Abstract
Photodynamic therapy (PDT) is a non-invasive and non-surgical method representing an attractive alternative choice for lung cancer treatment. Photosensitizers selectively accumulate in tumor tissue and lead to tumor cell death in the presence of oxygen and the proper wavelength of light.
To increase the therapeutic effect of PDT, we developed both photosensitizer- and anticancer agent-loaded lung cancer-targeted nanoparticles. Both enhanced permeability and retention (EPR) effect-based passive targeting and hyaluronic-acid-CD44 interaction-based active targeting were applied. CD44 is a well-known hyaluronic acid receptor that is often introduced as a biomarker of non-small cell lung cancer.
In addition, a combination of PDT and chemotherapy is adopted in the present study. This combination concept may increase anticancer therapeutic effects and reduce adverse reactions.
We chose hypocrellin B (HB) as a novel photosensitizer in this study. It has been reported that HB causes higher anticancer efficacy of PDT compared to hematoporphyrin derivatives1. Paclitaxel was selected as the anticancer drug since it has proven to be a potential treatment for lung cancer2.
The antitumor efficacies of photosensitizer (HB) solution, photosensitizer encapsulated hyaluronic acid-ceramide nanoparticles (HB-NPs), and both photosensitizer- and anticancer agent (paclitaxel)-encapsulated hyaluronic acid-ceramide nanoparticles (HB-P-NPs) after PDT were compared both in vitro and in vivo. The in vitro phototoxicity in A549 (human lung adenocarcinoma) cells and the in vivo antitumor efficacy in A549 tumor-bearing mice were evaluated.
The HB-P-NP treatment group showed the most effective anticancer effect after PDT. In conclusion, the HB-P-NPs prepared in the present study represent a potential and novel photosensitizer delivery system in treating lung cancer with PDT.
Keywords: Cancer Research, Issue 118, Photodynamic Therapy, Nanoparticle, Lung Cancer, Phototoxicity, A549, BALB/C Nude Mouse
Introduction
Photodynamic therapy (PDT) is composed of three major factors: photosensitizers, light, and oxygen. PDT is reported as a promising treatment for various cancers3. When the photosensitizers are administered into the cancer patient, they selectively accumulate in the tumor tissues. When the proper wavelength of light is applied, the highly reactive singlet oxygen and other free radicals lead to tumor cell damage4.
Lung cancer was introduced as one of the first applications for PDT in the early 1980s5. PDT provides several advantages in treating lung cancer. Since PDT is a non-invasive and non-surgical treatment, it is an attractive alternative choice for the patients in whom surgical resection is inappropriate.
There have been many challenges to enhance the cancer-targeting efficacy of the photosensitizers. Increasing photosensitizer accumulation in cancer sites and decreasing accumulation in normal tissues are the identical goals for the cancer-targeting studies. A variety of targeted drug delivery systems, such as polymers, liposomes, and nanoparticles are adopted as photosensitizer carriers6-8. In our previous studies, nanoparticles effectively increased the cancer-targeting abilities of the photosensitizers9,10. Nanoparticles are ideal cancer-targeting carriers since they possess both passive and active targeting abilities. The leaky tumor vessels provide opportunity for nano-sized carriers to accumulate easily in tumors, which is well-known as the enhanced permeability and retention (EPR) effect11,12. The interaction between the nanoparticles and the specific receptors on cancer cells enables active cancer targeting. In this study, we prepared hyaluronic acid-based nanoparticles to interact with CD44, the major hyaluronic acid receptor that is overexpressed on lung cancer cells13.
To maximize the anticancer efficacy, a combination of PDT and chemotherapy is adopted in the present study. This combination concept may permit an increased therapeutic effect. Furthermore, decreased doses of both the photosensitizer and the anticancer drug can diminish adverse effects. We selected hypocrellin B (HB) as a novel photosensitizer in the present study. HB is isolated from Chinese medicinal fungus Hypocrella bambuase. Shang et al. reported that HB-based PDT possesses a higher anticancer efficacy when compared to hematoporphyrin derivative-based PDT1. Paclitaxel was selected as the anticancer drug since it has proven to be a potential treatment for various cancers, including lung cancer2.
Herein, we compared the anticancer efficacies of photosensitizer (hypocrellin B, HB) solution, photosensitizer-encapsulated hyaluronic acid-ceramide nanoparticles (HB-NPs), and both photosensitizer- and anticancer agent (paclitaxel)-encapsulated hyaluronic acid-ceramide nanoparticles (HB-P-NPs) after PDT. The in vitro phototoxicity in A549 (human lung adenocarcinoma) cells and the in vivo antitumor efficacy in A549 tumor-bearing mice were evaluated.
Protocol
NOTE: All animal study protocols were approved by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital (BA1308-134/072-01).
1. Synthesis of Hyaluronic Acid-Ceramide (HACE)
Solubilize 12.21 mmol of hyaluronic acid (HA) oligomer and 9.77 mmol of tetra-n-butylammonium hydroxide (TBA) in 60 ml of double-distilled water (DDW). Stir for 30 min.
To synthesize the DS-Y30 linker, dissolve 8.59 mmol of DS-Y30 ceramide and 9.45 mmol of triethylamine in 25 ml of tetrahydrofuran (THF). Mix with 8.59 mmol of 4-chloromethylbenzoyl chloride in THF. Stir for 6 hr at 60 °C.
Dissolve the synthesized 8.10 mmol of HA-TBA and 0.41 mmol of DS-Y30 linker in a mixture of THF and acetonitrile (4:1, v/v). Stir for 5 hr at 40 °C.
Remove impurities by filtering with a filter agent, and eliminate the organic solvent by vacuum evaporation. Purify the product using a dialysis membrane (molecular weight cut-off: 3.5 kDa) and lyophilize.
2. Preparation of the Nanoparticles
Dissolve 1 mg of HB and 1 mg of paclitaxel in 0.5 ml of dimethyl sulfoxide (DMSO) and blend with 0.5 ml of DDW by vortex-mixing for 5 min. Then, solubilize HACE in that mixture by vortex-mixing for a further 5 min.
To eliminate the solvent, heat at 70 °C for 4 hr under a gentle stream of nitrogen gas.
Resuspend the film composed of HACE, HB, and paclitaxel with 1 ml of DDW. Filter with a syringe filter (0.45 µm pore size) to remove unencapsulated drugs.
3. In Vitro Phototoxicity
- Uptake of nanoparticles in lung cancer cell lines
- Prepare RPMI-1640 medium-containing 10% (v/v) fetal bovine serum and 1% (w/v) penicillin-streptomycin.
- Seed A549 cells in 24-well cell culture plates at a density of 1 × 105 cells/well (triplicates for each group). Incubate for 24 hr at 37 °C in a humidified 5% CO2 and 95% air atmosphere.
- After cell attachment, remove the medium and wash the cells by adding 1 ml of phosphate-buffered saline (PBS).
- Dissolve the nanoparticles in PBS to a final concentration of 2 µM/ml HB. Then, incubate the cells with 1 ml of PBS, empty NPs, HB-NPs, or HB-P-NPs in each well for 4 hr in the dark.
- Remove all of the solution and wash the cells by adding 1 ml of cold PBS. Repeat the washing step once more. Add fresh culture medium.
- Cell viability assay
- Place the cell culture plate under the PDT fiber (with 1 cm of distance from the PDT fiber to the well). Wear laser safety glasses and illuminate the cells with a PDT laser (630 nm, 400 mW/cm2) in the dark for various periods of time: 0, 5, 10, 20, 30, and 40 sec (0, 2, 4, 8, 12, and 16 J/cm2). Then, incubate the cells for 24 hr in the dark.
- Aspirate the medium and wash the cells by adding 1 ml of cold PBS. Repeat the washing step once more.
- Add 10 µl of cytotoxicity measuring solution to each well. Incubate in the dark for 2 hr.
- Measure the absorbance at 450 nm using a microplate reader.
- Microscopic analysis
- Place the cell culture plate under the PDT fiber (with 1 cm of distance from the PDT fiber to the well). Wear laser safety glasses and illuminate the cells with a PDT laser (630 nm, 400 mW/cm2) in the dark for 0, 20, or 40 sec (0, 8, or 16 J/cm2). Then, incubate the cells for 24 hr in the dark.
- Aspirate the medium and wash the cells by adding 1 ml of cold PBS. Repeat the washing step once more.
- Add 50 µl of annexin V-FITC and 50 µl of 4',6-diamidino-2-phenylindole (DAPI, 1.5 µg/ml). Gently shake the plate and incubate it for 15 min at room temperature in the dark. NOTE: Use annexin V-FITC from the fluorescence microscope kit.
- Wash the cells by adding 1 ml of cold PBS. Repeat the washing step once more. Keep the cells in fresh PBS. Identify the apoptotic cells using light microscopy at 100X magnification.
- Fluorescence activated cell sorting (FACS) analysis
- Place the cell culture plate under the PDT fiber (with 1 cm of distance from the PDT fiber to the well). Wear laser safety glasses and illuminate the cells with a PDT laser (630 nm, 400 mW/cm2) for 0, 20, or 40 sec (0, 8, or 16 J/cm2). Then, incubate the cells for 24 hr in the dark.
- Aspirate the medium and wash the cells by adding 1 ml of cold PBS. Repeat the washing step once more.
- Resuspend the cells in 1 ml of 1× binding buffer (dilute 1 part of the 10x binding buffer; 0.1 M Hepes/NaOH (pH 7.4), 1.4 M NaCl, and 25 mM CaCl2 to 9 parts distilled water) and transfer 100 µl of the sample solution to a 5-ml culture tube.
- Add 5 µl of annexin V-FITC and 5 µl of propidium iodide (PI). Gently vortex the tube and incubate for 15 min at room temperature(RT) in the dark. Add 400 µl of 1× binding buffer. NOTE: Use annexin V-FITC and PI from the fluorescence microscope kit.
- Identify the apoptotic cells by using FACS14. The excitation laser lines of PI and annexin V-FITC are 488 nm and 635 nm, respectively. Measure the fluorescence emission of PI and annexin V-FITC at 610 ± 20 nm and 660 ± 20 nm, respectively. Collect the acquired cells on the flow cytometer per 10,000 events.
4. In vivo anticancer efficacy in tumor-bearing mice
- Lung cancer-induced mouse model
- Prepare 1 × 106 A549 cells in 0.1 ml RPMI-1640 medium; keep it in ice.
- Anesthetize the mice with an i.p. injection of a xylazine and a mixture of tiletamine and zolazepam (1:2, 1 ml/kg). Confirm proper anesthetization by gently pinching a small fold of mouse skin. Use vet ointment on the eyes to prevent dryness while under anesthesia. NOTE: If no movement is observed, the animal is sufficiently anesthetized to start the experiments.
- Inject the cells subcutaneously into the left flanks of BALB/C male nude mice (6 - 7 weeks old, 20 - 22 g).
- Keep observing the mice until they start to move around the cage. NOTE: Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return an animal that has undergone surgery to the company of other animals until it has fully recovered. Keep the mice under specific pathogen-free (SPF) conditions.
- Measure the tumor size with calipers every day. Calculate the tumor volume (mm3) as (length × width2) / 2. When the tumor size reaches approximately 200 mm3 in volume, start the experiment.
- Anticancer efficacy study
- Randomly divide the mice into 4 groups (n = 10 for each group).
- Anesthetize the mice with an i.p. injection of a xylazine and a mixture of tiletamine and zolazepam (1:2, 1 ml/kg). Confirm proper anesthetization by gently pinching a small fold of mouse skin. Use vet ointment on the eyes to prevent dryness while under anesthesia. NOTE: If no movement is observed, the animal is sufficiently anesthetized to start the experiments.
- Dissolve the nanoparticles in PBS to a final concentration of 2 mg/ml HB. Inject PBS, free HB, HB-NPs, or HB-P-NPs via the tail vein (2 mg/kg as HB) twice on days 0 and 7.
- Keep observing the mice until they start to move around the cage. NOTE: Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return an animal that has undergone surgery to the company of other animals until fully recovered. Keep the cages dark and under specific SPF conditions.
- 24 hr after each injection, anesthetize the mice with an i.p. injection of a xylazine and a mixture of tiletamine and zolazepam (1:2, 1 ml/kg). Confirm proper anesthetization by gently pinching a small fold of mouse skin. Use vet ointment on the eyes to prevent dryness while under anesthesia. NOTE: If no movement is observed, the animal is sufficiently anesthetized to start the experiments.
- Place the tumor site under the PDT fiber (with 1 cm of distance from the PDT fiber to the tumor). Wear laser safety glasses, turn off the switch, and illuminate the tumor with a PDT laser (630 nm, 400 mW/cm2) for 500 sec (200 J/cm2) twice on days 1 and 8.
- Keep observing the mice until they start to move around the cage. Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
- Keep the cages under SPF conditions. Maintain the cages in the dark for 24 hr after laser treatment.
- Visually monitor the tumor volume and the changes at the tumor site every day. Measure the tumor size with calipers, and calculate the volume as (length × width2) / 2 (mm3). Take pictures of the tumor sites every day to check for tumor surface alterations after PDT.
- On day 16, sacrifice five mice per group by terminal anesthesia using isoflurane.
- With forceps and scissors, cut the outer skin and expose the tumors15. Carefully harvest them. Fix them in 10% formalin, embed them in paraffin, and stain them with hematoxylin and eosin (H&E) for the histological analysis16.
- After 45 days of monitoring, sacrifice the remaining mice by terminal anesthesia using isoflurane.
Representative Results
We prepared both HB-NPs and HB-P-NPs with the techniques mentioned above. The mean diameters of HB-NPs and HB-P-NPs were 220.9 ± 3.2 nm and 211.9 ± 1.6 nm, respectively.
The cell viability of A549 cells after 4 hr of incubation with PBS, empty NPs, HB-NPs, and HB-P-NPs followed by light irradiation (0 to 16 J/cm2) is shown in Figure 1. Without light, the HB-NP treatment group showed no cytotoxicity at all, while HB-P-NP-treated cells showed 81.28 ± 0.14% cell viability. This may be due to the anticancer effect of paclitaxel, which was released from the nanoparticles. Under PDT, the cell viability was decreased according to the light exposure time in both HB-NP- and HB-P-NP-treated cells. The HB-P-NP-treated cells showed increased phototoxicity than the HB-NPs group under the same irradiation conditions. When 16 J/cm2 of irradiation was given, only 7.52 ± 0.38% of the cells survived in the HB-P-NP treatment group.
The consistent results were visualized with annexin V-FITC staining (Figure 2). The PDT-induced apoptotic cells were stained with annexin V-FITC, expressing green fluorescence. The strongest green fluorescence signal was detected in the HB-P-NP-treated cells under the same irradiation conditions.
The stages of the PDT-induced apoptotic cells were classified by flow cytometry. By double-staining with annexin V-FITC and PI, early apoptotic cells (only annexin V-FITC positive), late apoptotic cells (both annexin-V and PI positive) and necrotic cells (only PI positive) were distinguished. Under the same PDT conditions, the portion of late apoptotic cells was increased in the HB-P-NP-treated group (Figure 3).
In vivo tumor growth in lung tumor-bearing mice after double intravenous injection of PBS, free HB, HB-NPs, and HB-P-NPs followed by light irradiation is shown in Figure 4. The surfaces of the tumor sites were also examined (Figure 5). The HB-P-NP-treated mice showed the fastest reactions, including hemorrhage and necrosis, and the slowest tumor growth. Moreover, histological analysis confirmed the most severely damaged tumor cells were in the HB-P-NP treatment group (Figure 6).
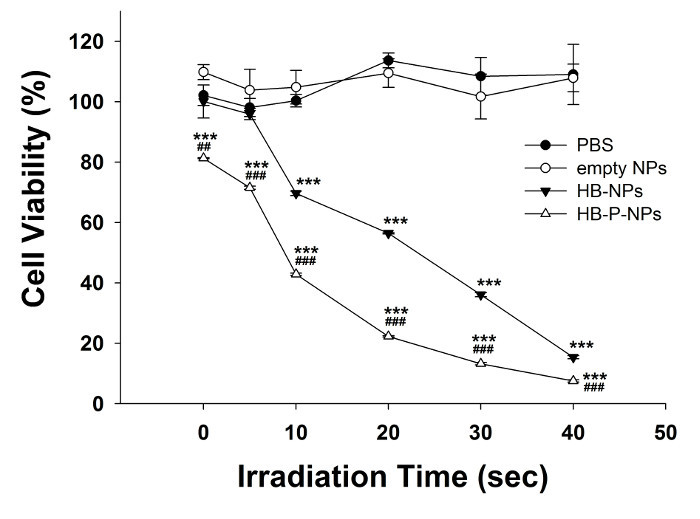 Figure 1:In vitro Phototoxicity of A549 Cells. A549 cells were treated with PBS, empty NPs, HB-NPs, or HB-P-NPs, and light irradiation was given for 0, 5, 10, 20, 30, or 40 sec (0, 2, 4, 8, 12, or 16 J/cm2). Under the same irradiation time, the HB-P-NP treatment group showed higher phototoxicity than the HB-NP treatment group. Values are expressed as means ± standard deviations (SD; n = 3). *** p < 0.001, compared to the PBS-treated group. ## p < 0.01, ### p < 0.001, compared to the HB-NP treatment group. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
Figure 1:In vitro Phototoxicity of A549 Cells. A549 cells were treated with PBS, empty NPs, HB-NPs, or HB-P-NPs, and light irradiation was given for 0, 5, 10, 20, 30, or 40 sec (0, 2, 4, 8, 12, or 16 J/cm2). Under the same irradiation time, the HB-P-NP treatment group showed higher phototoxicity than the HB-NP treatment group. Values are expressed as means ± standard deviations (SD; n = 3). *** p < 0.001, compared to the PBS-treated group. ## p < 0.01, ### p < 0.001, compared to the HB-NP treatment group. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
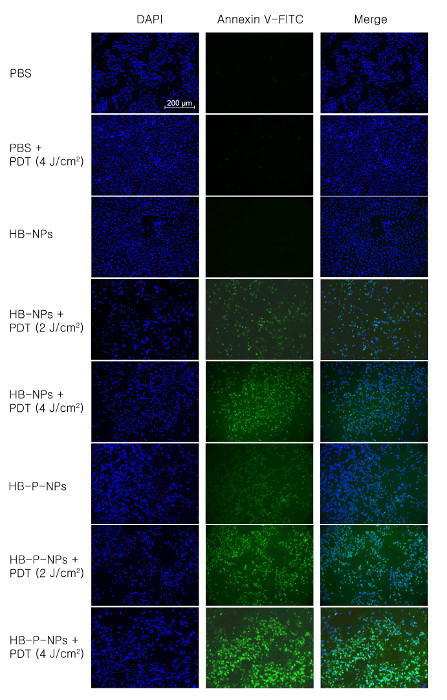 Figure 2:Microscopic Analysis of Apoptotic A549 Cells. A549 cells were treated with PBS, HB-NPs or HB-P-NPs, and light irradiation was given for 0, 2, or 4 J/cm2. The double-stain of DAPI (blue, first column) and annexin V-FITC (green, second column) indicate nuclei and apoptotic cells, respectively. The HB-P-NP-treated cells showed stronger green fluorescence signals than the HB-NP-treated cells under the same irradiation conditions, indicating a much higher phototoxicity. Scale bar = 200 µm, magnification = 100X. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
Figure 2:Microscopic Analysis of Apoptotic A549 Cells. A549 cells were treated with PBS, HB-NPs or HB-P-NPs, and light irradiation was given for 0, 2, or 4 J/cm2. The double-stain of DAPI (blue, first column) and annexin V-FITC (green, second column) indicate nuclei and apoptotic cells, respectively. The HB-P-NP-treated cells showed stronger green fluorescence signals than the HB-NP-treated cells under the same irradiation conditions, indicating a much higher phototoxicity. Scale bar = 200 µm, magnification = 100X. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
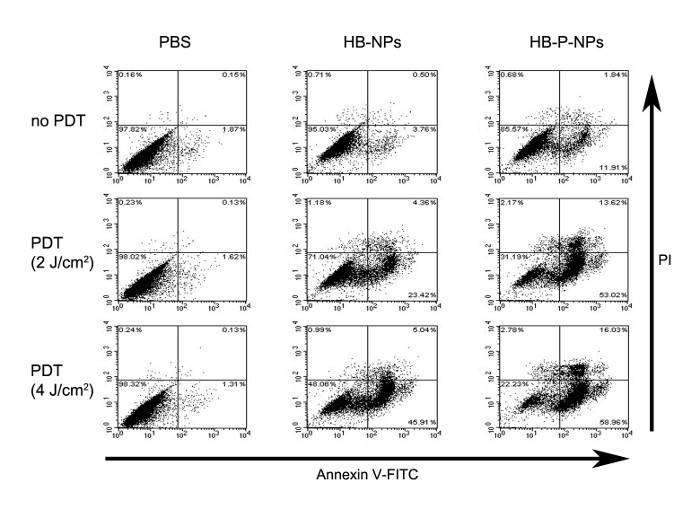 Figure 3:FACS Analysis of Apoptotic A549 Cells. A549 cells were treated with PBS, HB-NPs, or HB-P-NPs, and light irradiation was given for 0, 2, or 4 J/cm2. The cells were categorized into four groups. i) Both annexin V-FITC- and PI-negative cells are considered undamaged, ii) annexin V-FITC-positive and PI-negative cells are early apoptotic, iii) both annexin V-FITC- and PI-positive cells are late apoptotic, and iv) annexin V-FITC-negative and PI-positive cells are either late apoptotic or necrotic. In accordance with the microscopic analysis, under the same irradiation conditions, the HB-P-NP-treated cells showed higher phototoxicity than the HB-NP-treated cells, and late apoptotic cell levels were increased. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
Figure 3:FACS Analysis of Apoptotic A549 Cells. A549 cells were treated with PBS, HB-NPs, or HB-P-NPs, and light irradiation was given for 0, 2, or 4 J/cm2. The cells were categorized into four groups. i) Both annexin V-FITC- and PI-negative cells are considered undamaged, ii) annexin V-FITC-positive and PI-negative cells are early apoptotic, iii) both annexin V-FITC- and PI-positive cells are late apoptotic, and iv) annexin V-FITC-negative and PI-positive cells are either late apoptotic or necrotic. In accordance with the microscopic analysis, under the same irradiation conditions, the HB-P-NP-treated cells showed higher phototoxicity than the HB-NP-treated cells, and late apoptotic cell levels were increased. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
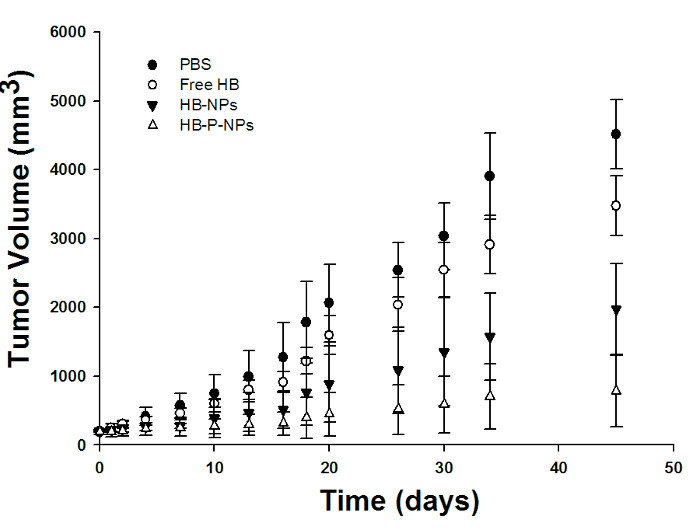 Figure 4:Tumor Volume Monitoring of A549 Tumor-bearing Mice. On days 0 and 7, the double injections of PBS, free HB, HB-NPs, and HB-P-NPs were performed. The PDT (200 J/cm2) was performed 1 day after each injection, on days 1 and 8. The treatments showed different levels of therapeutic effect, with HB < HB-NPs < HB-P-NPs. On day 45, the HB-P-NPs treatment group showed a significantly decreased tumor volume compared to the other groups. Values are presented as means ± standard deviations (SD; n = 4). The tumor volume (mm3) was defined as (length × width2) / 2. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
Figure 4:Tumor Volume Monitoring of A549 Tumor-bearing Mice. On days 0 and 7, the double injections of PBS, free HB, HB-NPs, and HB-P-NPs were performed. The PDT (200 J/cm2) was performed 1 day after each injection, on days 1 and 8. The treatments showed different levels of therapeutic effect, with HB < HB-NPs < HB-P-NPs. On day 45, the HB-P-NPs treatment group showed a significantly decreased tumor volume compared to the other groups. Values are presented as means ± standard deviations (SD; n = 4). The tumor volume (mm3) was defined as (length × width2) / 2. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
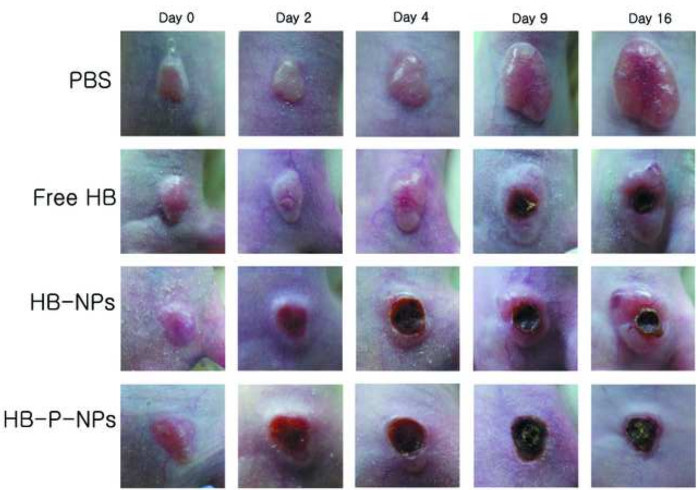 Figure 5:The Surface Alterations on the Tumor Site of A549 Tumor-bearing Mice. The tumor sites were monitored after double intravenous injections (on days 0 and 7) of PBS, free HB, HB-NPs, and HB-P-NPs with 200 J/cm2 light irradiation 1 day after each injection (on days 1 and 8). Both the HB-NPs and the HB-P-NPs treatment groups started to show reactions the day after the first PDT. The HB-P-NPs treatment group showed severe hemorrhaging and the fastest development of necrosis. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
Figure 5:The Surface Alterations on the Tumor Site of A549 Tumor-bearing Mice. The tumor sites were monitored after double intravenous injections (on days 0 and 7) of PBS, free HB, HB-NPs, and HB-P-NPs with 200 J/cm2 light irradiation 1 day after each injection (on days 1 and 8). Both the HB-NPs and the HB-P-NPs treatment groups started to show reactions the day after the first PDT. The HB-P-NPs treatment group showed severe hemorrhaging and the fastest development of necrosis. Adapted from Chang et al.10
Please click here to view a larger version of this figure.
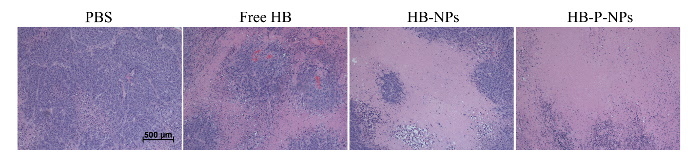 Figure 6:Histological Analysis of Tumor Tissues in A549 Tumor-bearing Mice. On day 16, the histological confirmation was performed by an H&E stain of excised tumor tissues. The HB-P-NPs treatment group demonstrated the most complete tumor cell death. Scale bar = 500 µm, magnification = 40X. Adapted from Chang et al.10 Please click here to view a larger version of this figure.
Figure 6:Histological Analysis of Tumor Tissues in A549 Tumor-bearing Mice. On day 16, the histological confirmation was performed by an H&E stain of excised tumor tissues. The HB-P-NPs treatment group demonstrated the most complete tumor cell death. Scale bar = 500 µm, magnification = 40X. Adapted from Chang et al.10 Please click here to view a larger version of this figure.
Discussion
The most critical step in this study is selecting the proper laser conditions: wavelength, power, and irradiation time. The proper wavelength of light suitable for the specific photosensitizer is necessary for the PDT. We used a 630-nm laser that was appropriate for hypocrellin B. The output power was another important factor, which was set at 400 mW/cm2 based on many pilot studies. Output powers exceeding 400 mW/cm2 damaged the cells or the skin surface of the animals due to the irradiation itself, while output powers below 400 mW/cm2 were too weak to show any therapeutic effect. The appropriate irradiation times for the in vitro and in vivo studies were 40 sec (16 J/cm2) and 500 sec (200 J/cm2), respectively. The distance from the PDT fiber to the cells or to the tumor tissues of the animal models was also a critical factor in this study. To cover the entire cell or tumor surface with the light, 1 cm was found to be the optimal distance.
If a different photosensitizer is applied, use of the proper wavelength of light can maximize the PDT efficacy. The output power and irradiation time should be fixed through trial and error. When the skin surface starts to burn during the light irradiation, the output power should be set lower. When the tumor surface shows no change at all the day after PDT, it may indicate the necessity to increase the output power.
This method has some limitations. First, though the distance from the PDT fiber to the target is an important factor in this study, it is hard to ensure uniformity. The distance may vary slightly because it is controlled by humans. Secondly, for the animal model with tumors in organs such as the lung, this protocol is difficult to apply since endoscopy is necessary and respiratory movement unavoidable.
Though surgical resection is the first option for treating lung cancer, PDT has several advantages as a non-invasive and non-surgical alternative treatment. When the operation is not appropriate, such as in multiple lesion cases, PDT can be a valid option. Also, preoperative PDT may reduce the tumor size and lessen the degree of surgery17. PDT has fewer side effects when compared to surgery, radiation therapy, or endobronchial brachytherapy. In addition, PDT is irrelevant to mutations that provide resistance to radiation therapy or chemotherapy18.
In this study, we suggested both photosensitizer- and anticancer drug-encapsulated lung cancer-targeted nanoparticles as a novel photosensitizer delivery system for PDT. This concept may be applied to other photosensitizers and anticancer drugs.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by grant no. 14-2014-017 from the SNUBH Research Fund.
The authors are indebted to J. Patrick Barron, Professor Emeritus, Tokyo Medical University and Adjunct Professor, Seoul National University Bundang Hospital for his pro bono editing of this manuscript.
References
- Shang L, Zhou N, Gu Y, Liu F, Zeng J. Comparative study on killing effect of esophageal cancer cell line between hypocrellin B-photodynamic therapy and hematoporphyrin derivative-photodynamic therapy. Chin J Cancer Prev Treat. 2005;12:1139–1142. [Google Scholar]
- Huisman C, et al. Paclitaxel triggers cell death primarily via caspase-independent routes in the non-small cell lung cancer cell line NCI-H460. Clin Cancer Res. 2002;8(2):596–606. [PubMed] [Google Scholar]
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Pass HI. Photodynamic therapy in oncology: mechanisms and clinical use. J Natl Cancer Inst. 1993;85(6):443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- Sutedja TG, Postmus PE. Photodynamic therapy in lung cancer. A review. J. Photochem. Photobiol. 1996;36(2):199–204. doi: 10.1016/s1011-1344(96)07372-1. [DOI] [PubMed] [Google Scholar]
- Peng CL, Shieh MJ, Tsai MH, Chang CC, Lai PS. Self-assembled star-shaped chlorin-core poly(epsilon-caprolactone)-poly(ethylene glycol) diblock copolymer micelles for dual chemo-photodynamic therapies. Biomaterials. 2008;29(26):3599–3608. doi: 10.1016/j.biomaterials.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Chen B, Pogue BW, Hasan T. Liposomal delivery of photosensitising agents. Expert Opin Drug Deliv. 2005;2(3):477–487. doi: 10.1517/17425247.2.3.477. [DOI] [PubMed] [Google Scholar]
- Lee SJ, et al. Comparative study of photosensitizer loaded and conjugated glycol chitosan nanoparticles for cancer therapy. J Control Release. 2011;152(1):21–29. doi: 10.1016/j.jconrel.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Chang JE, et al. Anticancer efficacy of photodynamic therapy with hematoporphyrin-modified, doxorubicin-loaded nanoparticles in liver cancer. J. Photochem. Photobiol. 2014;140:49–56. doi: 10.1016/j.jphotobiol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Chang JE, Cho HJ, Yi E, Kim DD, Jheon S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. 2016;158:113–121. doi: 10.1016/j.jphotobiol.2016.02.035. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- Penno MB, et al. Expression of CD44 in human lung tumors. Cancer Res. 1994;54(5):1381–1387. [PubMed] [Google Scholar]
- Wilkins R, Kutzner B, Truong M, Sanchez-Dardon J, McLean J. Analysis of radiation-induced apoptosis in human lymphocytes: Flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytometry. 2002;48(1):14–19. doi: 10.1002/cyto.10098. [DOI] [PubMed] [Google Scholar]
- Bannas P, et al. Validation of nanobody and antibody based in vivo tumor xenograft NIRF-imaging experiments in mice using ex vivo flow cytometry and microscopy. J Vis Exp. 2015. p. e52462. [DOI] [PMC free article] [PubMed]
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008. [DOI] [PubMed]
- Lam S. Photodynamic therapy of lung cancer. Semin Oncol. 1994;21(6 Suppl 15):15–19. [PubMed] [Google Scholar]
- Simone CB, 2nd, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis. 2012;4(1):63–75. doi: 10.3978/j.issn.2072-1439.2011.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]


