Abstract
Epithelial ovarian carcinoma (EOC) is associated with a poor prognosis because it shows peritoneal dissemination. To improve the prognosis, it is important to control peritoneal dissemination. However, it is still unclear how tumor cells detach from primary lesions and attach to the mesothelium. The establishment of an appropriate animal model is needed to gain an understanding of the mechanism of peritoneal dissemination in vivo. In the current study, we introduce the process from the local injection of EOC cells into the murine ovarian surface to the development of metastasis, including the peritoneum and distant organs. Female nude mice (BALB/c nu/nu) at 8 weeks of age were used. Under a microscopic field of view, EOC cells (1 x 105 cells/µl of medium-extracellular matrix (ECM)-based hydrogel/unilateral ovary/mouse) were injected into murine ovaries through a retroperitoneal approach from the dorsal flank. This proposed method is a less invasive procedure for the mouse and minimizes damage to the ovary. Here, we describe the methodological steps in the development of the original and metastatic tumor formation of EOC.
Keywords: Cancer Research, Issue 118, epithelial ovarian cancer (EOC), orthotopic inoculation, peritoneal dissemination, proliferation, metastasis, animal model
Introduction
Epithelial ovarian carcinoma (EOC) accounts for the highest rate of cancer-related mortality among gynecological malignancies1. EOC is associated with a poor prognosis, primarily due to its late symptomatology, and is often associated with multiple intraperitoneal disseminations and distant metastases2-4. Peritoneal dissemination is a multi-step process. Firstly, tumor cells detach from the primary lesions, and migrate into the abdominal cavity. When the tumor cells attach to the peritoneal mesothelium, they start to invade tissues through the mesothelium5,6. In order to better understand the tumor biology (e.g., cancer progression and therapeutic response), mouse models provide a wealth of information. A xenograft with human cancer cells is widely used for mouse models in which human cancer cells are inoculated subcutaneously, intraperitoneally, intravenously, and orthotopically. An animal model with an orthotopically grafted tumor can more efficiently and accurately generate results that reflect the tumor environment in humans in comparison with an animal model with a heterotopically grafted tumor. Therefore, for many human tumors, orthotopic transplantation models have been established7-11.
Here, we describe the orthotopic inoculation of human EOC cells through a retroperitoneal approach from the dorsal flank, which has limited invasiveness compared to ventral inoculation. This technique can provide a variety of useful information on EOC, especially the mechanism of intraperitoneal dissemination.
Protocol
The treatment protocol follows the guidelines for animal experimentation adopted by Nagoya University.
1. Preparation of Cell Suspensions
- Human ovarian clear cell carcinoma cell line.
- Maintain ES-2 cells12 in RPMI-1640 medium with 10% fetal bovine serum (FBS) and penicillin/streptomycin (penicillin: 100 units/ml, streptomycin: 0.1 mg/ml). Culture cells at 37 °C in a 5% CO2 humidified incubator.
- Collect cells in the logarithmic growth phase in 0.05% trypsin/0.02% EDTA solution (trypsin/EDTA).
- First, remove the old media from the cell culture vessel, then wash the cells once with PBS (PBS without Ca2+/Mg2+, 3 - 4 ml per 25 cm2).
- Next, add 1 ml per 25 cm2 of trypsin/EDTA. Rotate the cell culture vessel to cover the cell monolayer with trypsin/EDTA. After removing and discarding trypsin/EDTA, return the cell culture vessel to the incubator for 3 - 5 min or until cells are detached.
- Examine the cells under a microscope to ensure the cells are detached from the surface of the cell culture vessel and floating.
- Add fresh serum containing growth medium to inactivate the trypsin (5 ml per 25 cm2), and re-suspend the cells. Transfer re-suspended cells to a 15 ml conical tube.
- Centrifuge at 300 x g for 5 min. Perform centrifugation either at 4 °C or room temperature. Carefully remove supernatant and re-suspend cells with growth medium (5 ml per 25 cm2).
- Count cells (e.g., by trypan-blue exclusion) then re-suspend in growth medium at a density of 2 x 108 cell/ml. Put the suspended cells on ice.
Thaw frozen extracellular matrix (ECM)-based hydrogel aliquots in an ice bath.
Add the cell suspension to ECM-based hydrogel aliquots at a ratio of 1:1 (final cell concentration: 1 x 105 cells/µl of medium-ECM-based hydrogel) and mix thoroughly. Place the prepared cell suspensions in an ice bath until use.
2. Orthotopic Inoculation with Cell Suspensions
NOTE: Surgery does not require a dedicated suite. Surgery can be performed on a laboratory benchtop in an area of the room that is separate and has minimal activity. A laminar hood also may be used. Sterile surgical instruments must be used and scissors should not be used to make skin incisions.
Use female nude mice (BALB/c nu/nu) at 8 weeks of age for this orthotopic inoculation model.
First, anesthetize the mouse using inhalation with isoflurane (2 - 4%). Check anesthetic depth by verifying lack of withdrawal upon firm toe pinch. After the mouse is anesthetized, apply a bland sterile ophthalmic ointment to the eyes to prevent drying as necessary.
Place the anesthetized mouse on an operating stage belly-side down. Disinfect the surgical area several times with both alcohol and an iodine-based or chlorhexidine-based scrub.
Make a ~ 2-cm incision vertically near the spine between the right costal arch and femur. Use a sterile surgical drape around the incision site. Fix the incised skin surfaces with clamps to maintain the opening. Note: Retractors can be used instead of clamps.
Next, make a second incision (approximately 1 cm) at the parietal peritoneum under the first incision to open the abdominal cavity. Clamp the incised abdominal surfaces. Clip the fat tissue that surrounds the ipsilateral ovary and fallopian tube with a clamp to remove the ovary from the abdominal cavity. Carefully place the ovary and fallopian tube on gauze. Then, place the mouse belly-side down under the dissecting microscope.
Place the cell suspension in an insulin syringe (1/2 cc, 29 G) just before injection. Carefully inject the prepared cell suspension (1 - 2 µl) into the ovary. For several sec, retain the needle within the ovary to ensure gelation of the ECM-based hydrogel. After gelation, the injected tumor cells remain inside the ovary.
Carefully return the ovary into the body. Suture the second incision with sterile medical silk, and then seal the skin with wound clips.
After surgery, the mice will be administered an analgesia (e.g., meloxicam, intramuscular (i.m.) or subcutaneous (s.c.), 0.2 mg/kg) for initial 24 hr period.
3. Post-surgical Treatment
Place each mouse in a separate cage on clean paper with a hot pack to keep the mouse warm until it has regained sufficient consciousness to maintain sternal recumbency. Then, return the animals to their cages for rearing and monitoring. Monitor the mice daily for 3 - 7 days following surgical treatment.
- Monitoring of postoperative mice
- In case of signs of pain, administer analgesics daily (e.g., meloxicam, i.m. or s.c., 0.2 mg/kg).
- If mice have signs of infection (e.g., redness, swelling, discharge), administer daily injection with an anti-infective reagent (e.g., penicillin, i.m., 100,000 IU/kg).
Remove skin closure materials 10 - 14 days after post-surgical treatment.
4. Analysis of Tumors and Metastases
NOTE: The tumor will be formed at the injected ovary 2 - 3 weeks after inoculation.
Sacrifice the mice by carbon dioxide, and then collect the samples (e.g., tumors, metastases, organs)13-15 for further analysis14,16-20. NOTE: If an in vivo imaging system is available, cells bearing enzymes that produce bioluminescence (e.g., luciferase) or fluorescent protein (e.g., GFP) can be used in this protocol. This modified protocol will provide useful information for the dynamics of the spread of cancer cells from the primary tumor.
Representative Results
Six nude mice had their ovaries inoculated with the human clear cell carcinoma cell line ES-2, as described in this protocol. As shown in Figure 1A, after 2 weeks, 5 of the 6 mice had a tumor in the ovary inoculated with ES-2 cells, but there was no tumor in the contralateral ovary without inoculation (used as a control). Moreover, 2 of the 5 mice showed multiple peritoneal disseminations with ascites. Cells metastasize to the liver (Figure 1B). The ovaries, both with the tumor mass at the inoculation site and on the control side, were dissected, sectioned, stained with hematoxylin-eosin (HE), and analyzed under a microscope (Figure 2). A large number of ovarian cancer cells were observed in the inoculated ovary, but not on the contralateral side. The cell morphology was the same as that of the parental tumor.
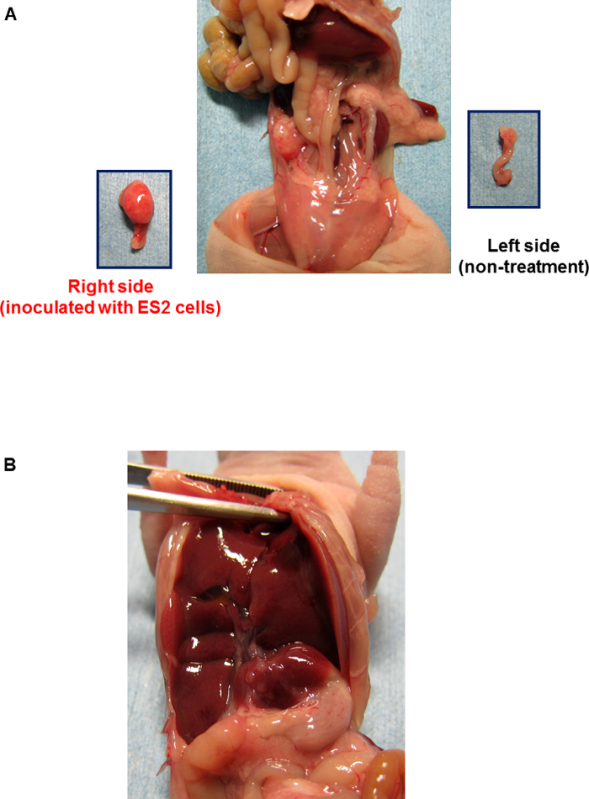 Figure 1:Tumor Formation by Orthotopic Inoculation with Human Clear Cell Carcinoma Cells ES-2. A and B, the BALB/c nu/nu mouse underwent orthotopic inoculation with ES-2 cells. After 2 weeks, the mouse was sacrificed for necropsy. B, Cells metastasize to the liver (as indicated by arrows). Please click here to view a larger version of this figure.
Figure 1:Tumor Formation by Orthotopic Inoculation with Human Clear Cell Carcinoma Cells ES-2. A and B, the BALB/c nu/nu mouse underwent orthotopic inoculation with ES-2 cells. After 2 weeks, the mouse was sacrificed for necropsy. B, Cells metastasize to the liver (as indicated by arrows). Please click here to view a larger version of this figure.
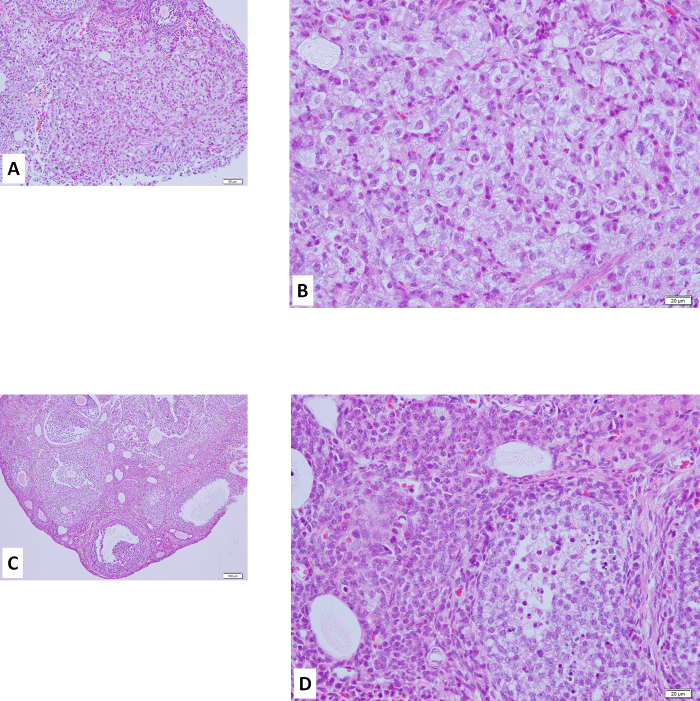 Figure 2:Hematoxylin-eosin Staining of Ovary with Orthotopic Inoculation with Human Clear Cell Carcinoma Cells ES-2.A and B, ovary with inoculation of ES-2 cells, stained with hematoxylin-eosin (HE). Similar staining was seen compared to that of clear cell carcinoma in the human ovary. C and D, HE staining of the ovary without inoculation as a control.Scale bars, 100 μm (A, C), 20 μm (B, D). Please click here to view a larger version of this figure.
Figure 2:Hematoxylin-eosin Staining of Ovary with Orthotopic Inoculation with Human Clear Cell Carcinoma Cells ES-2.A and B, ovary with inoculation of ES-2 cells, stained with hematoxylin-eosin (HE). Similar staining was seen compared to that of clear cell carcinoma in the human ovary. C and D, HE staining of the ovary without inoculation as a control.Scale bars, 100 μm (A, C), 20 μm (B, D). Please click here to view a larger version of this figure.
Discussion
Animal models are essential to analyze the mechanisms of tumor development, progression, metastasis, and drug efficacy against cancer cells. Mice bearing human ovarian cancer cells also have been used as an animal model. Here, we describe a less invasive procedure for the administration of ovarian tumor cells at an orthotopic site. Using this procedure, inoculation into the ovary through a retroperitoneal approach from the dorsal flank offers significant advantages over other commonly used techniques. Firstly, orthotopic inoculation can provide more efficient and accurate results that reflect the tumor environment in humans13,21. Secondly, this retroperitoneal approach from the dorsal flank only needs a small dissection (< 1 - 2 cm) so it is a rapid procedure compared to the peritoneal approach. Therefore, this retroperitoneal approach is less invasive for mice. Thirdly, the murine ovary is very small, and so unable to accept a large amount of reagents. In this presented procedure, we use highly concentrated cell suspensions (1 x 105 cells/µl) and a low inoculation volume (1 - 2 µl/injection) as a single shot; therefore, it minimizes damage to the ovary. It also avoids the leakage of inoculated cells from the ovary. Although further research is essential, the described murine orthotopic inoculation may be a useful approach to help develop new therapies for the treatment of EOC in the future.
There are two critical points in this protocol. Firstly, the cells are used in the logarithmic growth phase. Secondly, the cells suspended with ECM-based hydrogel are placed on ice until use. When the ECM-based hydrogel starts to warm, the gelation process will begin. To avoid the gelation of the ECM-based hydrogel, cells re-suspended with the ECM-based hydrogel and instruments (e.g., tubes, syringes, and needles) are kept on ice until use. There is one limitation of this technique. The injection volume of the cell suspension is less than 3 µl, because the mouse ovary cannot accept a large volume of inoculum.
This protocol can be modified in some points. If an in vivo imaging system is available, cells bearing enzymes that produce bioluminescence (e.g., luciferase) or fluorescent protein (e.g., GFP) can be used in this protocol. This modified protocol will provide useful information for the dynamics of the spread of cancer cells from the primary tumor. Also, this protocol uses ES-2 cells for inoculation with mouse ovary. ES-2 cells are derived from clear cell carcinoma, and this cell line exhibits aggressive growth in nude mouse. Other cell lines (e.g., serous carcinoma) also can be used this protocol instead of ES-2 cells.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors thank the members of the Department of Obstetrics and Gynecology and Cancer Biology for their helpful discussions and technical assistance. This study was supported by a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (15K15604; to H. Kajiyama).
References
- Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kajiyama H, et al. Long-term clinical outcome of patients with recurrent epithelial ovarian carcinoma: is it the same for each histological type. Int J Gynecol Cancer. 2012;22(3):394–399. doi: 10.1097/IGC.0b013e31823eed2c. [DOI] [PubMed] [Google Scholar]
- Kikkawa F, et al. Advances in treatment of epithelial ovarian cancer. Nagoya J Med Sci. 2006;68(1-2):19–26. [PubMed] [Google Scholar]
- Yoshikawa N, et al. Clinicopathologic features of epithelial ovarian carcinoma in younger vs. older patients: analysis in Japanese women. J Gynecol Oncol. 2014;25(2):118–123. doi: 10.3802/jgo.2014.25.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama H, et al. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122(1):91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- Terauchi M, et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007;24(5):329–339. doi: 10.1007/s10585-007-9070-1. [DOI] [PubMed] [Google Scholar]
- Bronstein L, Zechner C, Koeppl H. Bayesian inference of reaction kinetics from single-cell recordings across a heterogeneous cell population. Methods. 2015;85:22–35. doi: 10.1016/j.ymeth.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhang S, Liu S. Establishment of a novel orthotopic model of breast cancer metastasis to the lung. Oncol Rep. 2015;33(6):2992–2998. doi: 10.3892/or.2015.3927. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, et al. Src family kinases differentially influence glioma growth and motility. Mol Oncol. 2015. [DOI] [PMC free article] [PubMed]
- Saar M, et al. Orthotopic tumorgrafts in nude mice: A new method to study human prostate cancer. Prostate. 2015;75(14):1526–1537. doi: 10.1002/pros.23027. [DOI] [PubMed] [Google Scholar]
- Zou Y, et al. miR-29c suppresses pancreatic cancer liver metastasis in an orthotopic implantation model in nude mice and affects survival in pancreatic cancer patients. Carcinogenesis. 2015;36(6):676–684. doi: 10.1093/carcin/bgv027. [DOI] [PubMed] [Google Scholar]
- Lau DH, Lewis AD, Ehsan MN, Sikic BI. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991;51(19):5181–5187. [PubMed] [Google Scholar]
- Bao L, Matsumura Y, Baban D, Sun Y, Tarin D. Effects of inoculation site and Matrigel on growth and metastasis of human breast cancer cells. Br J Cancer. 1994;70(2):228–232. doi: 10.1038/bjc.1994.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MJ, et al. Epidemiological investigation on major depressive disorder in the most heavily damaged areas from Wenchuan earthquake in 2008. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31(2):167–170. [PubMed] [Google Scholar]
- Ma L, et al. Mortality of neonatal respiratory failure related to socioeconomic factors in Hebei province of China. Neonatology. 2011;100(1):14–22. doi: 10.1159/000320155. [DOI] [PubMed] [Google Scholar]
- Lin Z, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5(12):e15534. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, et al. Lumbar interspinous non-fusion techniques: comparison between Coflex and Wallis. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(11):2455–2458. [PubMed] [Google Scholar]
- Zhang Y, et al. Wave intensity analysis of carotid artery: a noninvasive technique for assessing hemodynamic changes of hyperthyroid patients. J Huazhong Univ Sci Technolog Med Sci. 2010;30(5):672–677. doi: 10.1007/s11596-010-0563-9. [DOI] [PubMed] [Google Scholar]
- Song GS, et al. Comparative transcriptional profiling and preliminary study on heterosis mechanism of super-hybrid rice. Mol Plant. 2010;3(6):1012–1025. doi: 10.1093/mp/ssq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, et al. The analysis of variation of Han female adolescent bone development in Henan and Zhejiang province. Fa Yi Xue Za Zhi. 2010;26(2):97–99. [PubMed] [Google Scholar]
- Wang HB, et al. Excitation-emission fluorescence characterization study of the three phenolic compounds. Guang Pu Xue Yu Guang Pu Fen Xi. 2010;30(5):1271–1274. [PubMed] [Google Scholar]


