984;56(2):136–141. [Google Scholar]
- 85.Gonon FG, Fombarlet CM, Buda MJ, Pujol JF. Anal. Chem. 1981;53(9):1386–1389. [Google Scholar]
- 86.Saraceno RA, Ewing AG. Anal. Chem. 1988;60(19):2016–2020. [Google Scholar]
- 87.Heien M, Phillips PEM, Stuber GD, Seipel AT, Wightman RM. Analyst. 2003;128(12):1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 88.Langley LA, Villanueva DE, Fairbrother DH. Chem. Mater. 2006;18(1):169–178. [Google Scholar]
- 89.Fagan DT, Kuwana T. Anal. Chem. 1989;61(9):1017–1023. [Google Scholar]
- 90.Pantano P, Kuhr WG. Anal. Chem. 1993;65(18):2452–2458. doi: 10.1021/ac00066a009. [DOI] [PubMed] [Google Scholar]
- 91.Pantano P, Kuhr WG. Anal. Chem. 1991;63(14):1413–1418. doi: 10.1021/ac00014a014. [DOI] [PubMed] [Google Scholar]
- 92.Ray KR, McCreery RL. J. Electroanal. Chem. 1999;469(2):150–158. [Google Scholar]
- 93.Rice ME, Nicholson C. Anal. Chem. 1989;61(17):1805–1810. doi: 10.1021/ac00192a005. [DOI] [PubMed] [Google Scholar]
- 94.Crespi F, Martin KF, Marsden CA. NeuroScience. 1988;27(3):885–896. doi: 10.1016/0306-4522(88)90191-1. [DOI] [PubMed] [Google Scholar]
- 95.Di Monte DA, DeLanney LE, Irwin I, Royland JE, Chan P, Jakowec MW, Langston JW. Brain Res. 1996;738(1):53–59. doi: 10.1016/0006-8993(96)00761-5. [DOI] [PubMed] [Google Scholar]
- 96.Miller DW, Abercrombie ED. J. Neurochem. 1999;72(4):1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.