Abstract
During a study of indoor fungi, 145 isolates belonging to Chaetomiaceae were cultured from air, swab and dust samples from 19 countries. Based on the phylogenetic analyses of DNA-directed RNA polymerase II second largest subunit (rpb2), β-tubulin (tub2), ITS and 28S large subunit (LSU) nrDNA sequences, together with morphological comparisons with related genera and species, 30 indoor taxa are recognised, of which 22 represent known species, seven are described as new, and one remains to be identified to species level. In our collection, 69 % of the indoor isolates with six species cluster with members of the Chaetomium globosum species complex, representing Chaetomium sensu stricto. The other indoor species fall into nine lineages that are separated from each other with several known chaetomiaceous genera occurring among them. No generic names are available for five of those lineages, and the following new genera are introduced here: Amesia with three indoor species, Arcopilus with one indoor species, Collariella with four indoor species, Dichotomopilus with seven indoor species and Ovatospora with two indoor species. The generic concept of Botryotrichum is expanded to include Emilmuelleria and the chaetomium-like species B. muromum (= Ch. murorum) in which two indoor species are included. The generic concept of Subramaniula is expanded to include several chaetomium-like taxa as well as one indoor species. Humicola is recognised as a distinct genus including two indoor taxa. According to this study, Ch. globosum is the most abundant Chaetomiaceae indoor species (74/145), followed by Ch. cochliodes (17/145), Ch. elatum (6/145) and B. piluliferum (5/145). The morphological diversity of indoor Chaetomiaceae as well as the morphological characteristics of the new genera are described and illustrated. This taxonomic study redefines the generic concept of Chaetomium and provides new insight into the phylogenetic relationships among different genera within Chaetomiaceae.
Key words: Chaetomiaceae, Indoor species, Morphological diversity, Phylogeny
Taxonomic novelties: New genera: Amesia X. Wei Wang, Samson & Crous; Arcopilus X. Wei Wang, Samson & Crous; Collariella X. Wei Wang, Samson & Crous; Dichotomopilus X. Wei Wang, Samson & Crous; Ovatospora X. Wei Wang, Samson & Crous
New species: Chaetomium tectifimeti X. Wei Wang & Samson; Collariella carteri X. Wei Wang, Houbraken & Samson; Dichotomopilus pseudoerectus X. Wei Wang & Samson; Dichotomopilus pseudofunicola X. Wei Wang & Samson; Humicola olivacea X. Wei Wang & Samson; Melanocarpus tardus X. Wei Wang & Samson; Ovatospora pseudomollicella X. Wei Wang & Samson
New combinations: Amesia atrobrunnea (Ames) X. Wei Wang & Samson, Amesia cymbiformis (Lodha) X. Wei Wang & Samson, Amesia nigricolor (Ames) X. Wei Wang & Samson, Amesia gelasinospora (Aue & Müller) X. Wei Wang & Samson, Arcopilus aureus (Chivers) X. Wei Wang & Samson, Arcopilus cupreus (Ames) X. Wei Wang & Samson, Arcopilus fusiformis (Chivers) X. Wei Wang & Samson, Arcopilus flavigenus (van Warmelo) X. Wei Wang & Samson, Arcopilus turgidopilosus (Ames) X. Wei Wang & Samson, Botryotrichum murorum (Corda) X. Wei Wang & Samson, Botryotrichum spirotrichum (R.K. Benjamin) X. Wei Wang & Samson, Collariella bostrychodes (Zopf) X. Wei Wang & Samson, Collariella causiiformis (Ames) X. Wei Wang & Samson, Collariella gracilis (Udagawa) X. Wei Wang & Samson, Collariella quadrangulata (Chivers) X. Wei Wang & Samson, Collariella robusta (Ames) X. Wei Wang & Samson, Collariella virescens (Arx) X. Wei Wang & Samson, Dichotomopilus dolichotrichus (Ames) X. Wei Wang & Samson, Dichotomopilus erectus (Skolko & J.W. Groves) X. Wei Wang & Samson, Dichotomopilus funicola (Cooke) X. Wei Wang & Samson, Dichotomopilus fusus (Ames) X. Wei Wang & Samson, Dichotomopilus indicus (Corda) X. Wei Wang & Samson, Dichotomopilus pratensis (X.W. Wang & L. Cai) X. Wei Wang & Samson, Dichotomopilus ramosissimus (X.W. Wang & L. Cai) X. Wei Wang & Samson, Dichotomopilus reflexus (Skolko & J.W. Groves) X. Wei Wang & Samson, Dichotomopilus subfunicola (X.W. Wang & L. Cai) X. Wei Wang & Samson, Dichotomopilus variostiolatus (Carter) X. Wei Wang & Samson, Ovatospora brasiliensis (Batista & Pontual) X. Wei Wang & Samson, Ovatospora medusarum (Meyer & Lanneau) X. Wei Wang & Samson, Ovatospora mollicella (Ames) X. Wei Wang & Samson, Ovatospora senegalensis (Ames) X. Wei Wang & Samson, Ovatospora unipora (Aue & Müller) X. Wei Wang & Samson, Subramaniula anamorphosa (S.A. Ahmed et al.) X. Wei Wang & Samson, Subramaniula cristata (Ames) X. Wei Wang & Samson, Subramaniula cuniculorum (Fuckel) X. Wei Wang & Samson, Subramaniula fusispora (G. Smith) X. Wei Wang & Samson
New name: Subramaniula flavipila X. Wei Wang & Samson
Neotypification: Chaetomium elatum Kunze
Introduction
Fungal contamination in damp or water-damaged buildings has become an increasing problem worldwide (Andersen et al. 2011). After water damage (e.g. leaking water pipes, flooding, faulty building constructions, or severe and prolonged condensation) many building materials become good substrates for certain fungi. These growing fungi can cause adverse effects not only on the buildings but also to their occupants (Samson et al., 1994, WHO, 2009, Samson et al., 2010, Flannigan and Miller, 2011, Andersen et al., 2011, Miller and McMullin, 2014). Members of the genus Chaetomium are capable of colonising various substrates and are well-known for their ability to degrade cellulose and to produce a variety of bioactive metabolites. More than 400 species have been described in Chaetomium. Some of these species have been reported to be important inhalant allergens. They contribute to the development of the symptoms of both rhinitis and asthma due to the production of mycotoxins and microbial volatile organic compounds as well as the liberation of ascospores and hyphal fragments in the indoor environment (Gonianakis et al., 2005, Apetrei et al., 2009, Polizzi et al., 2009, Mason et al., 2010, Andersen et al., 2011, Miller and McMullin, 2014). Chaetomium globosum is the most common species of the Chaetomiaceae in the indoor environment (Vesper et al., 2007, Ayanbimpe et al., 2010, Straus, 2011, McMullin et al., 2013, Miller and McMullin, 2014), and this species can already be present in new gypsum wallboard (Andersen et al. in press). Chaetomium globosum has been reported to produce a variety of toxic metabolites, such as chaetoglobosins, chaetomugilins, and chaetoviridins (Andersen et al., 2011, McMullin et al., 2013, Miller and McMullin, 2014), while both Ch. elatum and Ch. globosum were able to produce cochliodones in pure cultures as well as on naturally contaminated building materials (Došen et al. in press). Little is known about the other indoor Chaetomium species and their potential hazard to humans and buildings. Furthermore, Ch. globosum and several other Chaetomium species are reported as causal agents of onychomycosis or superficial infections (Koch and Haneke, 1965, Naidu et al., 1991, Aspiroz et al., 2007, Hubka et al., 2011, de Hoog et al., 2013), and some of them are capable of opportunistically causing deep or systemic infections (Hoppin et al., 1983, Barron et al., 2003, Guppy et al., 1998, Ahmed et al., 2016).
The genus Chaetomium is commonly recognised by having ostiolate ascomata with a membranaceous perithecial wall covered by relatively well-developed hairs, producing fasciculate and evanescent asci and single-celled, smooth and pigmented ascospores with germ pores (Ames, 1963, von Arx et al., 1986). Chaetomium globosum, the type species of the genus, was first described by Kunze (Kunze & Schmidt 1817). The taxonomy of Chaetomium has been studied by several authors (Corda, 1840, Zopf, 1881, Chivers, 1915, Skolko and Groves, 1948, Skolko and Groves, 1953, Sörgel, 1960, Ames, 1963, Mazzucchetti, 1965, Seth, 1970, Dreyfuss, 1976, Millner, 1977, Millner et al., 1977, von Arx et al., 1984). von Arx et al. (1986) re-defined the taxonomic concept of Ch. globosum. They included species that produce globose to ovate or obovate ascomata with a wall consisting of textura intricata, covered by a diverse morphology of ascomatal hairs ranging from erect, flexuous to regularly coiled. The ascomata contain clavate (or slightly fusiform), evanescent asci, and the ascospores are limoniform and bilaterally-flattened shaped, and have an apical germ pore. Following this concept 28 species were reduced to synonymy with Ch. globosum. The species concept of Ch. globosum sensu von Arx was not supported by a recent study (Asgari & Zare 2011). For example, von Arx et al. (1986) treated Ch. coarctatum as one of the synonyms of Ch. globosum. Based on three genomic loci (ITS region, partial LSU rDNA and partial β-tubulin gene sequences), the phylogenetic analysis of Asgari & Zare (2011) indicated a distant relationship between the authentic isolate of Ch. globosum (CBS 148.51) and the ex-type strain of Ch. coarctatum (CBS 162.62). On the basis of phylogenetic inference of six loci and morphological characters, Ch. globosum was again revised by Wang et al. (2016), and six species that were treated as synonyms of Ch. globosum by von Arx et al. (1986) were resurrected. Furthermore, the non-ostiolate genus Chaetomidium was also synonymised with Chaetomium (Wang et al. 2016).
The aim of the present study was to conduct a global investigation of the species diversity of indoor Chaetomiaceae in the context of advanced taxonomy and chemical analysis. The results would not only be a useful tool for the identification of indoor Chaetomiaceae and evaluation of their chemical potential, but also provide new insights into the phylogeny of the Chaetomiaceae.
Materials and methods
Isolates
This study is based on a collection of isolates from indoor environments of 19 countries which are housed in the working collection of the Department of Applied and Industrial Mycology (DTO), and of those which were assigned to species of Chaetomiaceae and housed in the public collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS). The strains isolated from dust were collected and isolated as previously described (Amend et al. 2010). Briefly, sterilised dust stream collectors (Indoor Biotechnologies) were attached to domestic vacuum cleaners for collection. Samples were filtered through a 2-mm sieve and refrigerated at 4 °C until further processing. The samples were analysed by a modified dilution-to-extinction plating technique (Visagie et al. 2014). Air samples were collected approx 1 m above the ground with a viable impaction sampler (MAS 100 Merck) and indoor surfaces (i.e. walls, ceilings) were sampled with a swab (Greiner Bio-One, Alphen aan de Rijn, The Netherlands). The air and swab samples were analysed using standard microbiological techniques. Agar media used for the isolation of the Chaetomiaceae strains include malt extract agar (Oxoid Ltd, Hampshire, UK) and dichloran 18 % glycerol (DG18: Oxoid Ltd, Hampshire, UK) agar. Petri dishes were incubated at room temperature or 25 °C, and inspected regularly. Metabolite extraction was performed on a subset of the representative isolates comprising the major indoor species in Chaetomiaceae. All the isolates used in this study are listed in Table 1.
Table 1.
Details of strains included in this study.
| Genus and species | Culture accession number(s)1 | Previous name | Origin | GenBank accession numbers2 |
|||
|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tub2 | ||||
| Achaetomium | |||||||
| Ach. globosum | CBS 332.67 T | Rhizosphere, Lucknow, India | KX976570 | KX976695 | KX976793 | KX976911 | |
| Ach. luteum | CBS 618.68 | Cucurbita rhizosphere, Delhi, India | KX976571 | KX976696 | KX976794 | KX976912 | |
| CBS 544.83 | Rosa stem, Lahore, Pakistan | KX976572 | KX976697 | KX976795 | KX976913 | ||
| Ach. macrosporum | CBS 152.97 T | Leaf litter, Uttar Pradesh, India | KX976573 | KX976698 | KX976796 | KX976914 | |
| CBS 532.94 | Mangrove mud, Japan | KX976574 | KX976699 | KX976797 | KX976915 | ||
| Ach. strumarium | CBS 333.67 T | Soil, Lucknow, India | AY681204 | AY681170 | KC503254 | AY681238 | |
| Amesia gen. nov. | |||||||
| Am. atrobrunnea | CBS 379.66*T | Ch. atrobrunneum | Mouldy mattress, Solomon Islands | JX280771 | JX280666 | KX976798 | KX976916 |
| CBS 250.75 | Air, Uttar Pradesh, India | KX976575 | KX976700 | KX976799 | KX976917 | ||
| Am. cymbiformis | CBS 175.84 | Ch. cymbiforme | Tent rope, Solomon Islands | KX976576 | KX976701 | KX976800 | KX976918 |
| CBS 176.84* | Case liner, Georgia, USA | KX976577 | KX976702 | KX976801 | KX976919 | ||
| Am. nigricolor | CBS 600.66 T | Ch. nigricolor | Vegetable detritus, India | KX976578 | KX976703 | KX976802 | KX976920 |
| CBS 291.83* | Paper, India | KX976579 | KX976704 | KX976803 | KX976921 | ||
| Am. gelasinospora | CBS 673.80 T | Ch. gelasinosporum | Soil, Qus, Egypt | KX976580 | KX976705 | KX976804 | KX976922 |
| CBS 643.83 | Sandy soil, Gawa, Nigeria | KX976581 | KX976706 | KX976805 | KX976923 | ||
| Arcopilus | |||||||
| Ar. aureus | CBS 153.52 | Ch. aureum | Virginia, USA | KX976582 | KX976707 | KX976806 | KX976924 |
| CBS 538.73 | Dung of hyrax, East Africa | KX976583 | KX976708 | KX976807 | KX976925 | ||
| Ar. cupreus | CBS 560.80 | Ch. cupreum | Dung of moose, Mietta Hot Springs, Canada | KX976584 | KX976709 | KX976808 | KX976926 |
| Ar. fusiformis | CBS 484.85 | Ch. fusiforme | Dung of rodent, Newberry Mts., Nevada, USA | KX976585 | KX976710 | KX976809 | KX976927 |
| CBS 485.85 | Wood chip, Hiltin Falls, Ontario, Canada | KX976586 | KX976711 | KX976810 | KX976928 | ||
| Ar. flavigenus | CBS 337.67 T | Ch. flavigenum | Soil, Johannesburg, South Africa | KX976587 | KX976712 | KX976811 | KX976929 |
| Ar. turgidopilosus | CBS 169.52*T | Ch. turgidopilosum | Top of storage tent, USA | KX976588 | KX976713 | KX976812 | KX976930 |
| Botryotrichum | |||||||
| B. atrogriseum | CBS 130.28 T | Dung of rabbit, The Netherlands | KX976589 | KX976714 | KX976813 | KX976931 | |
| CBS 604.69 | Corn field soil, Waterloo, Ontario, Canada | KX976590 | KX976715 | KX976814 | KX976932 | ||
| B. murorum | CBS 163.52 | Ch. murorum | Great Smoky Mts., Tennessee, USA | KX976591 | KX976716 | KX976815 | KX976933 |
| CBS 173.68 | Liquor cerebrospinalis of Homo sapiens, Netherlands | KX976592 | KX976717 | KX976816 | KX976934 | ||
| DTO 324-G9*; DTO 324-H9 | Air, China | KX976593 | KX976718 | KX976817 | KX976935 | ||
| DTO 333-E6*(= IBT 42175) | Ceiling tile, Denmark | KX976594 | KX976719 | KX976818 | KX976936 | ||
| B. peruvianum | CBS 460.90 | Dung of herbivore, Massanella, Spain | KX976623 | KX976720 | KX976819 | KX976937 | |
| CBS 421.93 | Air, La Habana, Cuba | KX976596 | KX976721 | KX976820 | KX976938 | ||
| B. piluliferum | CBS 654.79 | Pastry, Enschede, Netherlands | KX976597 | KX976722 | KX976821 | KX976939 | |
| CBS 105.14 | Unknown | KX976598 | KX976723 | KX976822 | KX976940 | ||
| DTO 194-F7 | Plaster wall, The Netherlands | KX976599 | KX976724 | KX976823 | KX976941 | ||
| DTO 254-B8*; DTO 254-B9 | Wall in villa, Utrecht, The Netherlands | KX976600 | KX976725 | KX976824 | KX976942 | ||
| B. spirotrichum | CBS 211.55 T | Emilmuelleria spirotricha | Dung of deer, California, USA | KX976601 | KX976726 | KX976825 | KX976943 |
| CBS 828.71 | Dung of donkey, Algeria | KX976602 | KX976727 | KX976826 | KX976944 | ||
| Chaetomium sensu stricto** | |||||||
| C. cervicicola | DTO 318-G6 | Dust, Mexico | KX976603 | KX976728 | KX976827 | KX976945 | |
| C. coarctatum | DTO 324-H2* | Air, China | KX976604 | KX976729 | KX976828 | KX976946 | |
| C. cochliodes | DTO 013-C2 | Air, Maastricht, The Netherlands | KX976605 | KX976947 | |||
| DTO 089-E2 | Air, Eindhoven, The Netherlands | KX976606 | KX976948 | ||||
| DTO 319-B5; DTO 319-B6 | Dust, South Africa | KX976607 | KX976730 | KX976829 | KX976949 | ||
| DTO 318-I1*; DTO 318-H2; DTO 318-H4;DTO 318-H5; DTO 318-H7; DTO 318-H8; DTO 318-I3; DTO 318-I5; DTO 318-I6;DTO 318-I8; DTO 318-I9; DTO 319-A1;DTO 319-B5; DTO 319-B6; DTO 325-F7 | Dust, USA | KX976608 | KX976950 | ||||
| C. elatum | DTO 318-H9*; DTO 318-G7 | Dust, USA | KX976609 | KX976731 | KX976830 | KX976951 | |
| DTO 319-B3* | Dust, Australia | KX976610 | KX976732 | KX976831 | KX976952 | ||
| DTO 333-E5 | Dust, Denmark | KX976611 | KX976953 | ||||
| CBS 142034 neoT (= DTO 333-E9 = IBT 42179) | Cardboard, Denmark | KX976612 | KX976733 | KX976832 | KX976954 | ||
| DTO 333-F8 (= IBT 42329) | Gypsum, Denmark | KX976613 | KX976955 | ||||
| C. globosum | DTO 134-D9; DTO 134-E1; DTO 134-E2; DTO 134-E3; DTO 134-E4; DTO 134-E5 | Air, Algeria | KX976614 | KX976956 | |||
| DTO 318-G3; DTO 318-G4; DTO 318-G5 | Dust, Canada | KX976615 | KX976957 | ||||
| DTO 324-D7; DTO 324-G8; DTO 324-H1; DTO 324-H4; DTO 324-H5; DTO 324-I1; DTO 324-I2; DTO 324-I3; DTO 324-I4; DTO 324-I5; DTO 324-I6; DTO 324-I7; DTO 324-I8; DTO 324-I9; DTO 325-A1; DTO 325-A2; DTO 325-A3; DTO 325-A4 | Air, China | KX976616 | KX976958 | ||||
| CBS 666.82* | Chili powder, China | KX976617 | KX976734 | KX976833 | KX976959 | ||
| DTO 333-D7 (= IBT 42328); DTO 333-D8 (= IBT 42326); DTO 333-D9 (= IBT 42327); DTO 333-F4 (= IBT 42297); DTO 333-F5 (= IBT 42299); DTO 333-F6 (= IBT 42301); DTO 333-F7 (= IBT 42325); DTO 333-F9 | Gypsum, Denmark | KX976618 | KX976960 | ||||
| DTO 333-E1 (= IBT 41766); DTO 333-E8 (= IBT 42177) | Plywood, Denmark | KX976619 | KX976961 | ||||
| DTO 333-E3*(= IBT 41800) | Linoleum, Denmark | KX976620 | KX976735 | KX976834 | KX976962 | ||
| DTO 333-E4 (= IBT 41801) | Carpet, Denmark | KX976621 | KX976963 | ||||
| DTO 333-E7 (= IBT 42176) | Oriented strand board, Denmark | KX976622 | KX976964 | ||||
| CBS 112386; DTO 340-I2 | Indoor environment, Germany | KX976623 | KX976965 | ||||
| DTO 012-F3 | Air, Hamburg, Germany | KX976624 | KX976966 | ||||
| DTO 012-D2 | Air, Koln, Germany | KX976625 | KX976967 | ||||
| DTO 237-D4 | Air, Indonesia | KX976626 | KX976968 | ||||
| DTO 319-B2*; DTO 319-A3; DTO 319-A4; DTO 319-A5; DTO 319-A6; DTO 319-A7; DTO 319-A8; DTO 319-A9; DTO 319-B1 | Dust, Mexico | KX976627 | KX976736 | KX976835 | KX976969 | ||
| DTO 085-E8; DTO 085-F5; DTO 085-F6 | Air, Baarn, The Netherlands | KX976628 | KX976970 | ||||
| DTO 122-H9 | Air, Gorinchem, The Netherlands | KX976629 | KX976971 | ||||
| DTO 123-D4 | Air, Zutphen, The Netherlands | KX976630 | KX976972 | ||||
| DTO 264-C1 | Wall in house, Wassenaar, The Netherlands | KX976631 | KX976973 | ||||
| DTO 272-I1 | Wall, Utrecht, The Netherlands | KX976632 | KX976974 | ||||
| DTO 086-D6 | Archive material, Gorinchem, The Netherlands | KX976633 | KX976975 | ||||
| DTO 126-B6 | Indoor environment, Den Haag, The Netherlands | KX976634 | KX976976 | ||||
| DTO 011-F7; DTO 012-F2 | Wall paper, Loosdrecht, The Netherlands | KX976635 | KX976977 | ||||
| DTO 319-B4 | Dust, South Africa | KX976636 | KX976978 | ||||
| DTO 319-C3 | Dust, Thailand | KX976637 | KX976979 | ||||
| DTO 319-C9 | Dust, Uruguay | KX976638 | KX976980 | ||||
| DTO 318-H3; DTO 318-H6; DTO 318-I4; DTO 319-C5; DTO 319-C6 | Dust, USA | KX976639 | KX976981 | ||||
| CBS 148.51 | Stored cotton, District of Columbia, USA | GU563374 | GU563363 | KF001801 | JF772459 | ||
| C. tectifimeti | CBS 142032 T (= DTO 318-G8)* | Dust, USA | KX976640 | KX976737 | KX976836 | KX976982 | |
| Collariella | |||||||
| Col. bostrychodes | CBS 163.73 | Ch. bostrychodes | Dung of antelope, East Africa | KX976641 | KX976738 | KX976837 | KX976983 |
| CBS 586.83 | Soil, Germany | KX976642 | KX976739 | KX976838 | KX976984 | ||
| DTO 319-C4 | Dust, Indonesia | KX976643 | KX976985 | ||||
| DTO 324-H3; DTO 324-H6* | Air, China | KX976644 | KX976740 | KX976839 | KX976986 | ||
| CBS 121706 | Commercial honey, Spain | KX976645 | KX976987 | ||||
| Col. causiiformis | CBS 792.83*T | Ch. causiiform | Sweatband of helmet liner, Solomon Islands | KX976646 | KX976741 | KX976840 | KX976988 |
| Col. carteri | CBS 128.85*T | Air, British Columbia, Canada | KX976647 | KX976742 | KX976841 | KX976989 | |
| Col. gracilis | CBS 146.60 T | Ch. gracile | Soil, Tsu, Mie, Japan | KX976648 | KX976743 | KX976842 | KX976990 |
| CBS 249.75* | Air, Uttar Pradesh, India | KX976649 | KX976744 | KX976843 | KX976991 | ||
| Col. quadrangulata | CBS 142.58 | Ch. quadrangulatum | Soil, French Polynesia | KX976650 | KX976745 | KX976844 | KX976992 |
| CBS 152.59 | Dung of rabbit, Derbyshire, Chatsworth Park, England | KX976651 | KX976746 | KX976845 | KX976993 | ||
| Col. robusta | CBS 551.83 T | Ch. robustum | Litter, Portland Parish, Jamaica | KX976652 | KX976747 | KX976846 | KX976994 |
| CBS 508.84 | Woodlot soil, Ocho Rios, Jamaica | KX976653 | KX976748 | KX976847 | KX976995 | ||
| Col. virescens | CBS 148.68 T | Ch. virescens | Agricultural soil, Lahore, Pakistan | KX976654 | KX976749 | KX976848 | KX976996 |
| CBS 547.75 | Wheat straw compost, Ludhiana, Punjab | KX976655 | KX976750 | KX976849 | KX976997 | ||
| Corynascella | |||||||
| Cor. humicola | CBS 337.72 T | Soil, Piedmont, North Carolina, USA | KX976656 | KX976751 | KX976850 | KX976998 | |
| CBS 379.74 | Soil, Piedmont, North Carolina, USA | KX976657 | KX976752 | KX976851 | KX976999 | ||
| Dichotomopilus | |||||||
| D. dolichotrichus | CBS 162.48 T | Ch. dolichotrichum | Great Smoky Mts., USA | HM449049 | HM449063 | KX976852 | JF772462 |
| CGMCC 3.14189 | Discarded cloth, Longjing, Jilin Province, China | HM449048 | HM449062 | KX976853 | JF772455 | ||
| D. erectus | CBS 140.56 T | Ch. erectum | Petroselinum sativum, USA | HM449044 | HM449058 | KX976854 | JF772458 |
| CGMCC 3.12900 | Soil, Anqiu, Shandong Province, China | KC109760 | KC109760 | KX976855 | KC109778 | ||
| D. funicola | CBS 159.52 eT | Ch. funicola | Germany | GU563369 | GU563354 | KX976856 | JF772461 |
| CBS 136.38 | Unknown | HM449046 | HM449060 | KX976857 | JF772457 | ||
| DTO 333-F1*; DTO 333-F2* | Dust, outdoors, Denmark | KX976658 | KX976753 | KX976858 | KX977000 | ||
| DTO 318-I2 | Dust, USA | KX976659 | KX977001 | ||||
| D. fusus | CBS 372.66 T | Ch. fusum | Leaf litter, Bataan, Costa Rica | KX976660 | KX976754 | KX976859 | KX977002 |
| CBS 114.83 | Tectona grandis or calyx, Jamaica | KX976661 | KX976755 | KX976860 | KX977003 | ||
| D. indicus | CGMCC 3.14184 eT | Ch. indicum | Rhizosphere of Panax notoginseng, Yunnan, China | GU563367 | GU563360 | KX976861 | JF772453 |
| CGMCC 3.14182 | Rhizosphere of Panax notoginseng, Yunnan, China | GU563366 | GU563358 | KX976862 | JF772451 | ||
| DTO 333-E2* | Feather, Denmark | KX976662 | KX976756 | KX976863 | KX977004 | ||
| DTO 333-F3* | Dust, outdoors, Denmark | KX976663 | KX976757 | KX976864 | KX977005 | ||
| DTO 319-B8* | Dust, South Africa | KX976664 | KX976758 | KX976865 | KX977006 | ||
| D. pratensis | CBS 133396 T (= CGMCC 3.14181) | Ch. pratense | Soil, Huangnan, Qinghai Province | GU563372 | GU563357 | KX976866 | JF772450 |
| CBS 804.83 | Wood of celar, Switzerland | KX976665 | KX976759 | KX976867 | KX977007 | ||
| CBS 860.68* | Ch. indicum | Air, Germany | KX976666 | KX976760 | KX976868 | KX977008 | |
| D. pseudoerectus | CBS 252.75*T | Air, Uttar Pradesh, India | KX976667 | KX976761 | KX976869 | KX977009 | |
| D. pseudofunicola | CBS 142033 T (= DTO 318-I7)* | Dust, USA | KX976668 | KX976762 | KX976870 | KX977010 | |
| D. ramosissimus | CGMCC 3.14183 T | Ch. ramosissimum | Rhizosphere of Panax Notoginseng, Yunnan, China | GU563371 | GU563361 | KX976871 | JF772452 |
| CGMCC 3.12930 | Soil, Huanggang, Hubei Province | HM449045 | HM449059 | KX976872 | JF772449 | ||
| D. reflexus | CBS 157.49 T | Ch. reflexum | Germinating seed, Toledo, Ohio, USA | HM449051 | HM449055 | KX976873 | JF772460 |
| CBS 141.56 | Seed, Edmonton, Alberta, Canada | KX976669 | KX976763 | KX976874 | KX977011 | ||
| D. subfunicola | CGMCC 3.12892 T | Ch. subfunicola | Soil, Shihezi, Xinjiang Autonomous Region | JX867125 | JX867125 | KX976875 | JX867122 |
| CGMCC 3. 9466 | Rhizosphere of Panax Notoginseng, Yunnan, China | GU563368 | GU563353 | KX976876 | JF772446 | ||
| CBS 812.73* | Pistol belt, New Guinea | KX976670 | KX976764 | KX976877 | KX977012 | ||
| CBS 794.83* | Paper, Switzerland | KX976671 | KX976765 | KX976878 | KX977013 | ||
| D. variostiolatus | CBS 179.84*T | Ch. variostiolatum | Tarpaulin, New Guinea | KX976672 | KX976766 | KX976879 | KX977014 |
| DTO 319-A2* | Dust, USA | KX976673 | KX976767 | KX976880 | KX977015 | ||
| DTO 319-B9*; DTO 319-C1 | Dust, Thailand | KX976674 | KX976768 | KX976881 | KX977016 | ||
| Humicola | |||||||
| H. fuscoatra | CBS 118.14 T | Soil, Norway | KX976675 | KX976769 | KX976882 | KX977017 | |
| H. olivacea | CBS 142031 T (= DTO 319-C7)* | Dust, USA | KX976676 | KX976770 | KX976883 | KX977018 | |
| Humicola sp. | DTO 318-G9; DTO 318-H1 | Dust, Mexico | KX976677 | KX976771 | KX976884 | KX977019 | |
| DTO 319-B7* | Dust, South Africa | KX976678 | KX976772 | KX976885 | KX977020 | ||
| Melanocarpus | |||||||
| Me. albomyces | CBS 638.94 T | Chicken nest straw, Nevada, USA | KX976679 | KX976773 | KX976886 | KX977021 | |
| CBS 747.70 | Coal pit refuse, UK | KX976680 | KX976774 | KX976887 | KX977022 | ||
| Me. tardus | CBS 541.76*T | Cotton jacket, Switzerland | KX976681 | KX976775 | KX976888 | KX977023 | |
| Myceliophthora | |||||||
| My. fergusii | CBS 406.69 T | Mushroom compost, Pennsylvania, USA | HQ871794 | KX976776 | HQ871815 | KX977024 | |
| My. heterothallica | CBS 202.75 | Garden soil, Giessen, Germany | HQ871771 | KM655354 | HQ871798 | KX977025 | |
| My. lutea | CBS 145.77 neoT | Hay, Newmarket, UK | HQ871775 | KM655351 | HQ871816 | KX977026 | |
| My. sepedonium | CBS 111.69 T | Soil, Allahabad, India | HQ871751 | KX976777 | HQ871827 | KX977027 | |
| My. thermophila | CBS 669.85 | Cellulase, USA | HQ871767 | KX976778 | HQ871806 | KX977028 | |
| CBS 381.97 | Homo sapiens, Unknown | HQ871766 | KX976779 | HQ871805 | KX977029 | ||
| Ovatospora | |||||||
| O. brasiliensis | CBS 130174 | Ch. brasiliense | Soil, Colombia | KX976682 | KX976780 | KX976895 | KX977030 |
| CBS 140.50* | Moist jute cloth, Calcutta, India | KX976683 | KX976781 | KX976896 | KX977031 | ||
| O. medusarum | CBS 148.67 T | Ch. medusarum | Soil, Zaire | KX976684 | KX976782 | KX976897 | KX977032 |
| O. mollicella | CBS 583.83 T | Ch. mollicellum | Dung of spotted skunk, Washington, USA | KX976685 | KX976783 | KX976898 | KX977033 |
| O. pseudomollicella | CBS 251.75*T | Air, Uttar Pradesh, India | KX976686 | KX976784 | KX976899 | KX977034 | |
| O. senegalensis | CBS 728.84 T | Ch. senegalense | Plant remains, Senegal | KX976687 | KX976785 | KX976900 | KX977035 |
| CBS 798.83 | Dung of gazelle, Israel | KX976688 | KX976786 | KX976901 | KX977036 | ||
| O. unipora | CBS 109.83 T | Ch. uniporum | Soil, Egypt | KX976689 | KX976787 | KX976902 | KX977037 |
| Subramaniula | |||||||
| S. anamorphosa | CBS 137114 T | Ch. anamorphosum | Peritonitis of Homo sapiens, Kuwait | KP862598 | KP970641 | KP900667 | KP900704 |
| S. asteroides | CBS 123294 T | Keratitis of Homo sapiens, USA | HQ906667 | JX280731 | KP900666 | KP900703 | |
| CBS 128466 | Corneal ulcer of Homo sapiens, USA | JX280843 | JX280732 | KP900656 | KP900695 | ||
| S. cristata | CBS 156.52 T | Ch. cristatum | Dung of rabbit, Virginia, USA | KX976690 | KX976788 | KX976903 | KX977038 |
| DTO 324-H8*; DTO 324-H7 | Air, China | KX976691 | KX976789 | KX976904 | KX977039 | ||
| S. cuniculorum | CBS 800.83 | Ch. cuniculorum | Soil, Spain | KX976692 | KX976790 | KX976905 | KX977040 |
| S. fusispora | CBS 199.84 | Ch. fusisporum | Dung of marmot, Alberta, Canada | KP862601 | KP970645 | KP900653 | KP900707 |
| S. flavipila | CBS 446.66 T | Ch. irregulare | Dead leaves, Bulgaria | KP862600 | KP970647 | KP900669 | KP900706 |
| CBS 227.82 | Dung, Spain | KP862599 | KP970646 | KP900668 | KP900705 | ||
| S. obscura | CBS 132916 T | Tinea pedis of Homo sapiens, Kuwait | KP862595 | KP970653 | KP900662 | KP900700 | |
| S. thielavioides | CBS 122.78 T | Dung of nilgai, Delhi Zoo, India | KP862597 | KP970654 | KP900670 | KP900708 | |
| CBS 560.84 | Dung of herbivore, Delhi, India | KP862596 | KP970655 | KP900672 | KP900710 | ||
| Thielavia | |||||||
| T. appendiculata | CBS 731.68 | Dung of rabbit, Wales | KM655330 | KM655369 | KX976906 | KX977041 | |
| T. fragilis | CBS 456.73 T | Rhizosphere of Pennisetum typhoideum in garden soil, Tamil Nadu, India | KX976693 | KX976791 | KX976907 | KX977042 | |
| T. hyrcaniae | CBS 353.62 T | Sand dune soil, Iran | KM655329 | KM655368 | KX976908 | KX977043 | |
| T. kuwaitensis | CBS 945.72 T | Desert soil, Kuwait | KM655332 | KM655371 | KX976909 | KX977044 | |
| T. terricola | CBS 165.88 | Barren soil, North Carolina, USA | KX976694 | KX976792 | KX976910 | KX977045 | |
| Microascus trigonosporus | CBS 218.31 T | USA | LM652443 | HG380436 | DQ470908 | LM652655 | |
T, eT and neoT denote ex-type, ex-epitype and ex-neotype cultures respectively.
The isolates from the indoor environments are highlighted in bold.
The newly generated sequences in this study are shown in bold; where multiple culture numbers are listed in the row only the sequences from the first culture was deposited in GenBank.
The isolates that were analysed for their metabolite production.
Here only the sequences of the representatives of indoor Chaetomium sensu stricto species are provided. For the information of other Chaetomium species please see in our previous study (Wang et al. 2016).
DNA phylogeny
Genomic DNA was extracted from 7- to 15-d-old cultures grown on oatmeal agar (OA) using the UltraClean™ Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA) following the manufacturer’s instructions. The primers used for PCR amplification and sequencing included: RPB2AM-1bf & RPB2AM-7R (Miller & Huhndorf 2005) for the second largest subunit of DNA-directed RNA polymerase II (rpb2) gene region; ITS5 & ITS4 (White et al. 1990) for the internal transcribed spacer regions (ITS) and intervening 5.8S nrRNA gene region, NL1 & NL4 (O’Donnell 1993) for the D1/D2 domains of the 28S nrDNA (LSU); T1 (O’Donnell & Cigelnik 1997) and TUB4Rd (Groenewald et al. 2013) for the partial beta-tubulin (tub2) gene region. The PCR conditions were the same as those described by Wang et al. (2016). Each of the amplicons was sequenced with the ABI Prism® Big Dye™ Terminator v. 3.1 Cycle Sequencing Kit. Samples were analysed on an ABI PRISM 3710 xl Genetic Analyzer. Consensus sequences for each locus were assembled using the forward and reverse sequences with the programme MEGA v. 6 (Tamura et al. 2013). Novel sequences generated in this study were deposited in GenBank (http://www.ncbi.nlm.nih.gov, Table 1).
Besides the sequences generated in this study, additional sequences were retrieved from GenBank. The sequence datasets were aligned using MAFFT v. 7 (Katoh & Standley 2013), and manually optimised using BioEdit v. 5.0.9 (Hall 1999). Congruency between the four loci was tested using the 70 % reciprocal bootstrap criterion (Mason-Gamer and Kellogg, 1996, Gueidan et al., 2007, Lombard et al., 2010).
The ITS region was used to initially screen the collection of fungi from the indoor environments in order to select members of the Chaetomiaceae. The tub2 gene region was used to recognise the species diversity within the indoor Chaetomiaceae isolates. The phylogenetic placement of the indoor isolates was determined using four loci (ITS, partial LSU, tub2 and partial rpb2) on the basis of the evaluation in a previous study (Wang et al. 2016), and representatives of related species and genera in the Chaetomiaceae were included as references in the final phylogenetic analyses. Phylogenetic analyses were based on Bayesian inference (BI), Maximum Likelihood (ML) and Maximum Parsimony (MP) as described previously (Wang et al. 2016). For BI, the best evolutionary model for each locus was determined using MrModeltest v. 2.0 (Nylander 2004). Obtained trees were viewed in FigTree v. 1.1.2 (Rambaut 2009). The alignment and derived trees were deposited in TreeBASE (submission ID 20347; http://treebase.org/treebase-web/home.html).
Morphology
Colony morphology was determined by inoculating strains onto four different media (Samson et al. 2010): OA, potato carrot agar (PCA), malt extract agar (MEA, Oxoid), and Dichloran 18 % glycerol agar (DG18), incubated in the dark at 25 °C for 7 d. Microscopic observation was performed using methods previously described (Wang et al. 2016). Morphological descriptions are mainly based on OA, sometimes on PCA. For the observations of the asexual morphology, SNA (spezieller nährstoffarmer agar) was used (Samson et al. 2010).
Metabolite extraction of pure cultures
Metabolite profiling was performed on 15-d-old cultures grown on MEA and potato dextrose agar (PDA) (Samson et al. 2010), where three agar plugs (6 mm diam) were cut across one colony from each agar medium and pooled in a 2 mL Eppendorf tube. One mL extraction solvent (ethyl acetate-2-propanol (3:1; vol/vol) containing 1 % formic acid) was added to each vial and the plugs were extracted in a sonication bath for 60 min. The extract was then transferred to a clean 2 mL Eppendorf tube and evaporated to dryness in a stream of N2. The dried extract was subsequently re-dissolved in 400 μL methanol in a sonication bath for 30 min, centrifuged for 3 min at 15 000 g, and transferred to a clean auto sampler vial.
UHPLC-DAD-QTOF-MS analyses
Samples (0.5 μL) were analysed using ultra-high performance liquid chromatography-diode array detection-quadrupole time of flight mass spectrometry (UHPLC-DAD-QTOF-MS) on an Agilent Infinity 1290 UHPLC system (Agilent Technologies, Santa Clara, California, USA) equipped with a DAD detector scanning 200–640 nm. Metabolites were separated on an Agilent Poroshell 120 phenyl-hexyl column (2.1 × 250 mm, 2.7 μm) using a linear gradient of solvents consisting of water (A) and acetonitrile (B) buffered with 20 mM formic acid. The gradient started at 10 % B and increased to 100 % in 15 min where it was held for 2 min (Kildgård et al. 2014). The flow rate was 0.35 mL/min and the column temperature was 60 °C. Mass spectrometry detection was performed in ESI+ mode on an Agilent 6545 QTOF MS equipped with Dual Jet Stream electrospray ion source, using hexakis-(2,2,3,3-tetrafluoropropoxy)phosphazene as the lock mass. Other MS parameters, including Auto-MS/HRMS, can be found in Kildgård et al. (2014).
Secondary metabolites were identified by aggressive dereplication of the full HRMS (high resolution mass spectrometry) data against a list of possible known compounds that have been described in the literature as well as comparison to 1 500 fungal secondary metabolites. All samples were further analysed for peaks not detected by the previous approach, and those were matched against Antibase2012 for a tentative identification. Metabolites that did not match were considered as novel compounds and their elemental composition was determined from the accurate mass (±2 ppm) and isotopic pattern (Kildgård et al., 2014, Došen et al., 2016). The peak areas of [M+H]+, [M+Na]+ or [M+H2O]+ of all the compounds, including the tentatively identified and the novel compounds, were then integrated in the Agilent MassHunter Quant software using extracted ion chromatograms ±12 ppm and the peak areas for multivariate data analysis.
Results
Isolates
A total of 145 indoor isolates (Table 1, in bold font) were identified as members of Chaetomiaceae after the ITS sequencing. A further selection of 45 representative indoor isolates was made based on the tub2 gene sequences, combined with an examination of the macro- and micromorphology. Similar isolates were excluded and strains that possibly represent different species were included in the detailed morphological examination and four-locus analyses. Thirty-eight representative isolates (Table 1, marked with *) were included in the metabolite analysis.
Phylogeny
The phylogenetic analysis of tub2 gene region placed the indoor isolates into 30 well-supported clades in 10 distinct monophyletic lineages (Table 1, Table 2). The preliminary identification based on the tub2 locus was confirmed by the four-locus analysis on the basis of a dataset consisting of 45 representative indoor isolates and representative isolates of related genera and species. Only the concatenated phylogenetic tree was presented with the bootstrap proportions (≥ 50 %) from ML or MP analyses and posterior probabilities (≥ 0.95) from Bayesian analyses plotted on the phylogramme to show statistical support (Fig. 1).
Table 2.
A summary of indoor Chaetomiaceae.
| Species names | Algeria | Australia | Canada | China | Cuba | Denmark | Germany | India | Indonesia | Mexico | The Netherlands | New Guinea | Solomon Islands | South Africa | Spain | Switzerland | Thailand | Uruguay | USA | Total |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per species | Per genus | ||||||||||||||||||||
| Amesia atrobrunnea | 1 | 1 | 2 | 5 | |||||||||||||||||
| Am. cymbiformis | 1 | 1 | 2 | ||||||||||||||||||
| Am. nigricolor | 1 | 1 | |||||||||||||||||||
| Arcopilus turgidopilosus | 1 | 1 | 1 | ||||||||||||||||||
| Botryotrichum murorum | 2 | 1 | 3 | 8 | |||||||||||||||||
| B. piluliferum | 4 | 5 | |||||||||||||||||||
| B. peruvianum | 1 | ||||||||||||||||||||
| Chaetomium cervicicola | 1 | 1 | 100 | ||||||||||||||||||
| Ch. coarctatum | 1 | 1 | |||||||||||||||||||
| Ch. cochliodes | 3 | 2 | 12 | 17 | |||||||||||||||||
| Ch. elatum | 1 | 3 | 2 | 6 | |||||||||||||||||
| Ch. globosum | 6 | 3 | 19 | 13 | 3 | 1 | 9 | 11 | 1 | 1 | 1 | 6 | 74 | ||||||||
| Ch. testifimeti | 1 | 1 | |||||||||||||||||||
| Collariella bostrychodes | 2 | 1 | 1 | 4 | 7 | ||||||||||||||||
| Col. causiiformis | 1 | 1 | |||||||||||||||||||
| Col. carteri | 1 | 1 | |||||||||||||||||||
| Col. gracilis | 1 | 1 | |||||||||||||||||||
| Dichotomopilus funicola | 2 | 1 | 3 | 15 | |||||||||||||||||
| D. indicus | 2 | 1 | 3 | ||||||||||||||||||
| D. pratensis | 1 | 1 | |||||||||||||||||||
| D. pseudoerectus | 1 | 1 | |||||||||||||||||||
| D. pseudofunicola | 1 | 1 | |||||||||||||||||||
| D. subfunicola | 1 | 1 | 2 | ||||||||||||||||||
| D. variostiolatus | 1 | 2 | 1 | 4 | |||||||||||||||||
| Humicola olivacea | 1 | 1 | 4 | ||||||||||||||||||
| Humicola sp. | 2 | 1 | 3 | ||||||||||||||||||
| Melanocarpus tardus | 1 | 1 | 1 | ||||||||||||||||||
| Ovatospora brasiliensis | 1 | 1 | 2 | ||||||||||||||||||
| O. pseudomollicella | 1 | 1 | |||||||||||||||||||
| Subramaniula cristata | 2 | 2 | 2 | ||||||||||||||||||
| Totals | 6 | 1 | 4 | 26 | 1 | 21 | 4 | 6 | 2 | 12 | 18 | 2 | 3 | 5 | 1 | 2 | 3 | 1 | 27 | 145 | |
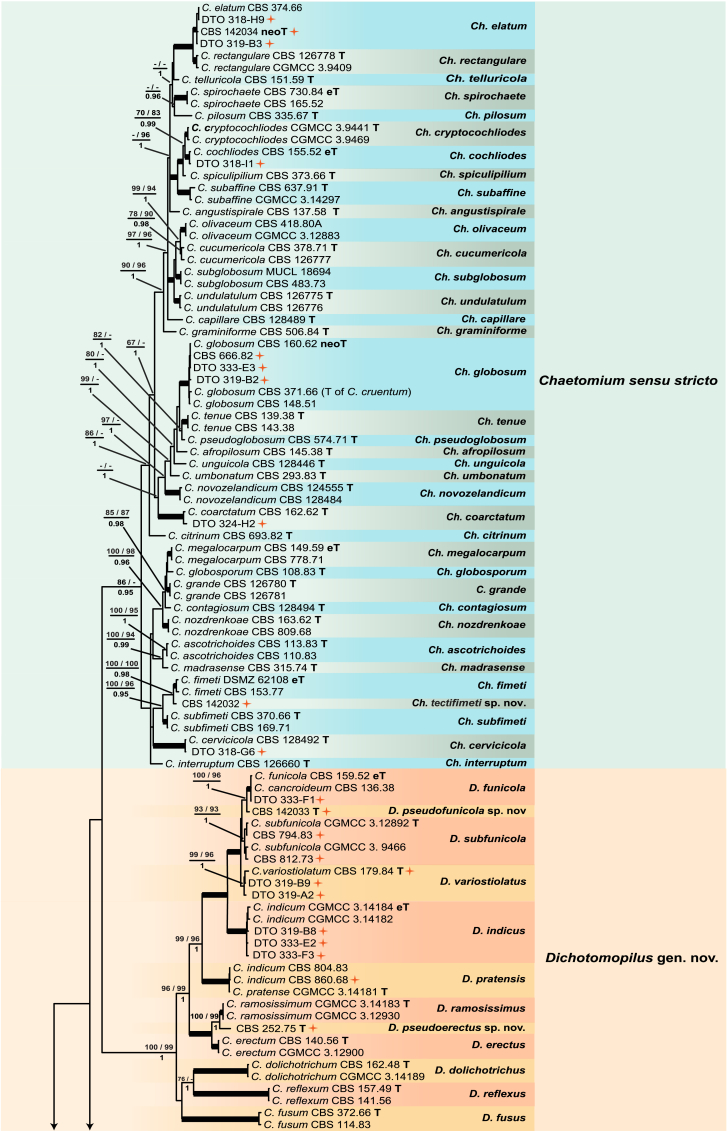
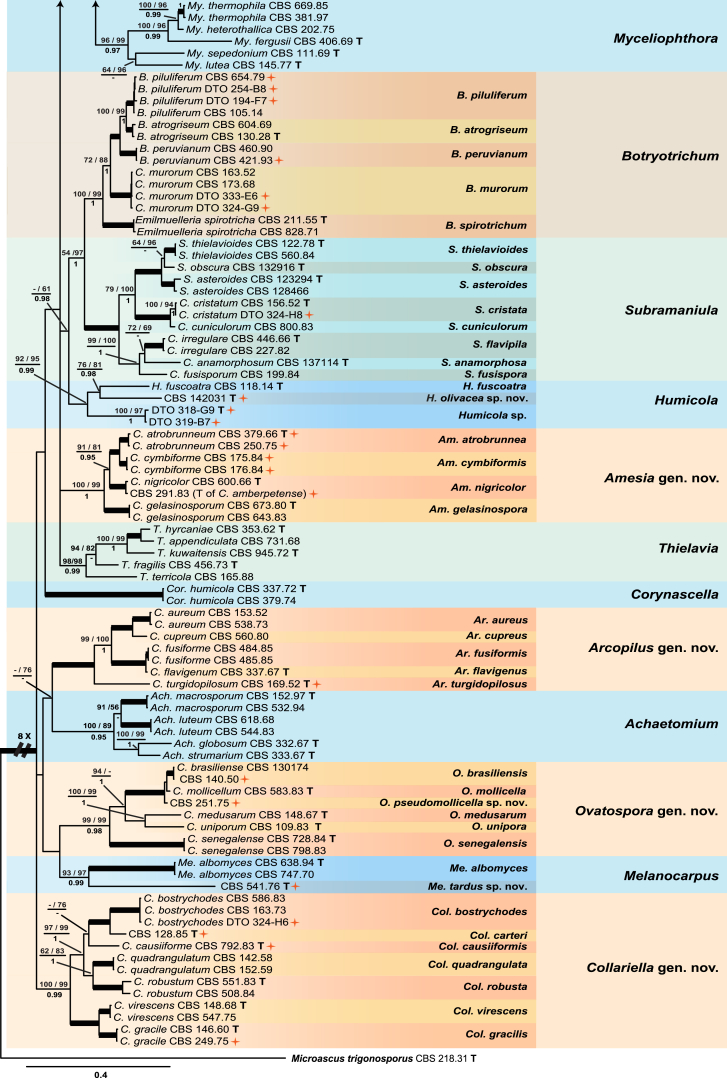
Fig. 1.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the concatenated rpb2, tub2, ITS and LSU gene region alignment, with the confidence values of bootstrap proportions from the MP analysis (before the backslash), the ML analysis (after the backslash) above branches, and the posterior probabilities from the Bayesian analysis below branches. The “−” means lacking statistical support (<50 % for bootstrap proportions from ML or MP analyses; <0.95 for posterior probabilities from Bayesian analyses). The branches with full statistical support (MP-BS = 100 %; ML-BS = 100 %; PP = 1.0) are highlighted by thickened branches. Generic novelties are indicated with “gen. nov.” after the genus name and the genus names of the species are abbreviated to facilitate layout of the tree. Genus and species clades are discriminated with boxes of different colours. The 45 isolates from the indoor environment are indicated with a red star on the right side of the culture number; these isolates are representative of all the indoor species recognised in this study. The scale bar shows the expected number of changes per site. The tree is rooted with Microascus trigonosporus strain CBS 218.31 (see Table 1 for GenBank accession numbers).
The multigene analyses contained 183 strains, including Microascus trigonosporus (CBS 218.31) as outgroup taxon. No topological conflicts were found when comparing the 70 % bootstrap reciprocal tree topologies based on the rpb2 and tub2 datasets. The minor incongruences observed for the ITS and LSU sequence data set failed to resolve some of the species, especially those in the Ch. globosum species complex recovered by each of the two protein-coding gene regions used here. All four loci were combined following recommendations of Cunningham (1997). The concatenated alignment consisted of 2 790 characters (including alignment gaps): 525, 968, 724 and 573 characters used in the rpb2, tub2, ITS and LSU partitions, respectively. Of these, 1 122 characters were constant, 339 characters parsimony-uninformative and 1 265 characters parsimony-informative. For the Bayesian inference, a GTR+I+G model was selected for rpb2, ITS and LSU and a HKY+I+G model for tub2. These models were incorporated into the analysis. A total of 23 822 trees were generated during the Bayesian inference, of which 5 956 trees were discarded as the “burnin-phase” and posterior probabilities (PP) were calculated from the remaining 17 866 trees. The BI consensus tree and PP confirmed the tree topologies and bootstrap support (BS) values obtained with the ML and MP analyses. The MP analysis yielded 136 equally most parsimonious trees (TL = 11 252; CI = 0.299; RI = 0.822; RC = 0.245). The BI consensus tree is presented (Fig. 1) with the respective MP- and ML-BS values indicated at the nodes.
The concatenated phylogenetic analyses revealed the Ch. globosum species complex (Wang et al. 2016) (MP-BS = 86; ML-BS ≤ 50; PP = 0.95) and 13 other monophyletic clades (Fig. 1). Six known genera were supported: Achaetomium including the type species A. globosum (MP-BS = 100; ML-BS = 89; PP = 0.95), Corynascella represented by the type species Cor. humicola (MP-BS = 100; ML-BS = 100; PP = 1.0), Humicola represented by the type species H. fuscoatra (MP-BS = 92; ML-BS = 95; PP = 0.99), Melanocarpus represented by the type species Me. albomyces (MP-BS = 93; ML-BS = 97; PP = 0.99), Myceliophthora including the type species My. lutea (MP-BS = 96; ML-BS = 99; PP = 0.97), and Thielavia including five species (MP-BS = 98; ML-BS = 98; PP = 0.99). Emilmuelleria and Ch. murorum clustered in the Botryotrichum clade (MP-BS = 100; ML-BS = 99; PP = 1.0), which is represented by the type species B. piluliferum and two other Botryotrichum species. Chaetomium cristatum, Ch. cuniculorum, Ch. irregulare, Ch. anamorphosum and Ch. fusisporum clustered in the Subramaniula clade (MP-BS = 100; ML-BS = 100; PP = 1.0) represented by the type species S. thielavioides. The Botryotrichum and Subramaniula clades formed sisters to each other (MP-BS = 54; ML-BS = 97; PP = 1), which clustered closely with the Humicola clade with relatively low statistic support (MP-BS ≤ 50; ML-BS = 61; PP = 0.98).
Five highly supported monophyletic clades represent possible novel genera. The clade represented by Ch. indicum (MP-BS = 100; ML-BS = 99; PP = 1.0) formed a sister to the Ch. globosum species complex, but with no statistical support for their relationships. Four others included: the Ch. atrobrunneum clade (MP-BS = 100; ML-BS = 99; PP = 1.0), the Ch. aureum clade (MP-BS = 100; ML-BS = 100; PP = 1.0), the Ch. brasiliense clade (MP-BS = 99; ML-BS = 99; PP = 0.98) and the Ch. bostrychodes clade (MP-BS = 100; ML-BS = 99; PP = 0.99). They were distant from the Ch. globosum species complex and separated by the Achaetomium, Botryotrichum, Corynascella, Melanocarpus, Myceliophthora, Subramaniula and Thielavia generic clades. The presented topology received little to no statistical support for most of these.
Four known species were originally isolated from indoor environments or from materials associated with human lives, namely Ch. atrobrunneum (ex-type CBS 379.66, from mouldy mattress), Ch. causiiforme (ex-type CBS 792.83, from sweatband of helmet liner), Ch. turgidopilosum (ex-type CBS 169.52, from top of a storage tent) and Ch. variostiolatum (ex-type CBS 179.84, from tarpaulin). Twenty-eight other representative indoor isolates clustered in 18 known species clades with high statistical support (MP-BS ≥ 93; ML-BS ≥ 93; PP = 1.0), which were represented by their ex-type cultures (seven species), ex-epitype cultures (three species), ex-neotype culture (Ch. globosum), or representative strains (seven species), respectively.
Two isolates (DTO 319-B7 and DTO 318-G9) formed a sister clade to another indoor isolate (DTO 319-C7) and clustered with H. fuscoatra (ex-type CBS 118.14), the type species of Humicola. The isolate CBS 541.76, which is deposited as Thielavia minuta in the CBS collection, formed a sister lineage to the type species of Melanocarpus (M. albomyces, ex-type CBS 638.94), which was distant from the core Thielavia clade. The isolate CBS 251.75 formed a sister lineage to the ex-type of Ch. mollicellum and the Ch. brasiliense clade (MP-BS = 100; ML-BS = 100; PP = 1.0). Three other isolates (DTO 318-G8, DTO 318-I7 and CBS 252.75) clustered close to but separated from their closest relatives: Ch. fimeti, Ch. funicola or Ch. ramosissimum, respectively. These isolates represent possible novel phylogenetic species.
Metabolite profiling
A subset of isolates (Table 3) was extracted and analysed in order to compare their metabolite production. The species names used in this paragraph are based on the newly proposed taxonomy mentioned below. The analyses showed that more than 68 metabolites could be detected, and the structure of 31 compounds is unknown. The majority of the detected metabolites (known and uncharacterised) were produced by isolates belonging to Chaetomium sensu stricto and Dichotomopilus. Table 3 shows the production of 35 known and six unknown metabolites at species level. In general there were no species specific metabolites produced by one species alone, with the exception of the production of chaetosemin A, chaetoquadrin A, I and K by H. olivacea (DTO 319-C7) and sterigmatocystin by Humicola sp. (DTO 319-B7). Other metabolites were produced by isolates belonging to various genera and species. For example, cochliodinol A was produced by C. globosum, D. pratensis, B. murorum, B. piluliferum, S. cristata, A. nigricolor, O. brasiliensis and O. pseudomollicella, and cochliodinol B was produced by C. cochliodes, C. pseudofimeti, D. subfunicola, D. variostiolatus, D. funicola, A. cymbiformis, Col. bostrychodes and Col. carteri. There were metabolites that were genus specific, as can be seen from Table 3. Some metabolites, like chaetoindicin, SB236049, SB236050 and SB238569, were only found in genus Dichotomopilus, while others were restricted to genus Chaetomium sensu stricto. Chaetoglobosins A and C, chaetomugilin D and prochaetoglobosins I-IV were only produced by C. coarctacum and C. globosum whereas chaetocin A, chaetomin and chaetoviridin B/C were only found in C. cochloides and C. pseudofimeti.
Table 3.
Metabolite production by representatives in each of the indoor genera.
| Metabolites | Genus |
Amesia |
Arcopilus |
Botryotrichum |
Chaetomium |
Collariella |
Dichotomopilus |
Humicola |
Melanocarpus |
Ovumospora |
Subramaniula |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Am. atrobrunnea | Am. cymbiformis | Am. nigricolor | Ar. turgidopilosus | B. murorum | B. piluliferum | Ch. coarctatum | Ch. cochliodes | Ch. elatum | Ch. globosum | Ch. testifimeti | Col. bostrychodes | Col. causiiformis | Col. carteri | Col. gracilis | D. funicola | D. indicus | D. pratensis | D. pseudoerectus | D. pseudofunicola | D. subfunicola | D. variostiolatus | H. olivacea | Humicola sp. | Melanocarpus tardus | O. brasiliensis | O. pseudomollicella | S. cristata | |
| Chaetochalasin A | − | − | − | − | − | − | + | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetocin A | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetocin C | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | |
| Chaetocochin C | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetoglobosins A and C | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetoindicin | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | − | − | − | |
| Chaetomin | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetomugilin D | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetoquadrin E | − | − | − | − | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | |
| Chaetoquadrins A, I and K | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | |
| Chaetosemin A | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | |
| Chaetoviridin A | − | − | − | − | + | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetoviridin B/C | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Chaetoviridin E | − | − | − | − | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | |
| Chetoseminudin A | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Cochliodinol A | − | − | + | − | + | + | − | − | − | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | + | + | |
| Cochliodinol B | − | + | − | − | − | − | − | + | − | − | + | + | − | + | − | + | − | − | − | − | + | + | − | − | − | − | − | − | |
| Cochliodones 1–3 | − | − | − | + | − | − | + | − | + | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Dihydroxychaetocin | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | |
| Mollicellin C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | |
| Mollicellin E | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | − | |
| Prenisatin | − | + | + | − | + | − | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | − | − | − | + | + | + | |
| Prochaetoglobosins I–IV | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Rotiorinol | − | − | − | − | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Sterigmatocystin | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | |
| SB236049/SB236050/SB238569 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | − | − | − | |
| C16H23NO2 | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | |
| C16H23NO3 | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | |
| C18H28O6 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + | − | − | − | |
| C28H40O7 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | |
| MW306 | + | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + | − | − | − | − | − | |
| MW665 | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | |
Taxonomy
Being the type species, Chaetomium globosum is affiliated with the Ch. globosum species complex (Wang et al. 2016). This species complex was confirmed as a monophyletic lineage in this study, which represents Chaetomium sensu stricto. Thirty species found in indoor environments could be accommodated in 10 genera of the Chaetomiaceae. Five new genera are established and seven new species described. One Humicola species represented by three indoor isolates remains to be compared with other known Humicola species and this will be done in future phylogenetic and morphological studies. All the species obtained from indoor substrates, and each of the recognised novel genera in this study are described and illustrated below. The generic concepts of Subramaniula and Botryotrichum are also expanded.
Amesia X. Wei Wang, Samson & Crous, gen. nov. MycoBank MB818829. Fig. 2.
Fig. 2.
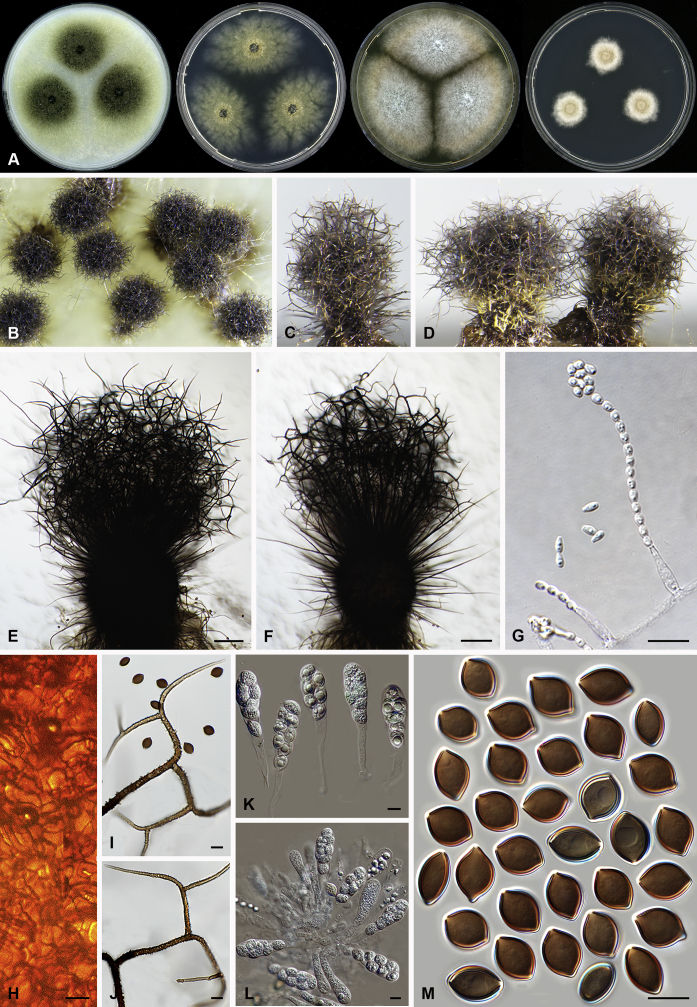
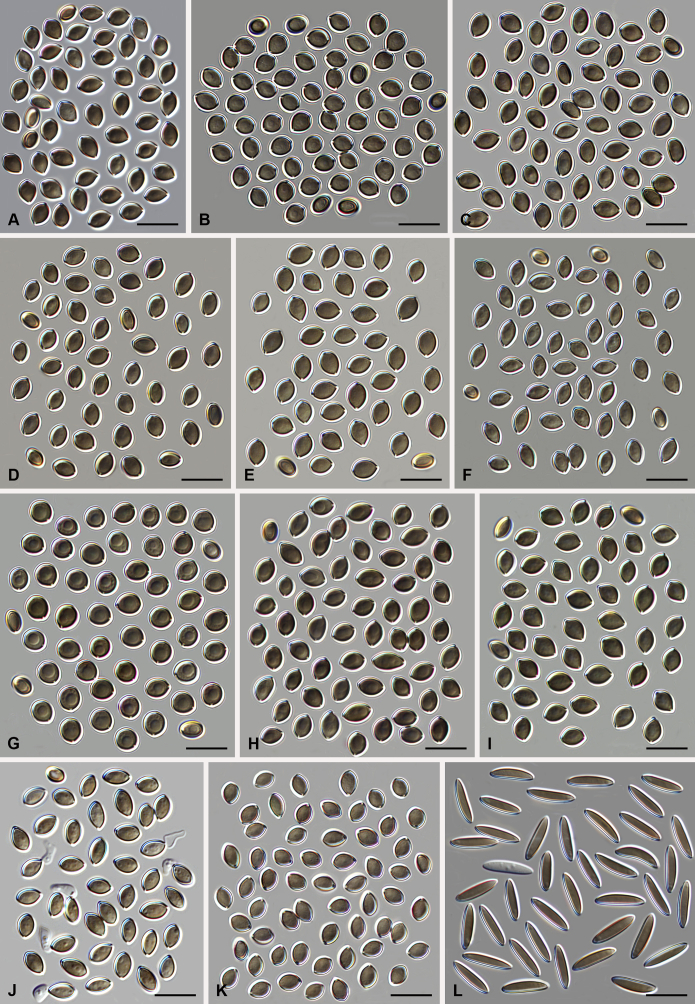
Morphology of the genus Amesia. Ascoma: A. Am. atrobrunnea (CBS 379.66T). B. Am. gelasinospora (CBS 643.83). C. Am. cymbiformis (CBS 176.84). D. Am. nigricolor (CBS 291.83). Ascospores: E. Am. atrobrunnea (CBS 379.66T). F. Am. gelasinospora (CBS 643.83). G. Am. cymbiformis (CBS 176.84). H. Am. nigricolor (CBS 291.83). Scale bars: A–D = 100 μm; E–H = 10 μm.
Etymology: Named after L.M. Marion Ames for his contribution to our knowledge of the taxonomy of the Chaetomiaceae.
Type species: Amesia atrobrunnea (Ames) X. Wei Wang & Samson (= Ch. atrobrunneum)
Ascomata superficial, ostiolate, spherical, ellipsoid or ovate with walls of textura angularis, intricata or epidermoidea in surface view. Terminal hairs straight, flexuous, undulate or spirally coiled. Lateral hairs straight, flexuous, or similar to terminal hairs, but shorter. Asci fasciculate, clavate, broadly clavate or fusiform, stalked, with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores brown at maturity, usually fusiform, elongate ovate to ovate, with an apical or sub-apical germ pore. Asexual morph unknown.
Notes: Several species of Amesia were investigated. These species exhibit a high morphological diversity in both ascomatal hairs and ascospore morphology. An ITS/LSU analysis indicated it to be a monophyletic lineage (de Hoog et al. 2013). Our four-locus phylogeny confirmed the ITS/LSU phylogeny. More investigation with larger sampling is required to find the close relative(s) of this genus.
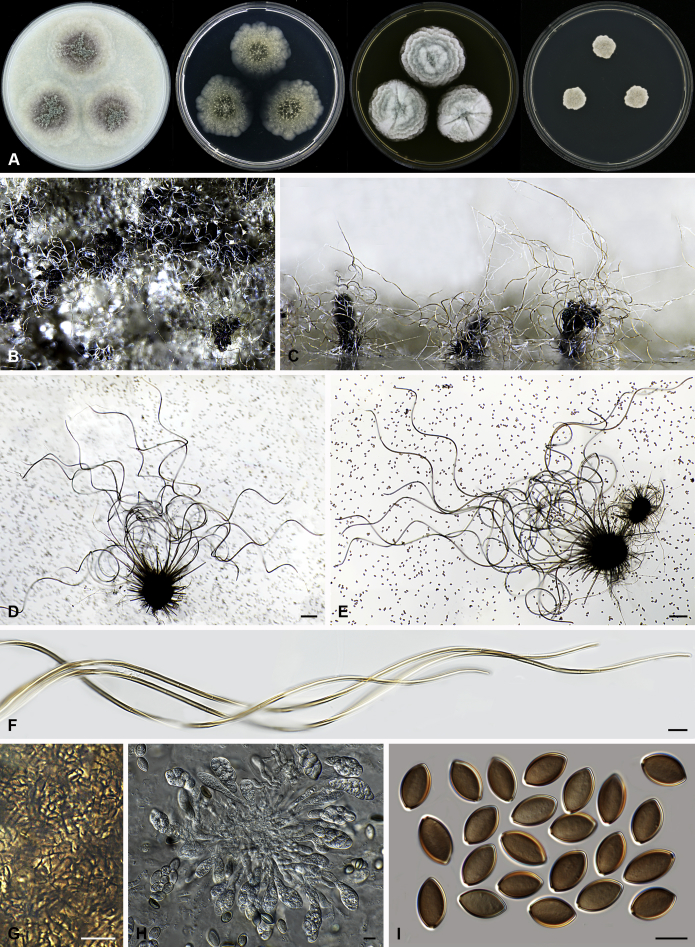
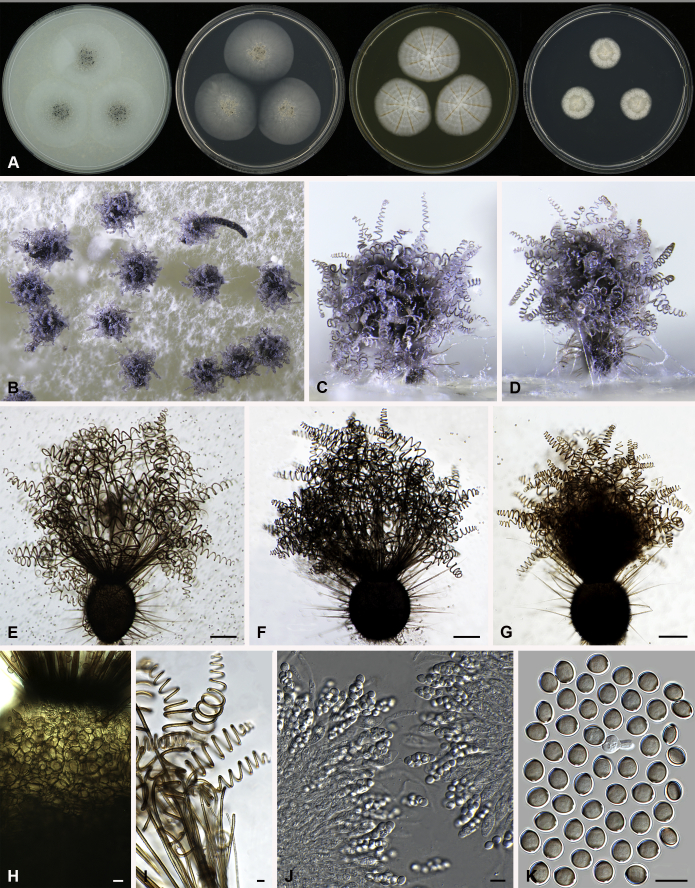
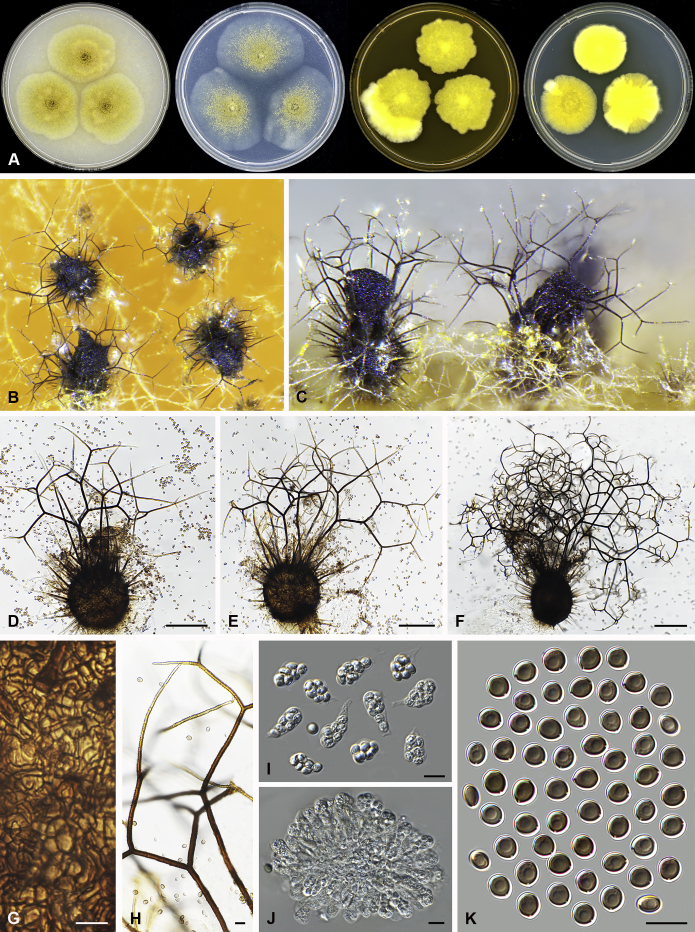
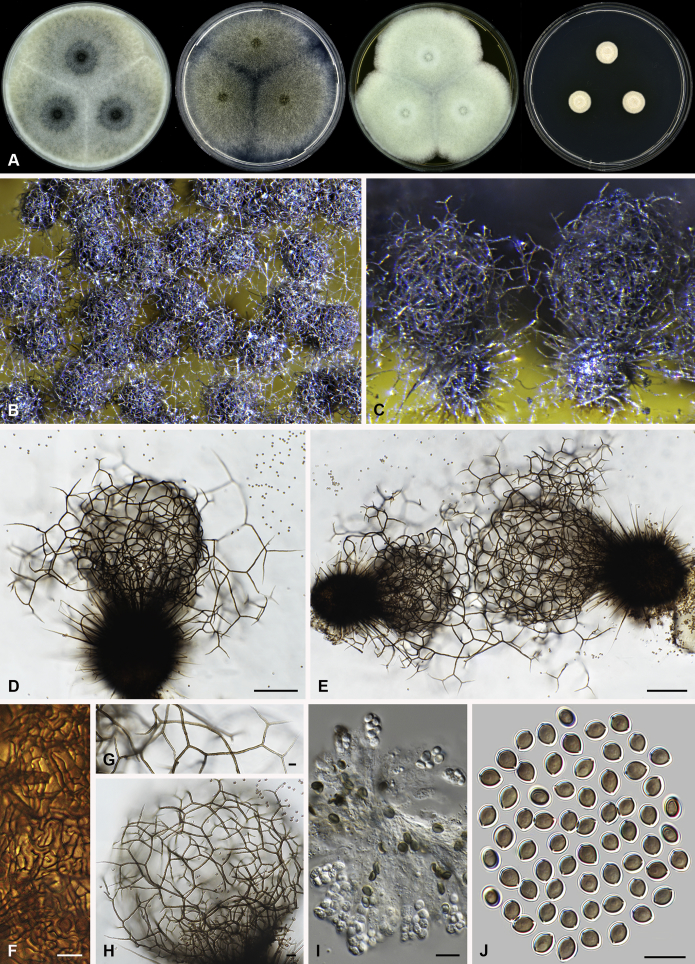
Amesia atrobrunnea (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818832. Fig. 3.
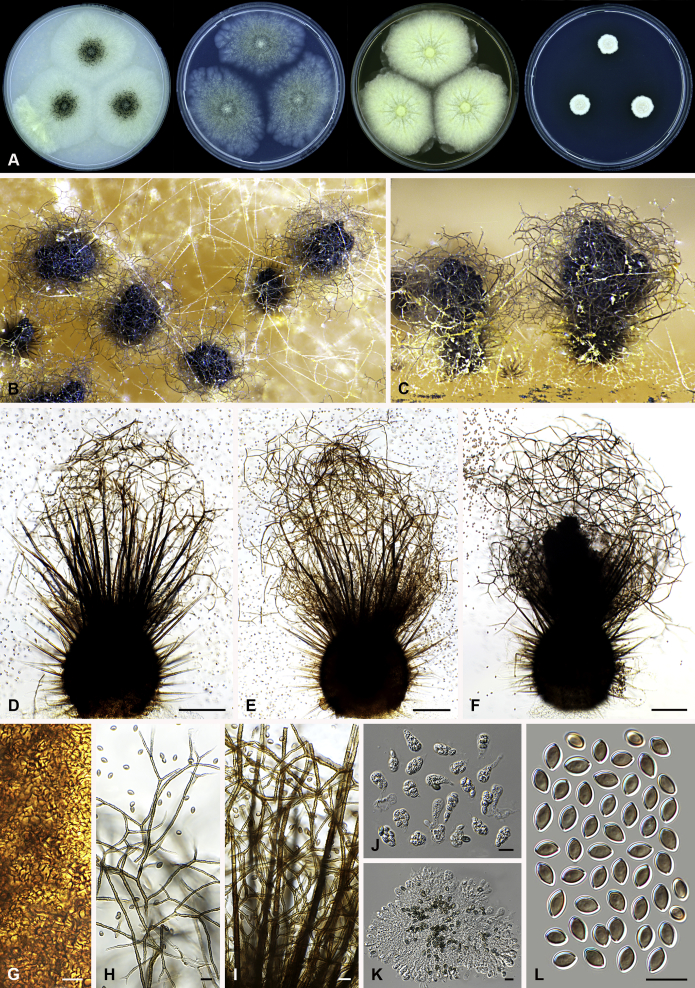
Fig. 3.
Amesia atrobrunnea (CBS 379.66). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Basionym: Chaetomium atrobrunneum Ames, Mycologia 41: 641. 1949.
Ascomata superficial, ostiolate, fuscous black to black in reflected light, subglobose or ovate, 80–160 μm high, 70–140 μm diam. Ascomatal wall brown, textura angularis in surface view. Terminal hairs seta-like or flexuous, sometimes branched, smooth, brown, 2.5–4 μm diam. near the base. Lateral hairs similar and shorter. Asci fasciculate, clavate or fusiform, spore-bearing part 18.5–29 × 8.5–11.5 μm, stalks 9.5–21 μm long, with 8 irregularly arranged ascospores, evanescent. Ascospores olivaceous brown when mature, fusiform or elongate pyriform, (8–)8.5–10.5(–11) × 4.5–5.5(–6) μm, with an apical or slightly subapical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, about 41–47 mm diam in 7 d at 25 °C, fuscous black to black owing to ascomata together with masses of ascoapores maturing within 7 d, without aerial mycelium, with fawn to greyish sepia exudates diffusing into the medium, reverse greyish sepia to black. Colonies on PCA with an entire edge, about 39–45 mm diam in 7 d at 25 °C, translucent, vinaceous buff and floccose due to ascomata mixed with aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA with an entire edge, about 36–42 mm diam in 7 d at 25 °C, with greyish white and floccose mycelium, looser and white to pale olivaceous buff texture in the central part due to ascomata mixed with aerial hyphae, reverse ochraceous. Colonies on DG18 with an entire edge, about 18–24 mm diam in 7 d at 25 °C, sterile, with white and floccose aerial mycelium, without coloured exudates; reverse uncoloured.
Specimens examined: India, isolated from air by Kamal; culture CBS 250.75. Solomon Islands, isolated from mouldy mattress by G.W. Martin and deposited in the CBS collection by H.K. Seth, ex-type culture CBS 379.66.
Notes: Several isolates of this species have been reported to cause human systemic and deep infection (Abbott et al., 1995, de Hoog et al., 2013). Our previous temperature test (Li et al. 2012) demonstrated its potential as an invasive human pathogen: the optimum growth temperature is 30–34 °C, and maximum growth temperature 47 °C.
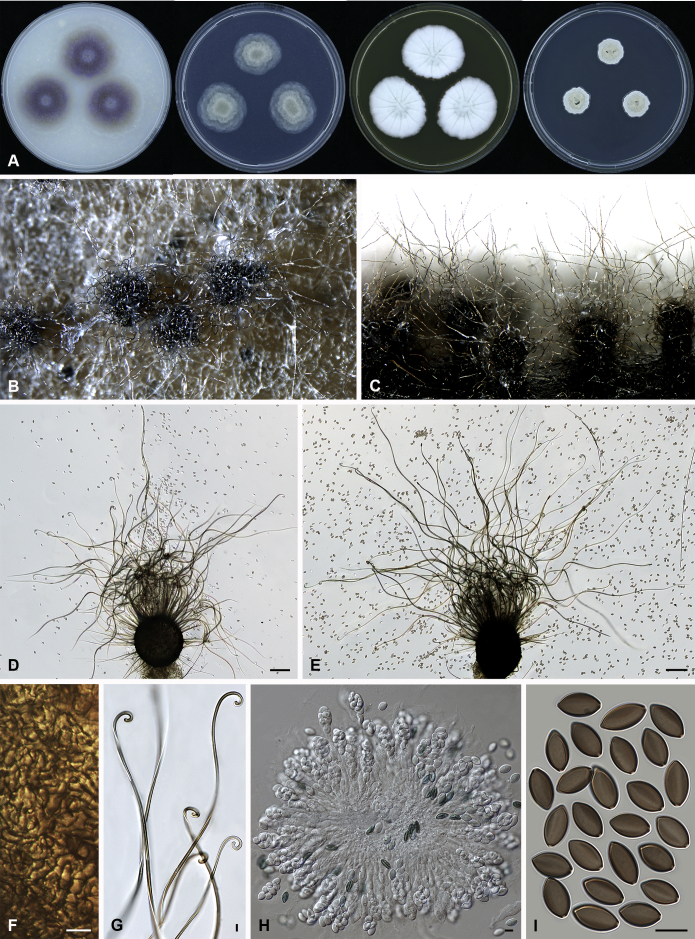
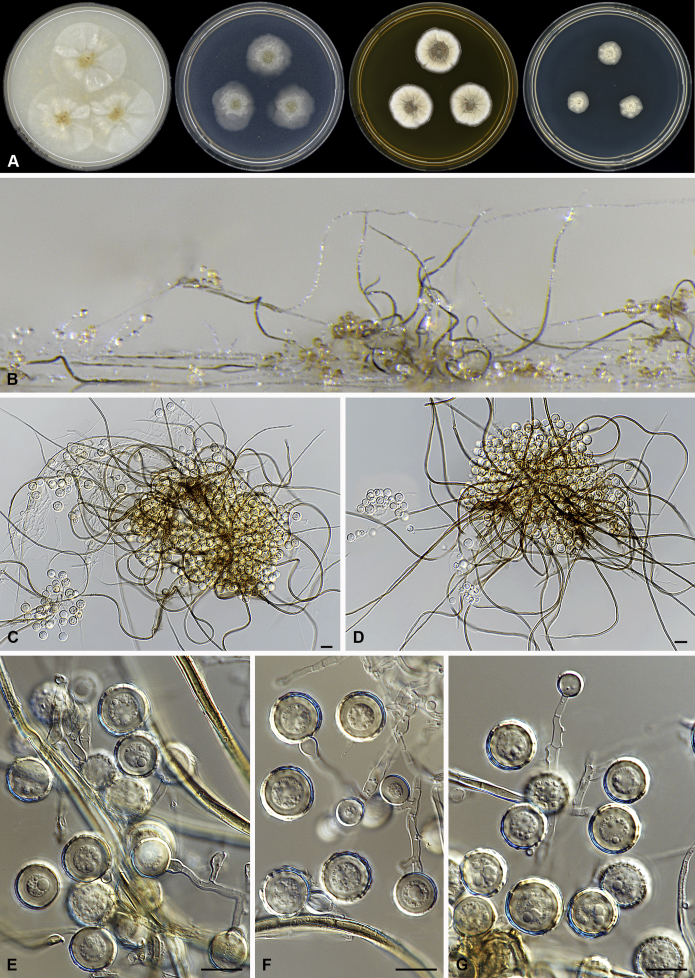
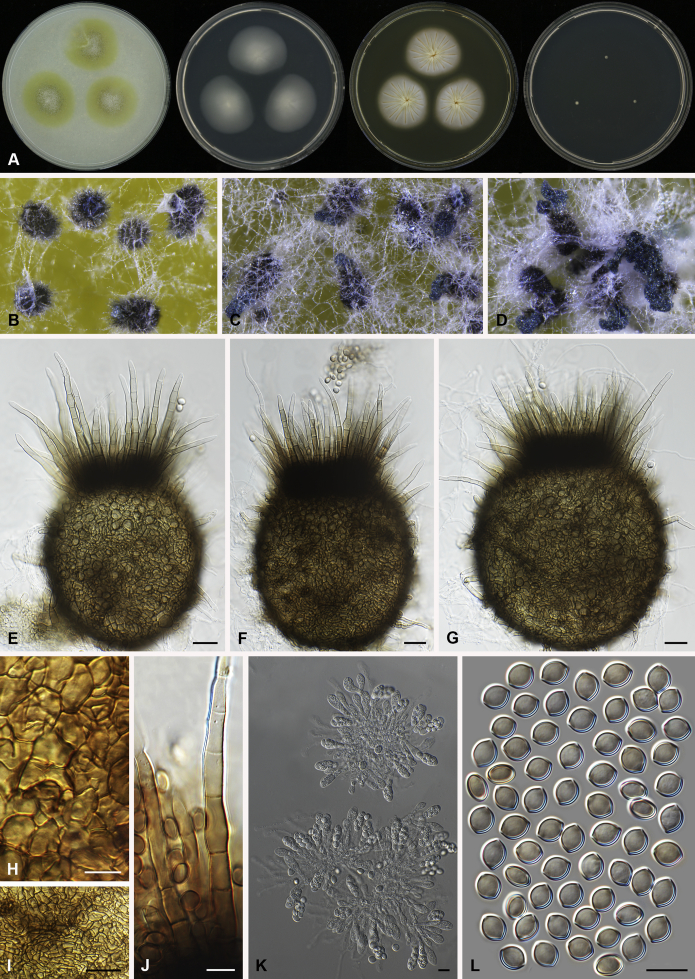
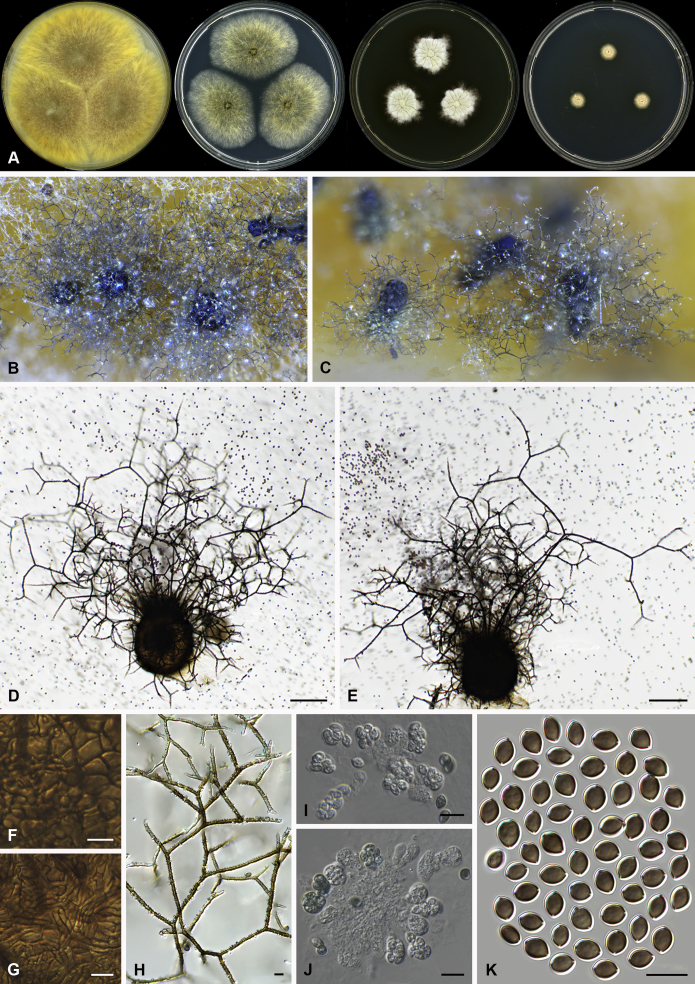
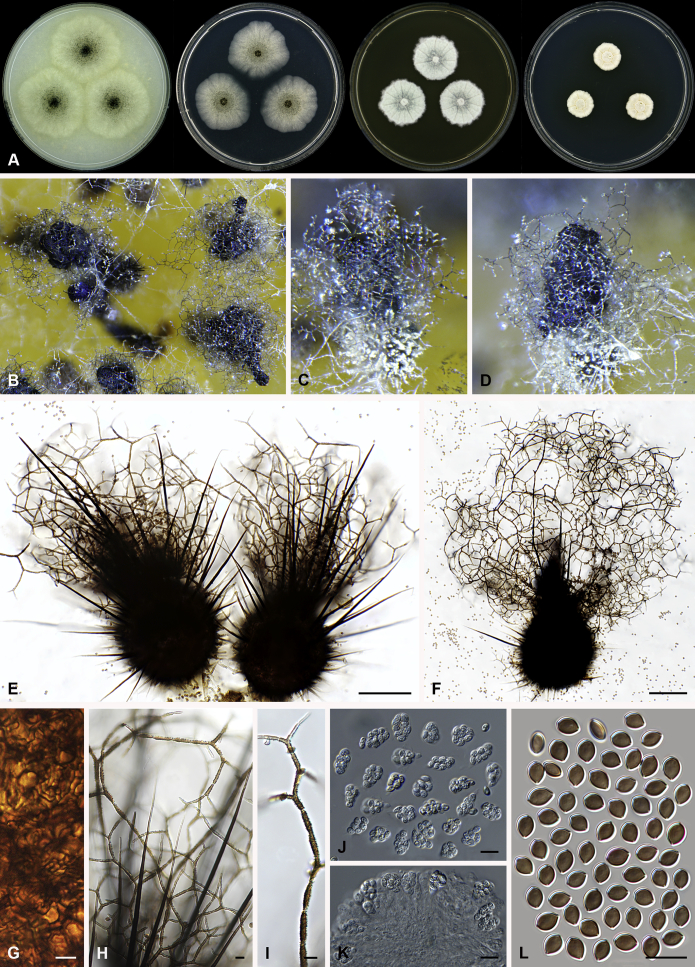
Amesia cymbiformis (Lodha) X. Wei Wang & Samson, comb. nov. MycoBank MB818833. Fig. 4.
Fig. 4.
Amesia cymbiformis (CBS 176.84). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Basionym: Chaetomium cymbiforme Lodha, J. Indian Bot. Soc. 43:127. 1963.
Synonym: Chaetomium serpentinum Ames ex Carter, Canad. J. Bot. 61: 2605. 1983.
Ascomata superficial, ostiolate, greyish sepia when young and olivaceous to dark olivaceous because of the ascospores mass in reflected light, subglobose or ovate, 95–180 μm high, 90–150 μm diam. Ascomatal wall brown, textura angularis in surface view. Terminal hairs flexuous, sometimes recurved, smooth, brown, 2–3.5 μm diam. near the base. Lateral hairs flexuous and shorter. Asci fasciculate, clavate or fusiform, spore-bearing part 16–26 × 10.5–13 μm, stalks 7–15 μm long, with 8 irregularly arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ovate or ellipsoidal, with attenuated ends, (7–)8–9(–9.5) × (5.5–)6–6.5(–7) μm, with an apical or slightly subapical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, about 33–39 mm diam in 7 d at 25 °C, translucent when young, then greenish olivaceous to olivaceous owing to ascomata together with masses of ascospores, without aerial mycelium, with olivaceous buff to pale luteous exudates diffusing into the medium, reverse pale luteous to grey olivaceous. Colonies on PCA with an entire edge, about 31–37 mm diam in 7 d at 25 °C, translucent, with loose and pale smoke grey aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA white with an entire edge, about 34–40 mm diam in 7 d at 25 °C, non-sporulating, with white and floccose aerial hyphae, without coloured exudates, reverse ochraceous. Colonies on DG18 white with an entire edge, about 11–17 mm diam in 7 d at 25 °C, non-sporulating, with white and floccose aerial mycelium, without coloured exudates; reverse uncoloured.
Specimens examined: Solomon Islands, isolated from tent rope, deposited in the CBS collection by J.C. Krug, culture CBS 175.84 (ex-culture of Ch. serpentinum). USA, Atlanta, isolated from case liner, deposited in the CBS collection by J.C. Krug, culture CBS 176.84.
Notes: Amesia cymbiformis is closely related to Am. atrobrunnea (Fig. 1), but can be easily distinguished from Am. atrobrunnea (Fig. 3) by the shape and size of its ascospores (Fig. 4). Isolate CBS 175.84 was originally studied by Ames and named as C. serpentinum without description. Carter (1983) validly published this name after the description of Ch. cymbiforme that was originally isolated from cow dung in india (Lodha 1964). After studying the type specimen of Ch. cymbiforme and the ex-culture of Ch. serpentinum, von Arx et al. (1986) synonymised Ch. cymbiforme with Ch. cymbiforme. Here we followed von Arx et al. (1986) to accept Ch. cymbiforme as the basionym of this species.
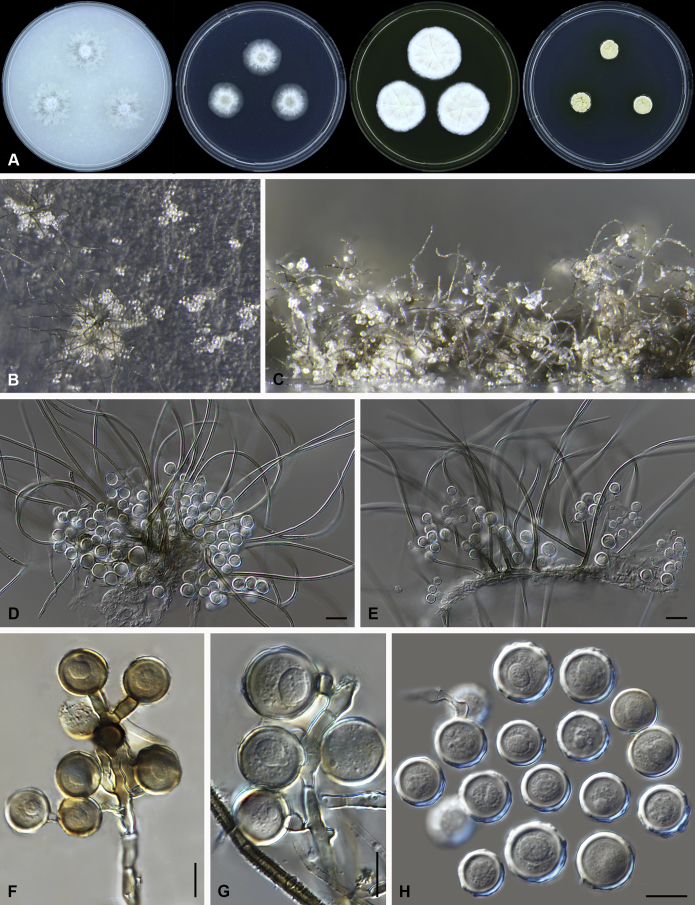
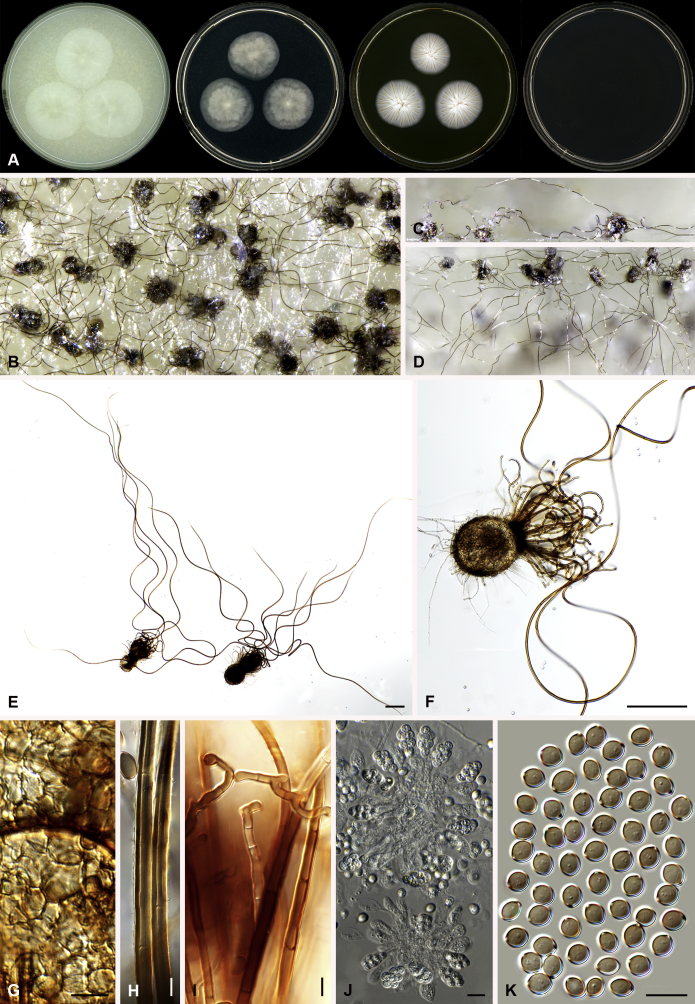
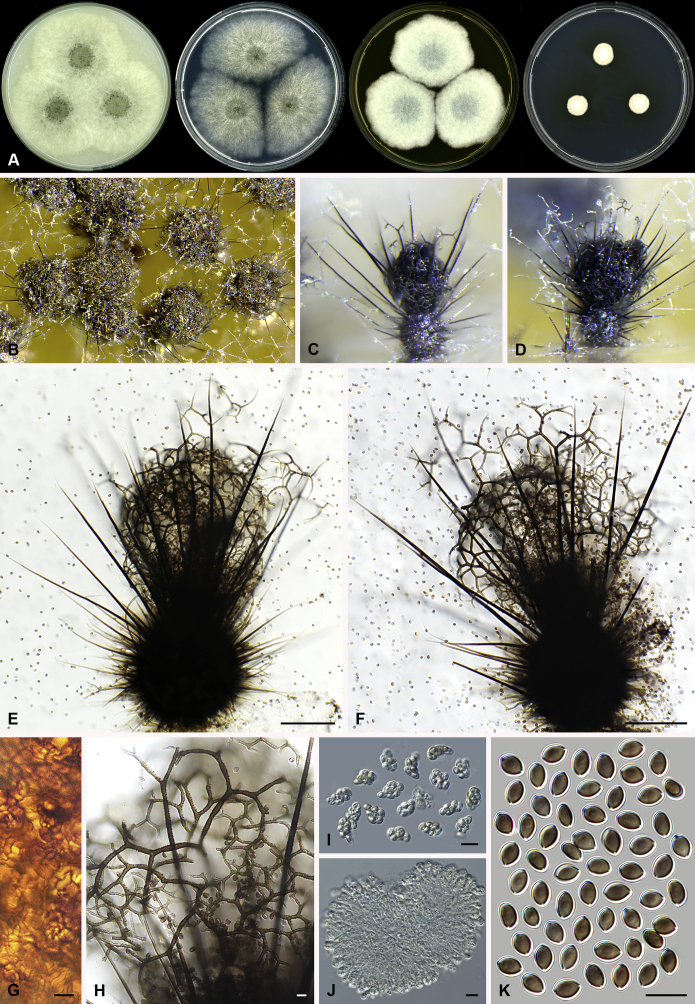
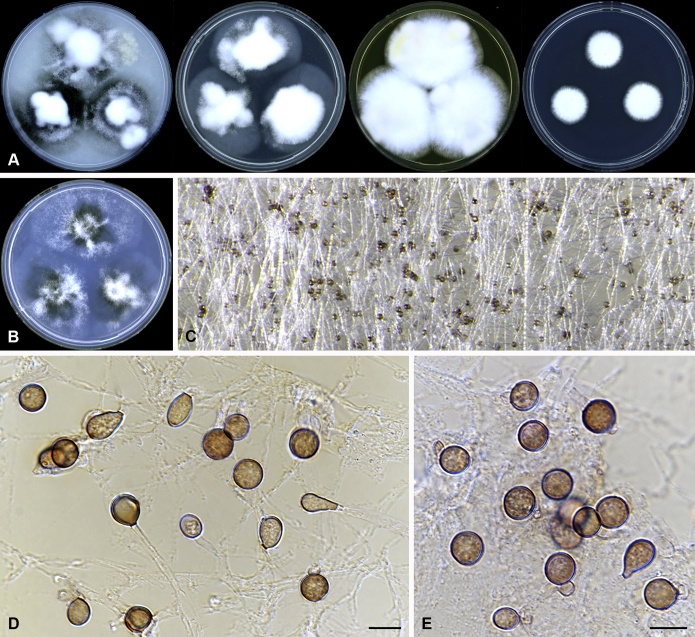
Amesia nigricolor (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818834. Fig. 5.
Fig. 5.
Amesia nigricolor (CBS 291.83). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Basionym: Chaetomium nigricolor Ames, Mycologia 42: 654. 1950.
Synonym: Chaetomium amberpetense Rao & Reddy, Mycopath. Mycol. Appl. 24: 114. 1964.
Ascomata superficial, ostiolate, olivaceous grey in reflected light due to ascomatal hairs, subglobose to ovate, 140–300 μm high, 100–255 μm diam. Ascomatal wall brown, textura intricata or epidermoidea in surface view. Terminal hairs undulate to loosely coiled with erect or flexuous lower part, conspicuously rough (granulate), greyish sepia to brown, septate, 3–4.5 μm diam in the undulate or coiled upper portion. Lateral hairs flexuous, undulate or apically circinate. Asci fasciculate, clavate to fusiform, spore-bearing part 13.5–21 × 7.5–10.5 μm, stalks 6–11.5 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ovate, (5.5–)6–7(–7.5) × 4–5(–5.5) μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge, about 42–48 mm diam in 7 d at 25 °C, with sparse white aerial hyphae, with ochraceous to fulvous exudates diffusing into the medium, reverse pale luteous to amber. Colonies on PCA with entire edge, about 35–41 mm diam in 7 d at 25 °C, with sparse white to pale buff aerial hyphae; reverse uncoloured. Colonies on MEA with entire edge, about 47–53 mm diam in 7 d at 25 °C, with white floccose aerial mycelium, reverse sienna with pale edge. Colony on DG18 with entire edge, about 17–23 mm diam in 7 d at 25 °C, buff with sparse white aerial hyphae, without coloured exudates; reverse uncoloured.
Specimen examined: India, Bihar, isolated from paper, culture CBS 291.83 (ex-type culture of Ch. amberpetense).
Notes: Amesia nigricolor is morphologically similar to members of the genus Ovatospora, especially in ascospore morphology. This species differs in possessing ascospores attenuated at one end and slightly apiculate at the other end. In contrast, the ascospores of the Ovatospora species are attenuated at one and typically round at the other end. A few isolates of Am. nigricolor were isolated from patients, both superficial and deep infections (de Hoog et al. 2013).
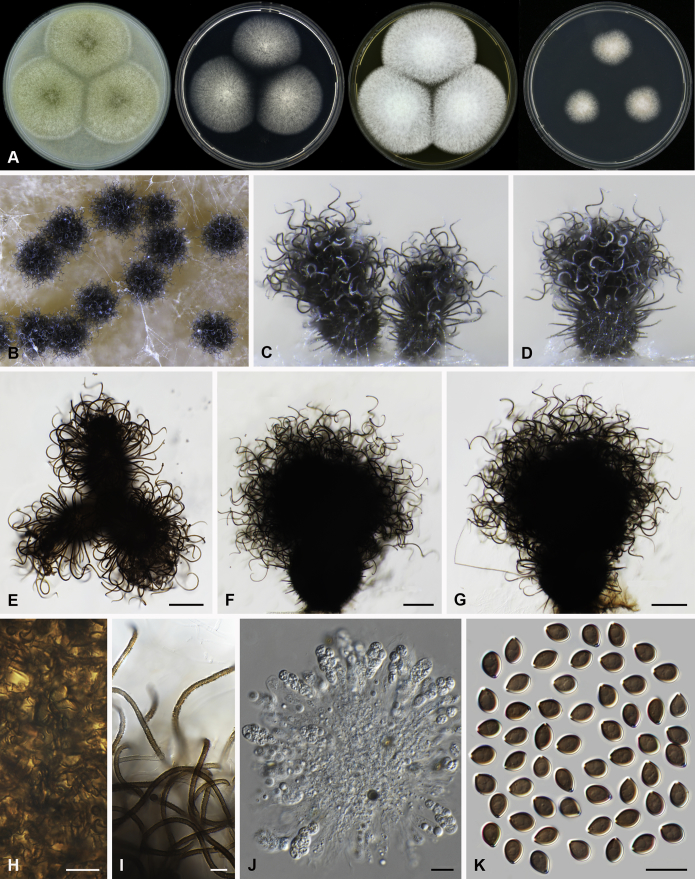
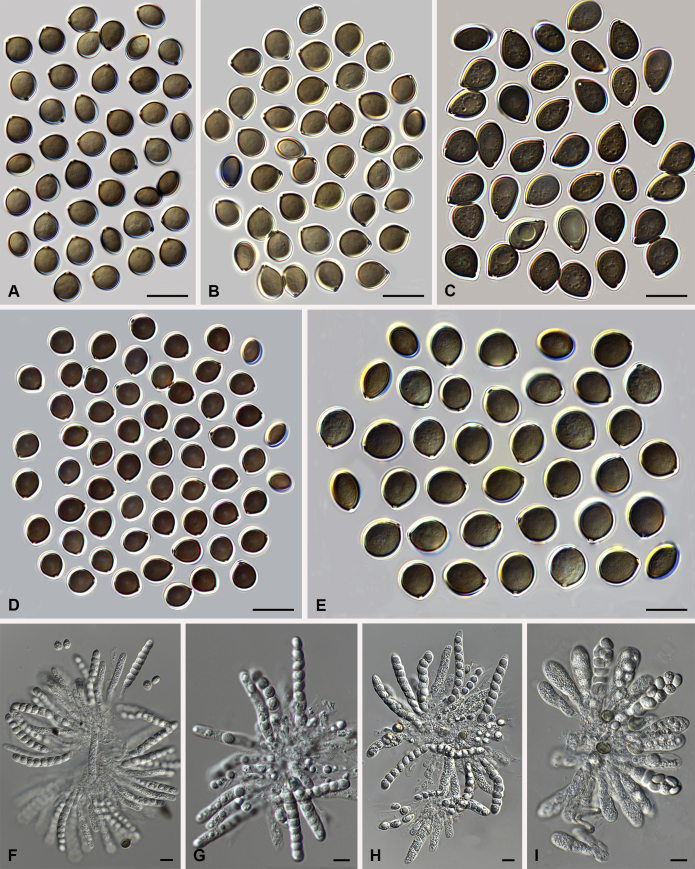
Arcopilus X. Wei Wang, Samson & Crous, gen. nov. MycoBank MB818835. Fig. 6.
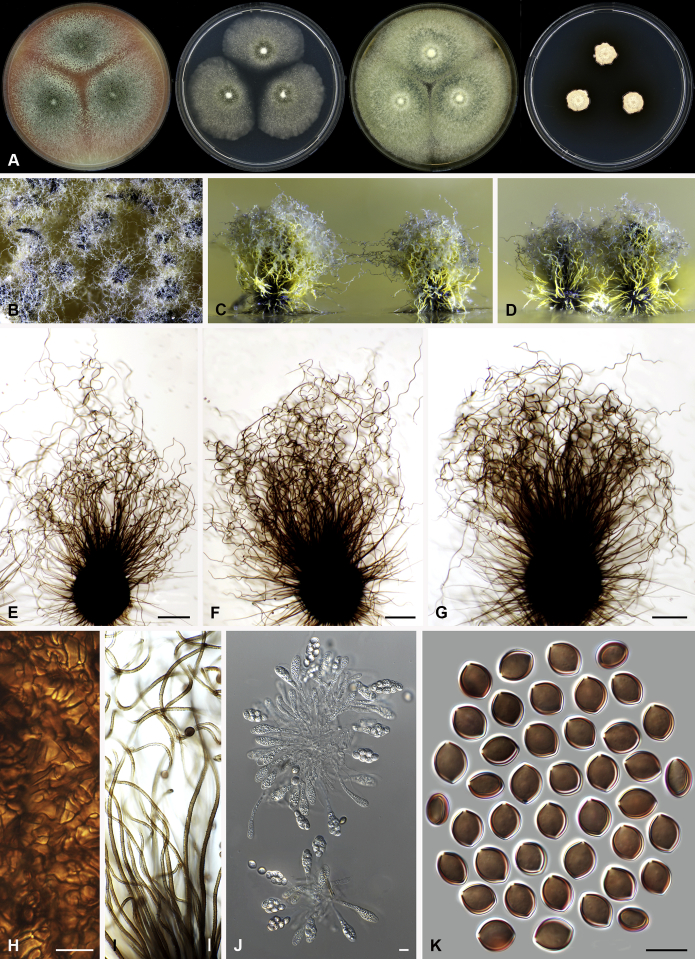
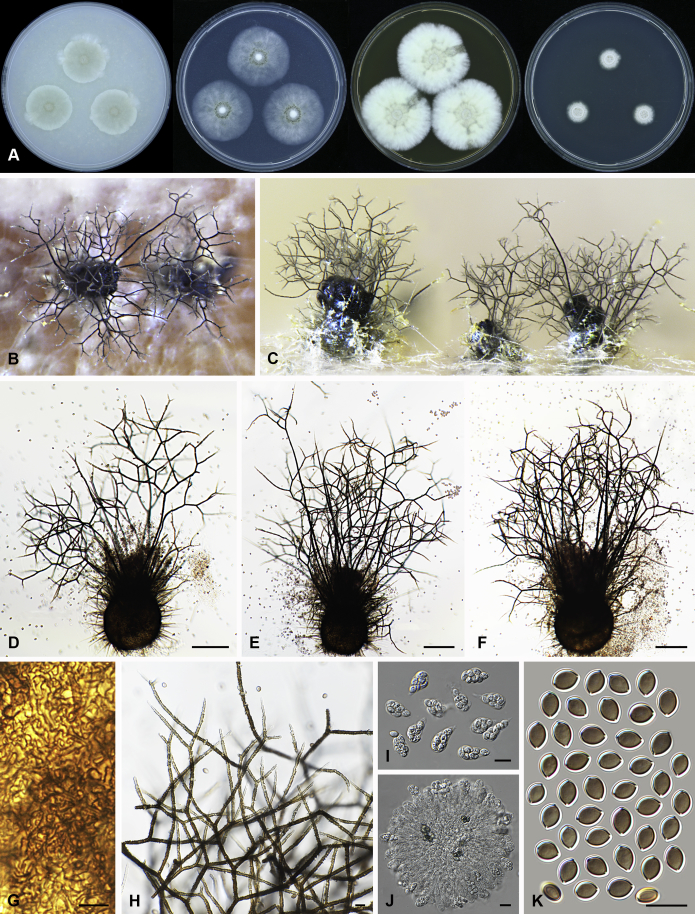
Fig. 6.
Morphological diversity in the genus Arcopilus. Ascoma: A. Ar. cupreus (CBS 560.80). B. Ar. aureus (CBS 153.52). C. Ar. flavigenus (CBS 337.67T). D. Ar. turgidopilosus (CBS 169.52T). Ascospores: E. Ar. cupreus (CBS 560.80). F. Ar. aureus (CBS 153.52). G. Ar. fusiformis (CBS 484.85). H. Ar. flavigenus (CBS 337.67T). I. Ar. turgidopilosus (CBS 169.52T). Scale bars: A–D = 100 μm; E–I = 10 μm.
Etymology: Name refers to the arcuate terminal ascomatal hairs in most of the species in this genus.
Type species: Arcopilus aureus (Chivers) X. Wei Wang & Samson (= Ch. aureum)
Colonies usually with yellow to orange or red to rust exudates. Ascomata superficial, ostiolate, subglobose or ovate with brown walls of textura angularis in surface view. Terminal hairs usually arcuate, with apeces incurved, circinate to coiled. Lateral hairs flexuous or apically incurved. Asci fasciculate, clavate, with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores brown when mature, more or less inequilateral, fusiform, elongate fusiform, navicular, reniform, lunate or limoniform, sometimes bilaterally flattened, with one or two apical germ pores. Asexual morph unknown.
Notes: This genus usually has arcuate ascomatal hairs, and often exhibits a colourful colony due to its ascomata and exudates. Ascospores of the species in this genus are relatively diverse (Fig. 6). Only one indoor species was examined in detail in this study. More morphological research is required to delimit species within the genus.
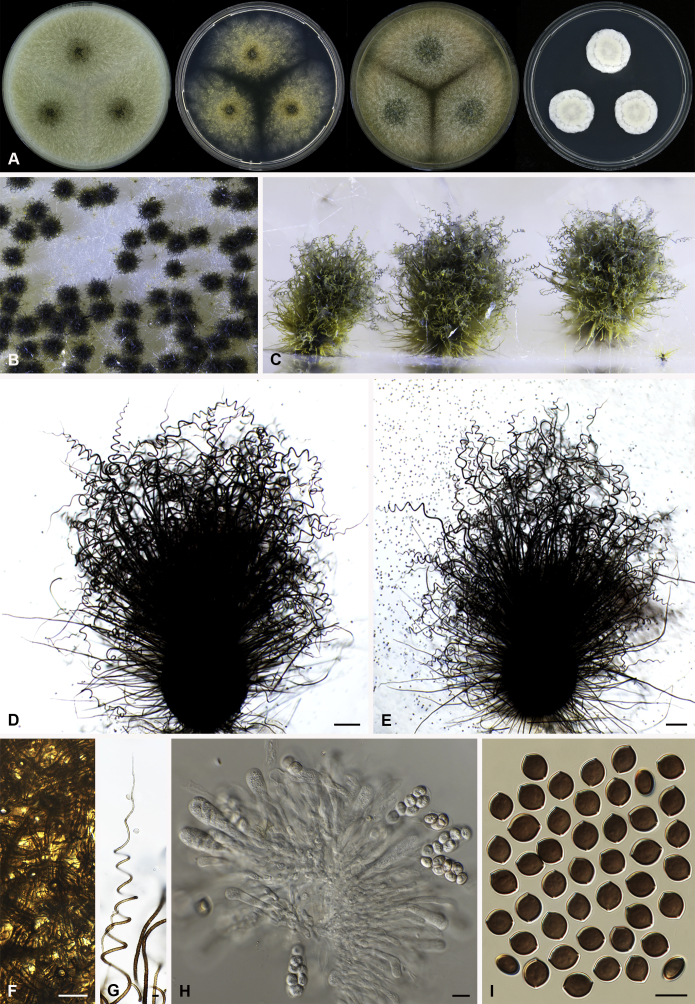
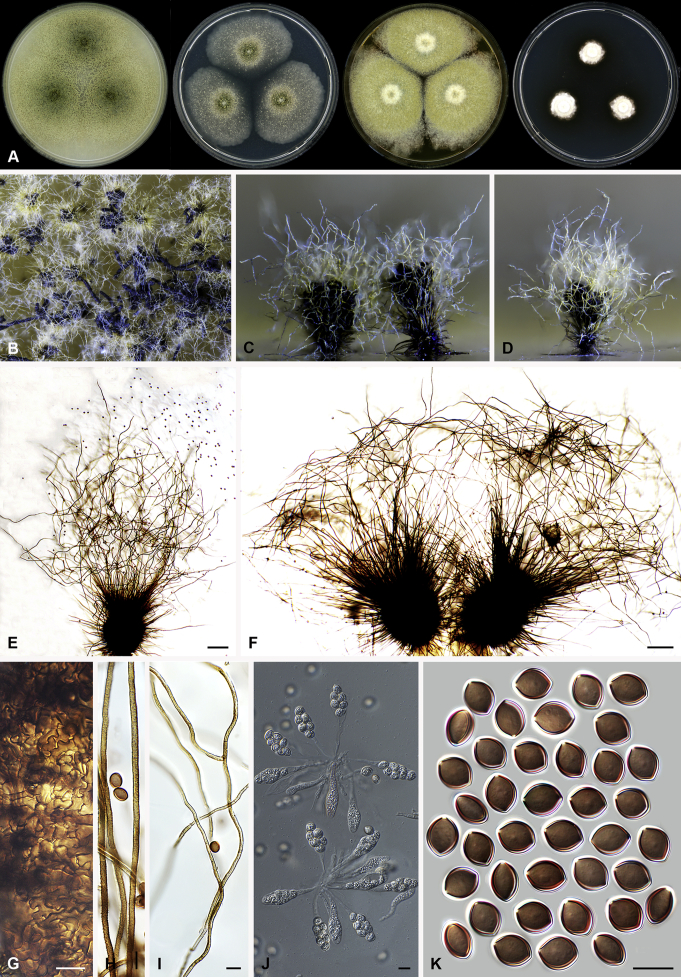
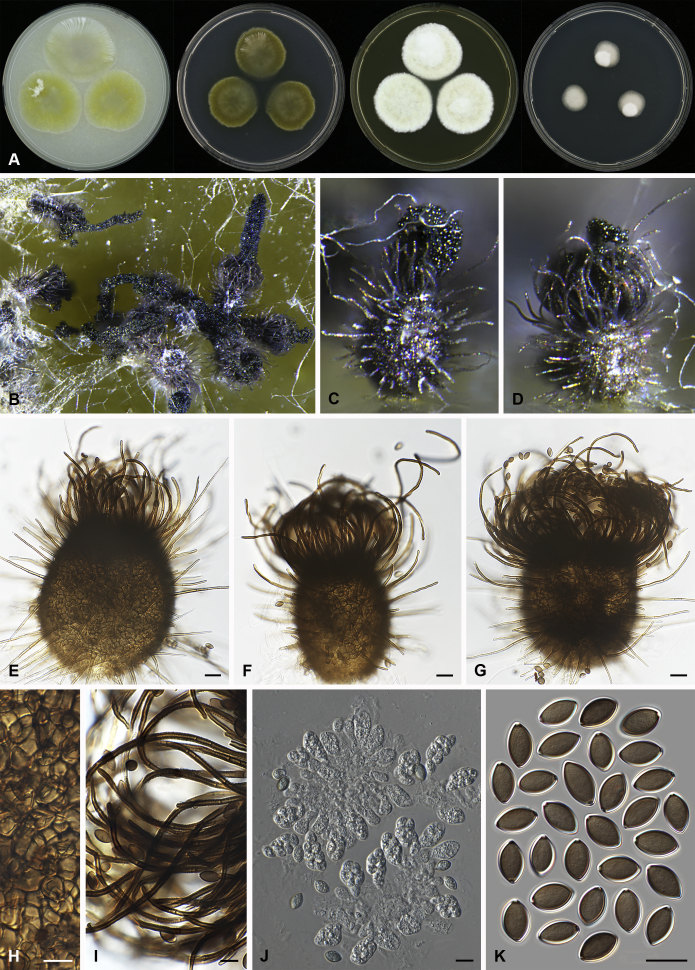
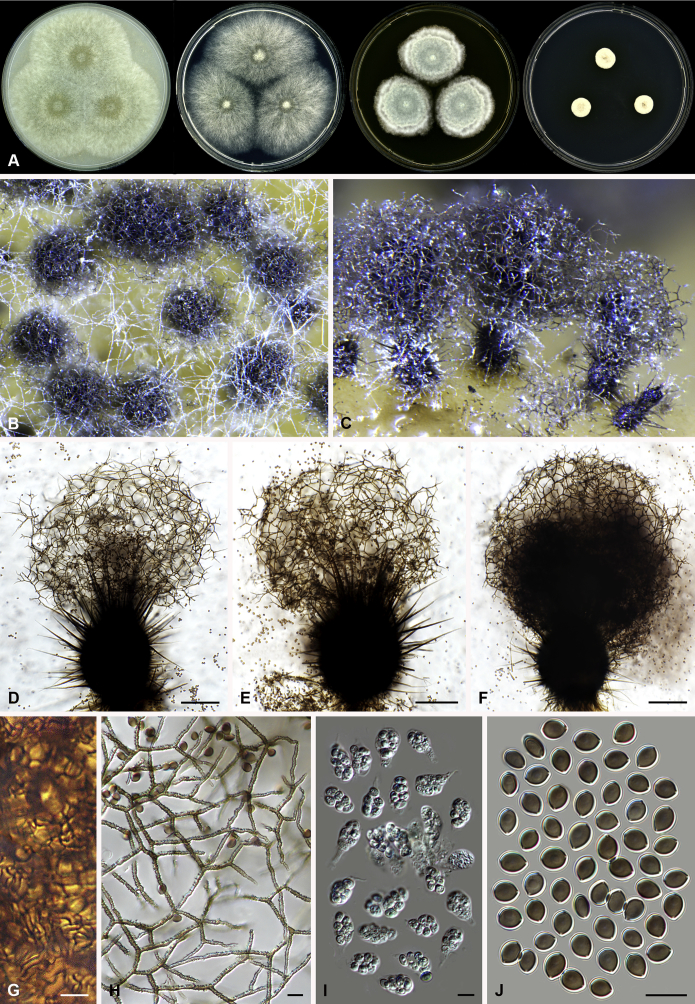
Arcopilus turgidopilosus (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818836. Fig. 7.
Fig. 7.
Arcopilus turgidopilosus (CBS 169.52T). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. An terminal ascomatal hair fading and tapering towards the tips. I. An terminal ascomatal hair with the widest lower middle portion. J. Asci. K. Ascospores. Scale bars: D = 100 μm; E–F = 20 μm; G–K = 10 μm.
Basionym: Chaetomium turgidopilosum Ames, Mycologia 41: 639. 1949.
Ascomata superficial, ostiolate, citrine or greenish olivaceous in reflected light due to ascomatal hairs, then becoming greenish black due to ascospores aggregating on the top, subglobose or ovate, 95–155 μm high, 85–150 μm diam. Ascomatal wall brown, textura angularis, sometimes mixed with hypha-like or amorphous cells in surface view. Terminal hairs arcuate, apically incurved, circinate to coiled, warty, or brown, distinctly septate, 3.5–6.5 μm diam near the base, partly ochraceous to cinnamon with the widest lower middle portion in 7–9 μm diam and a brown to dark brown tip; partly dark brown but fading and tapering towards the tips. Lateral hairs flexuous or recurved, ochraceous, fading and tapering towards the tips. Asci fasciculate, clavate, spore-bearing portion 19–28 × 8.5–12 μm, stalks 8–15 μm long, with 8 ascospores, evanescent. Ascospores dark brown when mature, limoniform, sometimes asymmetrical, bilaterally flattened, (8–)8.5–10.5(–11) × (6.5–)7–8(–8.5) × (5–)5.5–6.5 μm, with apical germ pores at both ends. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge, about 30–36 mm diam in 7 d at 25 °C, with floccose white aerial hyphae at the beginning, with amber to luteous exudates diffusing into the medium, reverse amber to luteous. Colonies on PCA with pale luteous to amber crenated edge, about 25–31 mm diam in 7 d at 25 °C, pale amber with floccose buff aerial hyphae mixed with ascomata, with amber exudates diffusing into the medium; reverse amber. Colonies on MEA with entire edge, about 34–40 mm diam in 7 d at 25 °C, with floccose white aerial mycelium, with red to pale rust exudates diffusing into the medium, reverse rust. Colony growth on DG18 pale rosy buff with entire edge, about 7–13 mm diam in 7 d at 25 °C, without coloured exudates; reverse uncoloured.
Specimen examined: USA, isolated from top of storage tent by G.W. Martin, ex-type culture CBS 169.52.
Notes: The ascospores of Ar. turgidopilosus are sometimes asymmetrical, but less inequilateral than most of the other species known in this genus (Fig. 6). Furthermore, this species is distinct from the other species by its swollen ascomatal hairs (Fig. 7I). Phylogenetic inference places this species in a basal position to the other known species in the genus.
Botryotrichum Sacc. & Marchal, Bull. Soc. R. Bot. Belg. 24(1): 66. 1885.
Synonym: Emilmuelleria Arx, Sydowia 38: 6. 1985.
Type species: Botryotrichum piluliferum Sacc. & Marchal
Notes: Botryotrichum piluliferum was first described as an asexual species based on an isolate from Belgium (Saccardo 1886). Daniels (1961) induced perithecia from soil-buried cellulose films with a culture of B. piluliferum, and named the induced organism as Ch. piluliferum, the sexual morph of B. piluliferum. Chaetomium piluliferum was noted to be closely related to Ch. murorum by ellipsoid ascospores and unbranched ascomatal hairs with circinate tips (Daniels, 1961, von Arx et al., 1986). Our phylogenetic analyses strongly support a monophyletic lineage containing B. piluliferum, two other Botryotrichum species, Ch. murorum and E. spirotricha, the type species of the monotypic genus Emilmuelleria. This lineage is more closely related to the genus Subramaniula than to the other lineages in the Chaetomiaceae. Since their sister relationships received low bootstrap support (MP-BS = 54), we prefer to keep these two clades as two separate genera for now.
Three indoor species of Botryotrichum are described below. More research based on a higher number of strains and species is required to better delimit this genus.
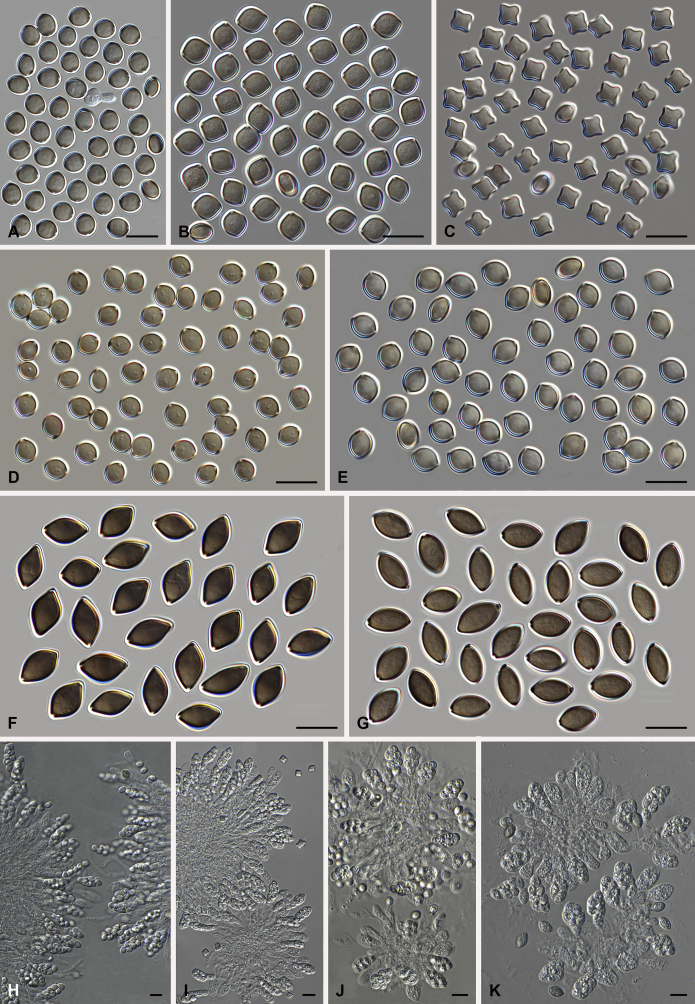
Botryotrichum murorum (Corda) X. Wei Wang & Samson, comb. nov. MycoBank MB818837. Fig. 8, Fig. 9.
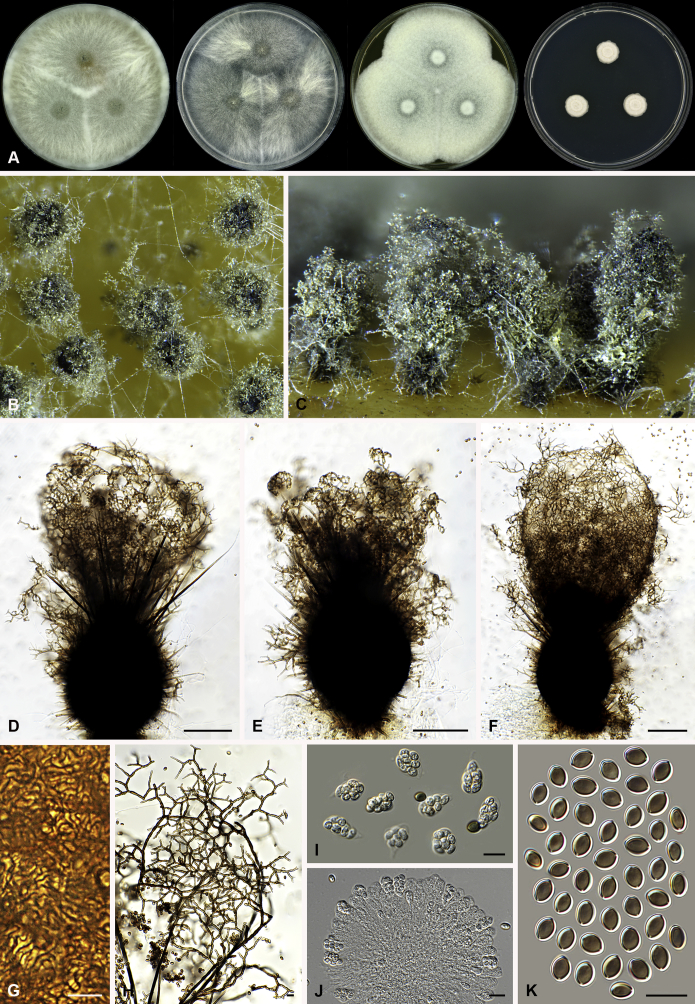
Fig. 8.
Botryotrichum murorum (DTO 324-G9). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Upper part of a terminal ascomatal hair. H. Asci. I. Ascospores. Scale bars: D–E = 100 μm; F–I = 10 μm.
Fig. 9.
Botryotrichum murorum (DTO 333-E6). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Upper part of terminal ascomatal hairs. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D–E = 100 μm; F–I = 10 μm.
Basionym: Chaetomium murorum Corda, Icon. Fung. 1: 24. 1837.
Ascomata superficial, ostiolate, grey olivaceous, olivaceous to brown vinaceous in reflected light due to ascomatal hairs, subglobose or ovate, 160–320 μm high, 150–270 μm diam. Ascomatal wall brown, textura intricata or epidermoidea in surface view. Terminal hairs usually over four times longer than ascoma, flexuous or undulate, often circinate at the apex (DTO 324-G9, Fig. 8), or undulate, usually apically straight, occasionally recurved or circinate at the apex (DTO 333-E6, Fig. 9), olivaceous brown, smooth, 4.5–7.5 μm diam near the base, up to about 3 mm long. Lateral hairs seta-like, shorter. Asci fasciculate, fusiform, sometimes clavate, spore-bearing portion 27–45 × 12.5–19 μm, stalks 12–36 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal-fusiform, (12–)12.5–15(–16.5) × (7–)7.5–8.5 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with a lobate or crenated edge, about 32–39 mm diam in 7 d at 25 °C, with sparse smoke grey aerial hyphae mixed with pale olivaceous grey ascomata, with vinaceous buff to livid purple or livid violet exudates diffusing into the medium, reverse pale purplish grey to fuscous black. Colonies on PCA showing an entire or slightly crenated edge with aerial hyphae olivaceous buff (DTO 324-G9, Fig. 8), or showing a irregularly or radially striated with lobate edge without aerial hyphae (DTO 333-E6, Fig. 9), with a few concentric and lobate rings on it, about 24–34 mm diam in 7 d at 25 °C, without coloured exudates; reverse olivaceous buff to honey. Colonies on MEA with a slightly undulate or lobate edge, about 30–37 mm diam in 7 d at 25 °C, possessing white and floccose with radial furrows (DTO 324-G9, Fig. 8) or pale olivaceous grey to white and floccose mycelium with concentric and floral or irregular rings on it (DTO 333-E6, Fig. 9), non-sporulating, without coloured exudates; reverse uncoloured at the beginning, then ochraceous, umber to cinnamon. Colonies on DG18 with an irregularly crenated edge, about 9–17 mm diam in 7 d at 25 °C, pale buff to buff, wrinkled on the surface, without aerial hyphae, non-sporulating, without coloured exudates; reverse uncoloured to pale luteous.
Specimens examined: China, isolated from air by A.J. Chen, culture DTO 324-G9. Denmark, isolated from ceiling tile by B. Andersen, culture DTO 333-E6 (= IBT 42175).
Notes: Isolates DTO 324-G9 and DTO 333-E6 have different colony morphologies and ascomatal hairs. The ascospores of DTO 324-G9 are also slightly bigger than those of DTO 333-E6. However, the phylogenetic analysis did not show any sequence differences between them, suggesting that the differences mentioned above represent the morphological diversity within the species.
Botryotrichum peruvianum Matsush., Icon. Microfung. Matsush. Lect. (Kobe): 17. 1975. Fig. 10.
Fig. 10.
Botryotrichum peruvianum (CBS 421.93). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Conidia on conidiophores together with setae on SNA, top view. C–D. Conidia on conidiophores together with setae mounted in lactic acid. E–G. Conidiophores with conidia. Scale bars: C–D = 20 μm; E–G = 10 μm.
Sexual morph unknown. Sterile setae often solitary, sometimes clustered with conidiophores, brown, verrucose, erect, flexuous or undulate, unbranched, 2.5–4.5 μm diam near the base, up to 2 200 μm long. Conidiophores descrete or clustered with a tuft of setae, hyaline, occasionally ochraceous, 2–4.5 μm near the base, up to 60 μm long, usually sympodially branched to produce several conidiogenous cells. Conidiogenous cells terminal or intercalary, monoblastic or sympodially polyblastic, cylindrical to degenerated form like a broad denticle, 0–14.5 × 2–3.5 μm, sometimes swollen beneath the conidium. Conidia single, occasionally two to three in chains, globose to subglobose, hyaline when young, then becoming pale luteous to ochraceous, conspicuously roughtened, (10–)12–16(–17.5) μm diam.
Culture characteristics: Colonies on OA with an entire edge, 36–41 mm diam in 7 d at 25 °C, with sparse white aerial hyphae, and then becoming ochraceous to pale mouse grey because of the formation of groups of conidia and setae, without coloured exudates; reverse uncoloured. Colonies on PCA with an undulate to lobate edge, about 22–28 mm diam in 7 d at 25 °C, with sparse white aerial hyphae, pale luteous in the centre, without coloured exudates; reverse honey in the centre. Colonies on MEA with a slightly lobate edge, about 21–28 mm diam in 7 d at 25 °C, with floccose, buff to pale ochraceous aerial hyphae, greyish sepia in the centre; without coloured exudates; reverse ochraceous to umber. Colonies on DG18 buff to honey, with a slightly crenated edge, about 9–15 mm diam in 7 d at 25 °C, slightly winkled without aerial hyphae; without coloured exudates; reverse buff.
Specimen examined: Cuba, isolated from air by R. Castañeda, culture CBS 421.93.
Note: This species can be distinguished from B. piluliferum by conspicuously roughened and pigmented conidia and by longer setae.
Botryotrichum piluliferum Sacc. & Marchal, Bull. Soc. R. Bot. Belg. 24(1): 66. 1885. Fig. 11.
Fig. 11.
Botryotrichum piluliferum (DTO 254-B8). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Conidia on conidiophores together with setae on SNA, top view; C. Conidia on conidiophores together with setae on SNA, side view. D–E. Conidia on conidiophores together with setae mounted in lactic acid. F–G. Conidiophores with conidia. H. Conidia. Scale bars: D–E = 20 μm; F–H = 10 μm.
Synonym: Chaetomium piluliferum Daniels, Trans. Br. Mycol. Soc. 44: 84. 1961.
Sexual morph absence in the examined specimens. Sterile setae solitary or in group from the hyaline hypha, brown, verrucose, erect or flexuous, unbranched or branched near the base, 3.5–7.5 μm diam near the base, usually less than 300 μm long, occasionally up to 690 μm long. Conidiophores usually produced together with a tuft of setae, or descreate, hyaline, occasionally ochraceous, 3–5 μm near the base, up to 40 μm long, usually sympodially branched to produce several conidiogenous cells. Conidiogenous cells terminal or intercalary, monoblastic or sympodially polyblastic, cylindrical to broad denticle, more or less constricted at the base, 0–13 × 2–4.5 μm. Conidia usually single, globose to subglobose, hyaline, occasionally ochraceous to umber, smooth to slightly roughtened, (9–)11–17.5(–18.5) μm diam.
Culture characteristics: Colonies on OA with an irregularly fimbriate edge, 22–28 mm diam in 7 d at 25 °C, with white and sparsely floccose aerial hyphae and then partially becoming pale mouse grey because of the formation of groups of setae, without coloured exudates; reverse uncoloured. Colonies on PCA with entire or slightly undulate edge, about 18–24 mm diam in 7 d at 25 °C, with white to buff, floccose aerial hyphae, without coloured exudates; reverse honey to pale luteous. Colonies on MEA with an entire edge, about 25–31 mm diam in 7 d at 25 °C, with floccose, white to rosy buff aerial hyphae; without coloured exudates; reverse ochraceous. Colonies on DG18 buff to honey, with an entire or lobate edge, about 7–13 mm diam in 7 d at 25 °C, winkled without aerial hyphae; without coloured exudates; reverse luteous.
Specimens examined: Netherlands, isolated from a wall, cultures DTO 194-F7, DTO 254-B8, DTO 254-B9.
Notes: The indoor isolates match the original description of B. piluliferum (Saccardo 1886). According to the observation of Daniels (1961), aside from the ascomata, the asexual morph of Ch. piluliferum produced two different types of asexual structures: one (text and fig. 1 in Daniels 1961) was similar to the description above, another was acremonium-like with hyaline conidia forming in chains on simple and hyaline phialides (text and fig. 2 in Daniels 1961). In our study, neither ascomata nor an acremonium-like morph was observed in the cultures of the indoor isolates.
Chaetomium Kunze, Mykol. Hefte 1: 16. 1817.
Ascomata globose, ellipsoid to ovate or obovate, ostiolate or non-ostiolate in a few species, with walls usually composed of textura intricata or epidermoidea in surface view, or of textura angularis in a few species. Ascomatal hairs hypha-like, flexuous, undulate, coiled to simply or dichotomously branched, with verrucose surface, or smooth in a few species. Asci clavate or fusiform with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores limoniform to globose, or irregular in a few species, bilaterally flattened, usually more than 7 μm in length. Asexual morphs, if present, acremonium-like.
Type species: Chaetomium globosum Kunze.
Notes: Since Saccardo (1882) separated Chaetomidium from Chaetomium as a distinct genus, Chaetomium had been defined to be a genus possessing typical ostiolate ascomata for over 100 years. Robust phylogenetic evidence (Wang et al. 2016) indicated that three species of Chaetomidium, including its type species Ch. fimeti, clustered in the Chaetomium globosum species complex. The data generated in this study further proved that the traditional morphologically-defined Chaetomium (Ames, 1963, von Arx et al., 1986) has been divided into several different lineages which are intermingled with several other genera in the Chaetomiaceae. On the other hand, the monophyly of the Chaetomium globosum species complex was confirmed by the phylogenetic analysis based on the expanded dataset in this study (Fig. 1), thus the genus Chaetomium is restricted to the Chaetomium globosum species complex.
Six indoor species were recognised in this genus.
Chaetomium cervicicola X. Wei Wang et al., Persoonia 36: 93. 2016.
Culture sterile.
Specimen examined: Mexico, isolated from dust, culture DTO 318-G6.
Notes: This isolate phylogenetically clustered in the Ch. cervicicola clade. The ex-type culture CBS 128492 was also sterile. See phylogenetic description in Wang et al. (2016).
Chaetomium coarctatum Sergejeva, Not. Syst. sect. Crypt. Inst. Bot. Acad. Sci. U.S.S.R. 14: 146. 1961. Fig. 12.
Fig. 12.
Chaetomium coactatum (DTO 324-H2). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Basal parts of terminal ascomatal hairs. J. Upper parts of terminal ascomatal hairs. K. Asci. L. Ascospores. Scale bars: E–G = 100 μm; H–L = 10 μm.
Ascomata superficial, ostiolate, greyish white to olivaceous buff in reflected light owing to ascomatal hairs, subglobose, 320–480 μm high, 250–390 μm diam. Ascomatal wall brown, textura epidermoidea or intricata in surface view. Terminal hairs conspicuously rough, brown, undulate, 3–4 μm near the base and tapering towards the tips. Lateral hairs erect or flexuous, tapering towards the tips. Asci fasciculate, fusiform or clavate, spore-bearing part 31–43 × 14–21 μm, stalks 20–38 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, broad limoniform to nearly globose, biapiculate, bilaterally flattened, (9–)10–12 × 9.5–11(–11.5) × (6–)6.5–7.5(–8) μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, spreading rapidly, over 70 mm diam in 7 d at 25 °C, with sparse white to buff aerial hyphae and pale greenish olivaceous to greenish olivaceous or citrine exudates diffusing into the medium; reverse ochraceous to citrine. Colonies on PCA honey to yellow with an irregular, fimbriate or rhizoid edge, about 42–48 mm diam in 7 d at 25 °C, with spare and pale luteous aerial hyphae, with amber exudates diffusing into the medium; reverse amber to luteous. Colonies on MEA buff with an entire edge, spreading rapidly, over 70 mm diam in 7 d at 25 °C, with floccose, greyish white aerial hyphae; reverse pale luteous to luteous owing to exudates diffusing into the medium. Colonies on DG18 buff or honey, with a thinner and irregular lobate or petaloid perisphere, about 17–23 mm diam in 7 d at 25 °C, with sparse white aerial hyphae; reverse scarlet owing to exudates diffusing into the medium.
Specimen examined: China, Beijing, isolated from air by A.J. Chen, culture DTO 324-H2.
Note: The morphology of the indoor isolates studied here fits with that of the ex-type culture, CBS 162.62 (fig. 8 in Wang et al. 2016), indicating that this species has a relatively stable morphology.
Chaetomium cochliodes Palliser, North Amer. Flora 3:61. 1910. Fig. 13.
Fig. 13.
Chaetomium cochliodes (DTO 318-I1). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Terminal ascomatal hairs. H. Asci. I. Ascospores. Scale bars: D–E = 100 μm; F–I = 10 μm.
Ascomata superficial, ostiolate, greenish olivaceous to dark citrine in reflected light owing to ascomatal hairs, ellipsoid or subglobose, 200–440 μm high, 140–420 μm diam. Ascomatal wall brown, textura intricata in surface view. Terminal hairs conspicuously rough, dark brown, erect in the lower part, 3.5–6 μm near the base, tapering and fading towards the tips, spirally coiled in the upper part, usually with coils regularly tapering in diameter towards the tips, occasionally with coiled branches. Lateral hairs brown, flexuous, undulate or loosely coiled, tapering and fading towards the tips. Asci fasciculate, fusiform or clavate, spore-bearing part 30–45 × 13.5–21 μm, stalks 28–56 μm long, with 8 biseriate ascospores, evanescent. Ascospores brown when mature, limoniform, usually biapiculate at both ends, bilaterally flattened, (8.5–)9–10.5(–11) × (7–)7.5–9(–9.5) × 5.5–6.5 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge and spreading rapidly, over 70 mm diam in 7 d at 25 °C, usually with sparse buff and floccose aerial hyphae, then without aerial hyphae when ascomata manure, with pale luteous or pale amber to citrine exudates diffusing into the medium, reverse pale amber to pale citrine. Colonies on PCA with a fimbriate to rhizoid edge, about 52–58 mm diam in 7 d at 25 °C, appearing olivaceous buff to greenish olivaceous texture owing to ascomata mixed with sparse aerial hyphae, with pale greenish olivaceous to pale luteous exudates diffusing into the medium; reverse honey to olivaceous buff. Colonies on MEA with an entire edge and spreading rapidly, over 70 mm diam in 7 d at 25 °C, with sparse white or greyish white and floccose aerial hyphae, with citrine exudates diffusing into the medium, reverse sienna with pale perisphere. Colony growth on DG18 with a slightly undulate edge, about 19–25 mm diam in 7 d at 25 °C, buffish white and floccose with white edge, without coloured exudates; reverse uncoloured.
Specimens examined: Netherlands, Maastricht, isolated from air, culture DTO 013-C2; Eindhoven, isolated from air, culture DTO 089-E2. South Africa, isolated from dust, cultures DTO 319-B5 and DTO 319-B6. USA, isolated from dust, cultures DTO 318-H2, DTO 318-H4, DTO 318-H5, DTO 318-H7, DTO 318-H8, DTO 318-I1, 318-I3, DTO 318-I5, DTO 318-I6, DTO 318-I8, DTO 318-I9 and DTO 319-A1. The description above is based on the isolate DTO 318-I1.
Notes: This is a common indoor species, especially in the USA. The indoor isolates showed consistent morphology, fitting with that of Ch. cochliodes (ex-epitype CBS 155.52, fig. 9 in Wang et al. 2016).
Chaetomium elatum Kunze, Deutsche Schwämme 8: 3, No. 184. 1818. Fig. 14, Fig. 15.
Fig. 14.
Chaetomium elatum (DTO 319-B3). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Asexual morph (Conidiophores and conidia). H. Structure of ascomatal wall in surface view. I–J. Upper part of terminal ascomatal hairs. K–L. Asci. M. Ascospores. Scale bars: E–F = 100 μm; G–M = 10 μm.
Fig. 15.
Chaetomium elatum (DTO 318-H9). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Asexual morph (Conidiophores and conidia). G. Structure of ascomatal wall in surface view. H. Upper part of terminal ascomatal hairs. I–J. Asci. K. Ascospores. Scale bars: D–E = 100 μm; F–K = 10 μm.
Ascomata superficial, ostiolate often covered by sparse white or buff aerial hyphae, grey olivaceous to isabelline in reflected light, globose or obovate, 280–440 μm high, 255–380 μm diam. Ascomatal wall brown, composed of hypha-like or amorphous cells, textura intricata or textura epidermoidea in surface view. Terminal hairs verrucose or warty, dark brown, tapering and fading towards the tips, erect or flexuous in the lower part, 3–5 μm diam near the base, repeatedly and dichotomously branched at right to nearly straight angles in the upper part, with erect to flexuous (DTO 319-B3) or undulate (DTO 318-H9) terminal branches taping and fading towards the tips. Lateral hairs brown, erect or flexuous, occasionally undulate, tapering towards the tips. Asci fasciculate, clavate or fusiform, spore-bearing part 36–49 × 12.5–16.5 μm, stalks 24–55 μm long, with 8 biseriate ascospores, evanescent. Ascospores brown when mature, limoniform, biapiculate or umbonate, bilaterally flattened, (10–)11.5–13.5(–16) × (8–)8.5–10(–11) × (6.5–)7–8 μm, with an apical germ pore. Asexual morph acremonium-like. Conidiophores phialidic, formed laterally from aerial hyphae, simple, 6–24.5 μm long, 1.5–3.5 μm diam at the base. Conidia formed basipetally in chains, hyaline, aseptate, smooth, ovate, often with a truncated base and a rounded apex, (2–)2.5–4(–5.5) × 1.5–2(–2.5) μm.
Culture characteristics: Colonies on OA with entire edge and spreading rapidly, over 70 mm diam in 7 d at 25 °C, with sparse, honey to greenish olivaceous aerial hyphae (DTO 319-B3), or forming sparse or radially sectorial, white to buff and floccose aerial hyphae (DTO 318-H9), without coloured exudates when young, or with greyish yellow-green, olivaceous buff to honey exudates diffusing into the medium, reverse honey to greenish olivaceous. Colonies on PCA pale luteous to luteous, with irregularly deep lobate edge, about 52–61 mm diam in 7 d at 25 °C, aerial hyphae sparse and honey, with luteous exudates diffusing into the medium; reverse pale luteous to amber. Colonies on MEA forming pale honey, citrine green to citrine and floccose mycelium with entire edge, about 58–66 mm diam in 7 d at 25 °C, reverse citrine green to citrine. Colony growth on DG18 forming white to buff aerial hyphae, with slightly lobate or rhizoid edge, 18–23 mm diam in 7 d at 25 °C, non-sporulating, without coloured exudates; reverse buff.
Specimens examined: Australia, isolated from dust, culture DTO 319-B3. Denmark, isolated from cardboard by B. Andersen, neotype designated here, herbarium CBS H-22851, MBT 373430, culture ex-neotype CBS 142034 = DTO 333-E9 (= IBT 42179); from dust, culture DTO 333-E5 (= IBT 41944); from gypsum, culture DTO 333-F8 (= IBT 42329). USA, isolated from dust, cultures DTO 318-G7; DTO 318-H9.
Notes: Chaetomium elatum was originally described based on an isolate collected from dead leaves in Germany. Our attempt to find the holotype of Ch. elatum housed in B (Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universität Berlin) was unsuccessful because the ascomycete collection was partly destroyed by a fire in 1943, in which the holotype of Ch. globosum might have been included. Therefore, a dried culture, CBS H-22851 from DTO 333-E9, is designated here as neotype of Ch. elatum, which was collected in Denmark geographically close to the collection location of the holotype.
As in our previous study (Wang et al. 2016), the phylogenetically defined Ch. elatum showed a diverse morphology in terminal ascomatal hairs. For example, CBS 910.70 (ex-type cultue of Ch. ramipilosum) exhibits relatively slender and flexible terminal hairs (2.5–4.5 μm diam near the base, fig. 10 in Wang et al. 2016), while DTO 319-B3, DTO 318-H9 and DTO 333-E9 exhibit relatively rigid terminal hairs which are more similar to Ch. rectangulare. Also DTO 319-B3 has erect to flexuous terminal branches of the hairs (Fig. 14), while DTO 318-H9 and DTO 333-E9 have undulate terminal branches of the hairs (Fig. 15). However, Ch. rectangulare can be distinguished from Ch. elatum by smaller ascospores (10–11 × 7–9 × 6–7.5 μm) and thicker terminal hairs (4.5–7 μm diam near the base fig. 28 in Wang et al. 2016).
Chaetomium globosum Kunze, Mykol. Hefte 1: 16. 1817. Fig. 16, Fig. 17, Fig. 18.
Fig. 16.
Chaetomium globosum (DTO 319-B2). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Fig. 17.
Chaetomium globosum (DTO 333-E3). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Basal parts of terminal ascomatal hairs. I. Upper parts of terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–F = 100 μm; G–K = 10 μm.
Fig. 18.
Chaetomium globosum (CBS 666.82). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Ascomata superficial, ostiolate, greenish olivaceous (DTO 319-B2), or slightly dark olivaceous buff to grey (DTO 333-E3), or dull green (CBS 666.82) in reflected light owing to ascomatal hairs which can possess sulphur yellow lower parts (DTO 319-B2) or appear sulphur yellow to yellow (CBS 666.82), subglobose, ovate or obovate, 140–270 μm high, 100–240 μm diam. Ascomatal wall brown, textura intricata in surface view. Terminal hairs finely warty, brown, erect (more often in CBS 666.82) or flexuous (more often in DTO 333-E3) or undulate to loosely coiled (most of the indoor isolates, see DTO 319-B2, Fig. 16) with erect or flexuous lower part, tapering and fading towards the tips, 2–5 μm diam near the base. Lateral hairs brown, flexuous, fading and tapering towards the tips. Asci fasciculate, fusiform or clavate, spore-bearing part 19–38 × 12–17 μm, stalks 22–48 μm long (most of the indoor isolates, Fig. 16, Fig. 17), or 10–19 μm long (CBS 666.82) with 8 irregularly-arranged ascospores, evanescent. Ascospores brown when mature, limoniform, usually biapiculate, bilaterally flattened, 8.5–11(–12) × 7–8.5(–9.5) × 5.5–7 μm, with an apical germ pore. Asexual morph absent.
Culture characteristics: Colonies on OA with an entire edge, spreading rapidly, over 70 mm diam in 7 d at 25 °C, with sparse white to olivaceous buff aerial hyphae when young, then without aerial hyphae and becoming citrine green, yellow, greenish olivaceous to grey olivaceous (most of the indoor isolates, Fig. 16, Fig. 17) or dull green (CBS 666.82) owing to the aggregation of ascomata, with fulvous, apricot to sienna exudates (DTO 319-B2), or olivaceous to olivaceous grey exudates (DTO 333-E3) or ochraceous to greenish olivaceous exudates (CBS 666.82) diffusing into the medium; reverse apricot, orange or sienna (DTO 319-B2), grey olivaceous to olivaceous (DTO 333-E3) or umber to dark brick (CBS 666.82). Colonies on PCA translucent, usually with a more or less lobate edge, about 39–48 mm diam in 7 d at 25 °C, without or with very sparse and floccose, pale yellow aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA with an entire (DTO 319-B2 and CBS 666.82) or a fimbriate to rhizoid (DTO 333-E3) edge, spreading rapidly, over 70 mm diam in 7 d at 25 °C, honey to olivaceous buff (DTO 319-B2 and DTO 333-E3), or malachite green to dull green (CBS 666.82) owing to the aggregation of ascomata mixed with floccose aerial hyphae, reverse fulvous, orange to sienna (DTO 319-B2 and DTO 333-E3) or scarlet to rust (CBS 666.82) owing to exudates diffusing into the medium. Colonies on DG18 with a lobate or slightly crenated edge, about 10–17 mm diam in 7 d at 25 °C, buff to pale luteous, with sparse white to greyish white aerial hyphae; reverse orange, rust to bay (DTO 319-B2), ochraceous (DTO 333-E3) or scarlet (CBS 666.82) owing to exudates diffusing into the medium.
Specimens examined: Algeria, isolated from air, cultures DTO 134-D9; DTO 134-E1; DTO 134-E2; DTO 134-E3; DTO 134-E4; DTO 134-E5. Canada, isolated from dust, cultures DTO 318-G3; DTO 318-G4; DTO 318-G5. China, isolated from chili powder, culture CBS 666.82; isolated from air by A.J. Chen in Beijing, cultures DTO 324-D7; DTO 324-G8; DTO 324-H1; DTO 324-H4; DTO 324-H5; DTO 324-I1; DTO 324-I2; DTO 324-I3; DTO 324-I4; DTO 324-I5; DTO 324-I6; DTO 324-I7; DTO 324-I8; DTO 324-I9; DTO 325-A1; DTO 325-A2; DTO 325-A3; DTO 325-A4. Denmark, isolated from carpet by B. Andersen, culture DTO 333-E4 (= IBT 41801); from linoleum by B. Andersen, culture DTO 333-E3 (= IBT 41800); from gypsum by B. Andersen, cultures DTO 333-D7 (= IBT 42328); DTO 333-D8 (= IBT 42326); DTO 333-D9 (= IBT 42327); DTO 333-F4 (= IBT 42297); DTO 333-F5 (= IBT 42299); DTO 333-F6 (= IBT 42301); DTO 333-F7 (= IBT 42325); DTO 333-F9; from plywood by B. Andersen, cultures DTO 333-E1 (= IBT 41766); DTO 333-E8 (= IBT 42177); from oriented strand board by B. Andersen, culture DTO 333-E7 (= IBT 42176). Germany, isolated from air, cultures CBS 112386; DTO 340-I2; DTO 012-F3; DTO 012-D2. Indonesia, isolated from air, culture DTO 237-D4. Mexico, isolated from dust, cultures DTO 319-A3; DTO 319-A4; DTO 319-A5; DTO 319-A6; DTO 319-A7; DTO 319-A8; DTO 319-A9; DTO 319-B1; DTO 319-B2. Netherlands, isolated from air, cultures DTO 085-E8; DTO 085-F5; DTO 085-F6; from indoor materials, cultures DTO 264-C1; DTO 272-I1; from swap, cultures DTO 086-D6; DTO 126-B6; from wall paper, cultures DTO 011-F7; DTO 012-F2. South Africa, isolated from dust, culture DTO 319-B4. Thailand, isolated from dust, culture DTO 319-C3. Uruguay, isolated from dust, culture DTO 319-C9. USA, isolated from dust, cultures DTO 318-H3; DTO 318-H6; DTO 318-I4; DTO 319-C5; DTO 319-C6; from stored cotton, culture CBS 148.51. The description above is based on the isolates DTO 319-B2 from dust in Mexico, DTO DTO 333-E3 from Linoleum in Denmark and CBS 666.82 from chili powder in China.
Notes: The general description and morphological diversity of Ch. globosum has been discussed by Wang et al. (2016). The information presented here gave more insight in the morphological diversity of this species, especially its colony morphology.
Chaetomium tectifimeti X. Wei Wang & Samson, sp. nov. MycoBank MB818838. Fig. 19.
Fig. 19.
Chaetomium tectifimeti (CBS 142032 = DTO 318-G8, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Inner and external layers of ascomatal wall in surface view. G. Structure of external layer of ascomatal wall in surface view. H. Part of a terminal ascomatal hair, longer type. I. Terminal ascomatal hairs, shorter type J. Asci. K. Ascospores. Scale bars: D–E = 100 μm; F–K = 10 μm.
Etymology: Name refers to an indoor species similar to Ch. fimeti.
Ascomata superficial, non-ostiolate, olivaceous grey to olivaceous black, with numerous short, pale olivaceous to olivaceous grey ascomatal hairs, and several long dark olivaceous hairs in reflected light, spherical or oblate, 210–340 μm diam. Ascomata walls composed of two layers separating easily from each other when old: the external wall thick, dark brown, composed of thick-walled, angular or irregular cells, textura angularis in surface view; the inner layer thin, luteous to pale brown, composed of amorphous cells, textura epidermoidea in surface view. Ascomatal hairs of two types: shorter type covering the whole ascomata, conspicuously rough, dark brown at the lower part, fading towards the tips, 3–4.5 μm near the base, up to 400 μm long; longer type arising from the bases of the ascomata, sometimes absent on OA, smooth, dark brown, 4.5–7 μm near the base, 400–2000 μm long. Asci fasciculate, fusiform or clavate, with 8 biseriate ascospores, spore-bearing part 26–40 × 16–20 μm, stalks 20–40 μm long, evanescent. Ascospores olivaceous brown to brown when mature, limoniform, bilaterally flattened, (12–)12.5–15(–16) × 10–11.5(–12) × (8–)8.5–10 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, about 30–36 mm diam in 7 d at 25 °C, with olivaceous buff aerial hyphae, producing green yellow to ochraceous exdudates diffusing into the medium, reverse pale luteous to pale amber. Colonies on PCA with a slightly crenated edge, about 19–25 mm diam in 7 d at 25 °C, with buff and floccose aerial hyphae and translucent perisphere; without coloured exudates; reverse buff to honey. Colonies on MEA with a slightly undulate edge, about 29–35 mm diam in 7 d at 25 °C, with floccose mycelium appearing pale olivaceous grey centre and vinaceous buff in perisphere, reverse fulvous to sienna with a pale edge. Colony growth on DG18 with a slightly crenated edge, about 13–19 mm diam in 7 d at 25 °C, with white aerial hyphae, without coloured exudates; reverse uncoloured.
Specimen examined: USA, isolated from dust by Whitfield & K. Mwange in 2009 (holotype CBS H-22844, culture ex-type CBS 142032 = DTO 318-G8).
Notes: Phylogenetic inference indicated that Ch. tectifimeti clustered close to but separate from Ch. fimeti (ex-epitype culture DSM 62108). This species can be distinguished by its smaller ascomata (210–340 μm diam) than those of Ch. fimeti (320–500 μm diam), and broader ascospores (8.5–10 μm wide in front view) than those of Ch. fimeti (7–8 μm wide in front view).
Collariella X. Wei Wang, Samson & Crous, gen. nov. MycoBank MB818839. Fig. 20, Fig. 21.
Fig. 20.
Morphological diversity of ascomata in the genus Collariella. A. Col. bostrychodes (DTO 326-H6). B. Col. robusta (CBS 551.83T). C. Col. quandrangulata (CBS 152.59). D. Col. causiiformis (CBS 792.83T). E. Col. intricata (CBS 128.85T). F. Col. virescens (CBS 148.68T). G. Col. gracilis (CBS 249.75). Scale bars: A–G = 100 μm.
Fig. 21.
Morphological diversity of ascospores and asci in genus Collariella. Ascospores: Col. bostrychodes (DTO 326-H6). B. Col. robusta (CBS 551.83T). C. Col. quandrangulata (CBS 152.59). D. Col. causiiformis (CBS 792.83T). E. Col. intricata (CBS 128.85T). F. Col. virescens (CBS 148.68T). G. Col. gracilis (CBS 249.75). Asci: H. Col. bostrychodes (DTO 326-H6). I. Col. quandrangulata (CBS 152.59). J. Col. causiiformis (CBS 792.83T). K. Col. gracilis (CBS 249.75). Scale bars: A–K = 10 μm.
Etymology: Name refers to a dark collar-like apex around the ostiolar pore of the ascomata in most of the species in this genus.
Type species: Collariella bostrychodes (Zopf) X. Wei Wang & Samson (= Ch. bostrychodes)
This genus is divided into two subclades by both phylogenetic and morphological evidence. The two subclades are closely related to each other with high statistical support (Fig. 1), indicating that they share a recent common ancestor. We therefore decided to keep them in the same genus. Different descriptions are provided here for each subclade:
Subclade 1: Ascomata superficial, ostiolate, ovate, obovate, ampulliform or cylindrical with brown walls of textura angularis in surface view. Apices of ascomata truncated, usually with a darkened collar around the ostiolar pore, and easy to rupture and to be dispersed together with ascomatal hairs as a whole. Terminal hairs highly diverse, straight, flexuous, undulate or spirally coiled or presenting two different types. Lateral hairs straight, flexuous. Asci fasciculate, fusiform or clavate, stalked, with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores olivaceous brown at maturity, broadly limoniform to quadrangular, bilaterally flattened, with an apical germ pore, usually less than 7.5 μm in length. Asexual morph unknown.
Subclade 2: Ascomata superficial, ostiolate, subglobose or ovate, with brown walls of textura angularis in surface view. Terminal hairs straight, flexuous, undulate or arcuate. Lateral hairs straight, flexuous. Asci fasciculate, clavate, fusiform or obovate, spore-bearing portion 18–26 × 11–14.5 μm, stalks 7–13 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores brown when mature, ellipsoidal or fusiform, never bilaterally flattened, with one or two apical or sometimes slightly sub-apical germ pores, usually more than 9 μm in length. Asexual morph unknown.
Collariella bostrychodes (Zopf) X. Wei Wang & Samson, comb. nov. MycoBank MB818862. Fig. 22.
Fig. 22.
Collariella bostrychodes (DTO 324-H6). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of upper part of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Basionym: Chaetomium bostrychodes Zopf, Abh. Bot. Ver. Prov. Brandenburg 19:173. 1877.
Ascomata superficial, pale greenish grey or pale purplish grey in reflected light owing to ascomatal hairs, subglobose, ovate or obovate, ostiolate, 210–300 μm high (including the collar), 160–240 μm diam. Wide, apically black due to a darkened collar in 25–55 μm high and 75–170 μm wide around the ostiolar pore with the area near the apical collar often in paler colour than the lower part of the ascoma. Ascomatal wall brown, textura angularis in surface view. Terminal hairs arising from the apical collar, conspicuously rough, dark brown, septate, erect in the lower part, 4–7 μm near the base, spirally coiled in the upper part, often with coiled branches. Lateral hairs seta-like, tapering and fading towards the tips. Asci fasciculate, fusiform or clavate, spore-bearing part 22–33 × 7–11 μm, stalks 9–20 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores olivaceous when mature, limoniform, bilaterally flattened, 6–7(–7.5) × (5–)5.5–6.5 × (4–)4.5–5.5 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge, about 34–40 mm diam in 7 d at 25 °C, usually without or with sparse white aerial hyphae, without coloured exudates, reverse uncoloured. Colonies on PCA translucent, with entire edge, about 33–39 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA translucent and membranous, with entire edge, about 29–35 mm diam in 7 d at 25 °C, forming radiating and luteous or fulvous furrows, non-sporulating, often with sparse and concentric white aerial hyphae; reverse uncoloured. Colonies on DG18 with entire edge, about 14–20 mm diam in 7 d at 25 °C, with white floccose aerial hyphae especially at the edge, non-sporulating, reverse uncoloured.
Specimens examined: Indonesia, isolated from dust, DTO 319-C3. China, Beijing, isolated from air by A.J. Chen, cultures DTO 324-H3; DTO 324-H6. The description above is based on the isolate DTO 324-H6.
Notes: This species was originally described with ellipsoid ascomata (Saccardo 1882), and later Ames (1963) described the species with subglobose to ovoid ascomata. von Arx et al. (1986) placed several species in synonymy with Col. bostrychodes (= Ch. bostrychodes), and their description covered a high morphological diversity in the shape of ascomata. Our description based on the indoor isolates fitted with that of Ames. Examination on more isolates of related species is required to delimit this species more accurately.
Collariella carteri X. Wei Wang, Houbraken & Samson, sp. nov. MycoBank MB818863. Fig. 23.
Fig. 23.
Collariella carteri (CBS 128.85, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H–I. Structure of ascomatal wall in surface view. J. Terminal ascomatal hairs. K. Asci. L. Ascospores. Scale bars: E–G = 20 μm; H–L = 10 μm.
Etymology: Named after Dr Adrian Carter, who recognised this species as new in his PhD thesis.
Synonym: Chaetomium intricatum A. Carter, A Taxonomic Study of the Ascomycete genus Chaetomium Kunze, unpublished PhD thesis of the University of Toronto. 1982. nom. inval.
Ascomata superficial, often covered by sparse white aerial hyphae, easily falling down rather than erect when mature, dark brick to sepia in reflected light, globose or subglobose, ostiolate, occasionally pyriform owing to a short ostiolar beak, 175–320 μm high (including the collar), 110–220 μm diam, apically black due to a darkened collar in 20–60 μm high and 40–120 μm wide around the ostiolar pore. Ascomatal wall brown, textura intricata mixed with angularis or epidermoidea in surface view. Terminal hairs arising from the apical collar, seta-like or flexuous, 30–180 μm long, usually shorter than the height of an ascoma, smooth, dark brown, distinctly septate, 4–8.5 μm near the base, tapering and fading towards the nearly hyaline tips. Lateral hairs similar but quite sparse. Asci fasciculate, clavate or slightly fusiform, spore-bearing part 17–26 × 8.5–11.5 μm, stalks 10–18 μm long, with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores olivaceous when mature, limoniform, usually biapiculate at both ends, bilaterally flattened, (6–)6.5–7.5(–8.5) × (4.5–)5–6(–7.5) × 4–5.5 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge, about 24–30 mm diam in 7 d at 25 °C, with white aerial hyphae and citrine green or greenish yellow to citrine exudates diffusing into the medium, reverse greenish yellow to greenish olivaceous. Colonies on PCA translucent, with entire edge, about 28–34 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA translucent and membranous, with entire edge, about 29–33 mm diam in 7 d at 25 °C, forming radiated and luteous or ochraceous furrows; non-sporulating, reverse uncoloured. Colony growth on DG18 very limited, less 3 mm diam after 7 d, without coloured exudates; reverse uncoloured.
Specimen examined: Canada, Vancouver, isolated from air of Bacteriology Lab in the University of British Columbia by R.J. Bandoni on 14 Feb. 1966 (holotype CBS H-22845, culture ex-type CBS 128.85).
Notes: Collariella carteri is easily recognised by its short and seta-like terminal ascomatal hairs. Carter (1982) noted that the ascomatal wall of this species consists of textura intricata in surface view. Our observation showed that the wall structure of CBS 128.85 consisted of textura intricate, angularis and epidermoidea (Fig. 23E–I). This species formed a sister lineage to Col. bostrychodes.
Collariella causiiformis (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818864. Fig. 24.
Fig. 24.
Collariella causiiformis (CBS 792.82). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Basal parts of terminal ascomatal hairs. I. Upper parts of terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–F = 100 μm; G–K = 10 μm.
Basionym: Chaetomium causiiforme Mycologia 41: 644. 1949.
Ascomata superficial, usually forming a layer on the surface of OA medium owing to adjacent long ascomatal hairs intermingling with each other, hazel to olivaceous in reflected light due to ascomatal hairs, globose or subglobose, ostiolate, 70–140 μm high (including the darkened collar), 60–120 μm diam, apically black due to a darkened collar in 10–24 μm high and 32–65 μm wide around the ostiolar pore. Ascomatal wall translucent, ochraceous, textura angularis in surface view. Terminal hairs arising from the apical collar, a few type I: up to 2 600 μm long, undulate, olivaceous, smooth, septate, 3.5–5 μm diam near the base; most type II: usually less than 700 μm long, olivaceous, smooth, septate, flexous or geniculate, often branched, sometimes spically recurved or circinate, 2–3 μm diam near the base. Lateral hairs seta-like, sparse. Asci fasciculate, fusiform, sometimes clavate, spore-bearing portion 15–22 × 8–13 μm, stalks 6–13 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores olivaceous when mature, broad limoniform or ovate, bilaterally flattened, (5–)5.5–6.5 × (4.5–)5–5.5(–6) × (3.5–)4–5 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA translucent at the beginning, with entire edge, 27–33 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates, reverse uncoloured. Colonies on PCA translucent, with entire edge and lobate and relatively thicker texture in the centre, about 24–30 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA translucent and membranous, with entire edge, about 20–26 mm diam in 7 d at 25 °C, forming radiating and luteous or fulvous furrows, non-sporulating; reverse uncoloured. No visible growth of colonies on DG18 after 7 d at 25 °C.
Specimen examined: Solomon Islands, isolated from sweatband of helmet liner by G.W. Martin, ex-type culture CBS 792.83.
Notes: Collariella causiiformis forms two types of terminal ascomatal hairs and this feature is not observed in the other known Collariella species. This species formed a sister lineage to the clade in which both Col. carteri and Col. bostrychodes are included.
Collariella gracilis (Udagawa) X. Wei Wang & Samson, comb. nov. MycoBank MB818865. Fig. 25.
Fig. 25.
Collariella gracilis (CBS 249.75). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 20 μm; H–K = 10 μm.
Basionym: Chaetomium gracile Udagawa, J. Gen. Appl. Microbiol. 6: 235. 1960.
Ascomata superficial, often covered by white aerial hyphae, ostiolate, olivaceous in reflected light due to ascomatal hairs and with black ascospores aggregating on the top, subglobose or ovate, 120–200 μm high, 100–180 μm diam, often with truncated and darkened apices, but not easy to rupture and to be dispersed. Ascomatal wall brown, textura angularis. Terminal hairs numerous, arcuate, partly apically flexuous, incurved or circinate, fine rough, brown, septate, 3.5–5.5 μm diam near the base. Lateral hairs relatively sparse, seta-like or flexuous. Asci fasciculate, clavate, fusiform or obovate, spore-bearing portion 18–26 × 11–14.5 μm, stalks 7–13 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores brown when mature, ellipsoidal or broadly fusiform, (9.5–)10–12.5(–13) × 6–7.5(–8) μm, with an apical or sometimes slightly sub-apical germ pore. Asexual morph unknown.
Culture characteristics: Colonies on OA with white aerial hyphae, translucent at the beginning, with an entire or slightly undulate edge, about 32–38 mm diam in 7 d at 25 °C, irregularly producing white aerial hyphae later, with citrine green or amber to citrine exudates diffusing into the medium, reverse yellow to amber. Colonies on PCA citrine, with or slightly crenated edge, about 23–29 mm diam in 7 d at 25 °C, with sparse white to straw aerial hyphae, reverse citrine. Colonies on MEA with entire edge, about 29–35 mm diam in 7 d at 25 °C, with white to yellowish buff and floccose aerial mycelium, reverse saffron due to exudates diffusing into the medium. Colony growth on DG18 with entire edge, about 11–17 mm diam in 7 d at 25 °C, pale rosy buff with white aerial hyphae, without coloured exudates; reverse uncoloured.
Specimen examined: India, Uttar Pradesh, isolated from air by Kamal, culture CBS 249.75.
Notes: von Arx et al. (1986) suggested that this species was closely related to Col. virescens. Our phylogenetic analysis confirmed their close relationship. The two species differ in ascomata and ascospores (Fig. 20, Fig. 21).
Dichotomopilus X. Wei Wang, Samson & Crous, gen. nov. MycoBank MB818840. Fig. 26, Fig. 27.
Fig. 26.
Morphological diversity of ascomata in the genus Dichotomopilus. A. D. variostiolatus (CBS 179.84T). B. D. variostiolatus (DTO 319-A2). C. D. subfunicola (CBS 812.73). D. D. pseudofunicola (DTO 318-I7). E. D. funicola (CBS 136.38 = C. cancroideum). F. D. indicus (DTO 319-B8). G. D. pratensis (CBS 860.68). H. D. erectus (CGMCC 3.12900). I. D. ramosissimus (CGMCC 3.14183T). J. D. dolichotrichus (CBS 162.48T). K. D. reflexus (CBS 157.49T). L. D. fusus (CBS 114.83). Scale bars: A–L = 100 μm.
Fig. 27.
Morphological diversity of ascospores in the genus Dichotomopilus. A. D. variostiolatus (CBS 179.84T). B. D. variostiolatus (DTO 319-A2). C. D. subfunicola (CBS 812.73). D. D. funicola (DTO 333-F1). E. D. indicus (DTO 319-B8). F. D. indicus (DTO 333-F3). G. D. pratensis (CBS 860.68). H. D. erectus (CGMCC 3.12900). I. D. ramosissimus (CGMCC 3.14183T). J. D. dolichotrichus (CBS 162.48T). K. D. reflexus (CBS 157.49T). L. D. fusus (CBS 114.83). Scale bars: A–L = 10 μm.
Etymology: Name refers to the shape of terminal ascomatal hairs which are usually dichotomously branched.
Ascomata superficial, ostiolate, spherical, ellipsoid or ovate with walls of textura intricata or epidermoidea in surface view, or of textura angularis in a few species. Terminal hairs seta-like and the beginning, then the majority developing into dichotomously or irregularly branched to form a net structure to hold ascospores, sometimes branched and seta-like together, occasional stayinging seta-like, usually punctulate or verrucose, with the exception of those of D. dolichotrichus (= Ch. dolichotrichum) presenting smooth. Lateral hairs unbranched, seta-like, tapering towards tips. Asci fasciculate, clavate, broadly clavate or ovate, stalked, with 8 biseriate or irregularly arranged ascospores, evanescent quickly. Ascospores brown at maturity, narrowly ovate, ovate or broad ovate, bilaterally flattened, attenuate at one or both ends, with an apical or slightly sub-apical germ pore at the most attenuated end, the opposite end more or less apiculate if not attenuate, usually less than 7.5 μm long, with the exception of D. fusus (= Ch. fusum) possessing cylindrical ascospores without visible germ pores. Asexual morph unknown.
Type species: Dichotomopilus indicus (Corda) X. Wei Wang & Samson = (Ch. indicum)
Notes: Skolko & Groves (1948) provided the first overview of this group of fungi. Based on the presence or absence of unbranched terminal hairs and the characteristics of the branched hairs, they circumscribed and accepted six species. Their classification was followed by Ames, 1963, Mazzucchetti, 1965 and Seth (1970). However, von Arx et al. (1986) only accepted two species: Ch. indicum and Ch. funicola. Chaetomium indicum was described to show typical dichotomously branched terminal ascomatal hairs, while Ch. funicola was described to produce both unbranched seta-like and dichotomously branched terminal hairs. Based on these definitions, Ch. dolichotrichum was treated as a synonym of Ch. funicola, and the rest of the species accepted by Skolko & Groves (1948) were questioned. Wang et al. (2014) re-assessed all these species based on a five-locus phylogenetic analysis. The results indicated that all these species clustered in a monophyletic clade. Five of the six species of Skolko & Groves (1948) were recognised with the exception of Ch. cancroideum, which clustered in the Ch. funicola clade. At the same time, three more species: Ch. subfunicola, Ch. ramosissimum and Ch. pratense were discovered, which were phylogenetically close to but separated from Ch. funicola, Ch. erectum and Ch. indicum, respectively (Wang et al. 2014).
In the present study, a new genus Dichotomopilus is proposed to accommodate this monophyletic lineage. Within the genus, two more species D. pseudofunicla and D. pseudoerectus were described as new. Two other species, D. variostiolatus (= Ch. variostiolatum) and D. fusus (Ch. fusum), also clustered in this genus. The former was originally described to possess unbranched seta-like terminal hairs (Fig. 26A), and the latter produces dichotomously-branched terminal hairs (Fig. 26L), and quite distinct ascospores (Fig. 27L).
Seven indoor species were recognised in Dichotomopilus. A high morphological diversity in ascomatal hairs, asci and ascospores was observed within indoor isolates of D. funicola, D. indicus, D. subfunicola and D. variostiolatus. Our morphological examination indicated extremely high intra-/ inter-species diversity and also overlap occurs between the indoor isolates of the four species. In contrast, the species D. pratensis (Fig. 26, Fig. 27G), D. dolichotrichus (Fig. 26, Fig. 27J), D. reflexus (Fig. 26, Fig. 27K) and D. fusus (Figs 26L, 27L) from different substrates and different locations are morphologically stable, and easy to be recognised on the basis of morphology. It is a challenge to identify D. funicola, D. indicus, D. subfunicola and D. variostiolatus only based on morphology. We highly recommend the use of sequence data to recognise these species in the genus Dichotomopilus.
Dichotomopilus funicola (Cooke) X. Wei Wang & Samson, comb. nov. MycoBank MB818841. Fig. 28.
Fig. 28.
Dichotomopilus funicola (DTO 333-F1). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper part of a terminal ascomatal hair. I–J. Asci. K. Ascospores. Scale bars: D–F = 100 μm; G–K = 10 μm.
Basionym: Chaetomium funicola Cooke, Grevillea 1: 176. 1873.
Synonym: Chaetomium cancroideum Tschudy, Am. J. Bot. 24: 478. 1937.
Ascomata superficial, ostiolate, greenish olivaceous to grey olivaceous in reflected light, subglobose to ellipsoid or ovate, 195–255 μm high, 180–240 μm diam. Ascomatal wall composed of brown, irregular or elongate cells (textura intricata or epidermoidea). Terminal hairs composed of two types: (1) wider, dark brown and erect, 4–5.5 μm diam near the base, tapering and fading towards tips, dichotomously branched profusely at acute angles starting from the upper half part, punctulate; (2) thinner, luteous to brown, 2–3.5 μm diam near the base, dichotomously branched at wide to acute angles starting near the base; the terminal branches relatively short, erect, incurved or reflexed, often in dense clusters. Lateral hairs simply branched or seta-like, tapering and fading towards tips. Asci fasciculate, with 8 irregularly-arranged ascospores, clavate, pyriform to ovate, spore-bearing portion 11–18 × 7–11.5 μm, stalks 6–10 μm long, evanescent quickly. Ascospores olivaceous when mature, ovate to slightly elongate ovate, bilaterally flattened, 5–6 × 3.5–4.8 × 3–3.8 μm, with an apical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, spreading rapidly, over 70 mm diam in 7 d at 25 °C, with pale yellow, sparse and floccose aerial hyphae, producing greenish olivaceous to citrine exudates diffusing into the medium; reverse ochraceous to cinnamon. Colonies on PCA olivaceous buff to greenish olivaceous because of the sparse aerial hyphae and ascomata, with irregularly crenated edge, spreading rapidly, over 70 mm diam in 7 d at 25 °C, without coloured exudates; reverse olivaceous buff to greenish olivaceous. Colonies on MEA with entire edge, about 57–63 mm diam in 7 d at 25 °C, forming profuse and pale yellow mycelium and then greenish olivaceous because of the formation of ascomata, without coloured exudates, reverse ochraceous. Colonies on DG18 buff with slightly lobate or crenated edge, about 10–16 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates; reverse pale buff.
Specimens examined: Denmark, isolated from dust by B. Andersen, cultures DTO 333-F1 (=IBT 42276); DTO 333-F2 (= IBT 42277). USA, isolated from dust, culture DTO 318-I2.
Notes: The terminal hairs of the indoor isolate examined above look like intermediates between the ex-epitype culture of D. funicola (fig. 3i–o in Wang et al. 2014) and the authentic culture of Ch. cancroideum (Fig. 26E in this study), especially in the terminal branches. This implied intra-species variation within this species, and encouraged us to synonymise Ch. cancroideum into D. funicola. This treatment is consistent with the phylogenetic data (Fig. 1).
Dichotomopilus indicus (Corda) X. Wei Wang & Samson, comb. nov. MycoBank MB818842. Fig. 29, Fig. 30, Fig. 31.
Fig. 29.
Dichotomopilus indicus (DTO 319-B8). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper part of a terminal ascomatal hair. I–J. Asci. K. Ascospores. Scale bars: D–F = 100 μm; G–K = 10 μm.
Fig. 30.
Dichotomopilus indicus (DTO 333-E2). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper part of a terminal ascomatal hair. I–J. Asci. K. Ascospores. Scale bars: E–F = 100 μm; G–K = 10 μm.
Fig. 31.
Dichotomopilus indicus (DTO 333-F3). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper parts of terminal ascomatal hairs. I. Basal parts of terminal ascomatal hairs. J–K. Asci. L. Ascospores. Scale bars: D–F = 100 μm; G–L = 10 μm.
Basionym: Chaetomium indicum Corda, Icon. Fung. 4: 38. 1840.
Ascomata superficial, ostiolate, greenish olivaceous to grey olivaceous in reflected light, subglobose or ovate, 160–310 μm high, 140–275 μm diam. Ascomatal wall composed of brown, irregular or elongate cells (textura intricata or epidermoidea). Terminal hairs composed of two types: (1) wider, dark brown and erect, 4–5 μm diam near the base, tapering and fading towards tips, dichotomously branched at acute angles starting from the upper half part, punctulate; (2) thinner, luteous to brown, 2–3.5 μm diam near the base, dichotomously branched at wide to acute angles starting near the base (DTO 333-F3), or only composed of one type: dark brown and erect, 3–5.5 μm diam near the base, dichotomously branched at acute to wide angles starting from the upper half part, verrucose, tapering and fading towards tips (DTO 319-B3) or partly shorter and dichotomously branched 1–4 times at acute angles starting from the upper half part, partly longer, unbranched and seta-like (DTO 333-E2). Lateral hairs unbranched, seta-like, tapering and fading towards tips. Asci fasciculate, with 8 irregularly-arranged ascospores visible, clavate, broadly clavate, fusiform to pyriform, spore-bearing portion 11–25.5 × 6.5–13.5 μm, stalks 6–15 μm long, evanescent quickly. Ascospores olivaceous when mature, ovate to slightly elongate ovate, bilaterally flattened, 5.5–6.5 × 3.5–4.5 × 2.5–3 μm (DTO 333-F3), or 5.5–6.5 × 4–5 × 3–4 μm (DTO 319-B3), or (6–)6.5–7.5(–8) × 4.5–5.5 × 3.5–4.5 μm (DTO 333-E2), with an apical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge, about 20–48 mm diam in 7 d at 25 °C, with sparse buff, pale yellow to pale primrose aerial hyphae, without coloured exudates at the beginning, and then producing pale luteous, amber, ochraceous to cinnamon exudates diffusing into the medium, reverse buff, pale luteous, luteous, ochraceous to cinnamon. Colonies on PCA usually with entire, slightly undulate, or irregularly crenated edge, about 25–54 mm diam in 7 d at 25 °C, olivaceous buff, pale yellow to greenish olivaceous because of the sparse aerial hyphae together with ascomata and ascospores, without coloured exudates; reverse olivaceous buff, pale yellow to greenish olivaceous. Colonies on MEA with entire or slightly lobate edge, about 36–53 mm diam in 7 d at 25 °C, forming thick yellowish white to pale yellow mycelium, sometimes with radiating furrows, reverse pale luteous to ochraceous. Isolate DTO 333E2 showed distinct colony morphology: white and a little bit wrinkled with entire edge, about 12–18 mm diam in 7 d at 25 °C, forming a relatively thin layer of aerial hyphae, reverse pale luteous. Colonies on DG18 white with entire to crenated edge, about 8–15 mm diam in 7 d at 25 °C, with sparse white aerial hyphae, reverse pale yellow, pale luteous or rust. DTO 333E2 showed distinct colony morphology: buff and wrinkled with crenated edge, about 3–9 mm diam in 7 d at 25 °C, without aerial hyphae, reverse buff.
Specimens examined: Denmark, isolated from feather by B. Andersen, cultures DTO 333-F3 (IBT 42278); DTO 333-E2 (IBT 41796). South Africa, isolated from dust, culture DTO 319-B8.
Notes: Three isolates phylogenetically recognised as D. indicus were examined critically. Each of them showed distinct morphology. Isolate DTO 319-B8 presented relatively sparse terminal hairs with the branched part not significantly thinner than the basal part of the hairs (Fig. 29). DTO 333-E2 showed seta-like terminal hairs, and only parts of them were branched near the top (Fig. 30). DTO 333-F3 presented relatively numerous terminal hairs dichotomously branched in two different types, the thicker ones with the branched part conspicuously thinner than the basal parts (Fig. 31). The examined isolates also showed difference to some extent in ascospores. DTO 333-E2 produced the largest ascospores (6.5–7.5 × 4.5–5.5 × 3.5–4.5 μm) compared to those of the two other isolates, while DTO 333-F3 produced narrower ascospores (5.5–6.5 × 3.5–4.5 × 2.5–3 μm) than DTO 319-B8 (5.5–6.5 × 4–5 × 3–4 μm).
Dichotomopilus pratensis (X.W. Wang & L. Cai) X. Wei Wang & Samson, comb. nov. MycoBank MB818843. Fig. 32.
Fig. 32.
Dichotomopilus pratensis (CBS 860.68). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper parts of terminal ascomatal hairs. I–J. Asci. K. Ascospores. Scale bars: D–F = 100 μm; G–K = 10 μm.
Basionym: Chaetomium pratense X.W. Wang & L. Cai, Mycol. Prog. 13: 723. 2014.
Ascomata superficial, ostiolate, greenish olivaceous to olivaceous in reflected light, subglobose or ovate, 135–230 μm high, 120–200 μm diam. Ascomatal wall composed of brown, irregular or elongate cells (textura intricata or epidermoidea). Terminal hairs dark brown and often attached by yellow but soluble crystals which would soon dissolve in the mounting solution, 4–6 μm diam near the base, erect, dichotomously branched at wide (nearly straight) to right angles primarily starting from the upper half part, verrucose. Lateral hairs unbranched, seta-like, tapering and fading towards tips. Asci fasciculate, clavate, sometimes fusiform or pyriform, with 8 irregularly-arranged ascospores, spore-bearing portion 14–22 × 8–11.5 μm, stalks 5–10 μm long, evanescent quickly. Ascospores olivaceous when mature, broad ovate to nearly globose, bilaterally flattened, 5–6.5 × 4.5–5.5 × 3.5–4.5 μm, with an apical germ pore at the more attenuated end, and slight apiculate at the other end. Asexual morph unknown.
Culture characteristics: Colonies on OA pale luteous to amber with entire or slightly undulate edge, about 34–40 mm diam in 7 d at 25 °C, with sparse aerial hyphae, with amber exudates diffusing into the medium, reverse amber to luteous. Colonies on PCA translucent with entire edge, about 43–49 mm diam in 7 d at 25 °C, pale luteous to greenish olivaceous because of the formation of ascomatal, without aerial hyphae, without coloured exudates; reverse buff to pale luteous under the clusters of ascomata. Colonies on MEA amber, with irregularly lobate edge, about 29–35 mm diam in 7 d at 25 °C, with sparse yellow aerial hyphae, reverse luteous to ochraceous. Colonies on DG18 with entire edge, about 28–34 mm diam in 7 d at 25 °C, with yellow to amber aerial hyphae, reverse amber to luteous.
Specimen examined: Germany, Kiel-Kitzeberg, isolated from air by K.H. Domsch, culture CBS 860.68.
Notes: Comparing several isolates of D. pratensis indicated a stable morphology. This species can be easily distinguished by its yellow to amber colony, broad ovate to nearly globose ascospores and the terminal hairs usually branched at wide (straight to right) angle. Prevously, a mistake in sequencing gave a wrong identification for the indoor isolate CBS 860.68 (was wrongly identified as Ch. indicum in Wang et al. 2014). We corrected this mistake in the present study.
Dichotomopilus pseudoerectus X. Wei Wang & Samson, sp. nov. MycoBank MB818844. Fig. 33.
Fig. 33.
Dichotomopilus pseudoerectus (CBS 252.75, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Textura angularis structure of ascomatal wall in surface view. G. Textura intricata structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I–J. Asci. K. Ascospores. Scale bars: D–E = 100 μm; F–K = 10 μm.
Etymology: Name refers to its similarity to D. erectus (= Ch. erectum).
Ascomata superficial, ostiolate, greenish olivaceous to olivaceous grey in reflected light, subglobose or ovate, 135–220 μm high, 125–195 μm diam. Ascomatal wall composed of brown, angular or elongate hypha-like cells (textura angularis mixed with textura intricata). Terminal hairs dark brown, irregular in length and often attached by amber but soluble crystals which would soon dissolve in the mounting solution, 3–4.5 μm diam near the base, dichotomously branched at wide to acute angles starting near the bases, verrucose. Lateral hairs similar to the terminal ones but shorter, or seta-like. Asci fasciculate, with 8 irregularly-arranged ascospores visible, clavate, broadly clavate, pyriform to ovate, spore-bearing portion 10–15 × 7.5–10.5 μm, stalks 4.5–10.5 μm long, evanescent quickly. Ascospores olivaceous when mature, ovate to broad ovate, bilaterally flattened, 5.5–6.5(–7) × 5–5.5 × 3.5–4 μm, with an apical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge and spreading rapidly, over 70 mm diam in 7 d at 25 °C, with luteous to ochraceous and floccose aerial hyphae, with amber to ochraceous exudates diffusing into the medium, reverse ochraceous. Colonies on PCA olivaceous with entire edge, about 45–51 mm diam in 7 d at 25 °C, with pale yellow to pale luteous, sparse and floccose aerial hyphae, without coloured exudates; reverse honey. Colonies on MEA white to pale yellow, with irregularly fimbriate edge, wrinkled with irregularly radiating furrows, about 22–26 mm diam in 7 d at 25 °C, with sparse aerial hyphae, reverse luteous to fulvous. Colonies on DG18 pale ochraceous with entire edge, about 7–13 mm diam in 7 d at 25 °C, without aerial hyphae, reverse pale luteous.
Specimen examined: India, Uttar Pradesh, isolated from air by Kamal (holotype CBS H-22846, culture ex-type CBS 252.75).
Notes: the ex-type culture CBS 252.75 is deposited in the CBS collection as Ch. erectum. This species is indeed morphologically similar to D. erectus (= Ch. erectum). However, the phylogenetic analysis (Fig. 1) indicated that it is closer to D. ramosissimus than to D. erectus. Dichotomophilus pseudoerectus can be differentiated from D. ramosissimus by relatively sparse terminal ascomatal hairs which are often different in length.
Dichotomopilus pseudofunicola X. Wei Wang & Samson, sp. nov. MycoBank MB818845. Fig. 34.
Fig. 34.
Dichotomopilus pseudofunicola (CBS 142033 = DTO 318-I7, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–F = 100 μm; G–J = 10 μm.
Etymology: Name refers to its similarity to D. funicola.
Ascomata superficial, ostiolate, grey olivaceous to dark grey olivaceous in reflected light, subglobose or ovate, 140–260 μm high, 130–235 μm diam. Ascomatal wall composed of brown, hypha-like or irregular cells (textura intricata). Terminal hairs olivaceous brown, 4–7 μm diam near the base, punctulate, partly longer and erect, seta-like, unbranched or dichotomously branched 1–3 times at wide to acute angles usually only at the top part; partly shorter, dichotomously branched profusely starting near the base and forming a globose net to enfold ascospores. Lateral hairs unbranched, seta-like, tapering and fading towards tips. Asci fasciculate, clavate, broadly clavate to pyriform or ovate, with 8 irregularly-arranged ascospores, spore-bearing portion 12–18 × 7–10.5 μm, stalks 6–11 μm long, evanescent quickly. Ascospores olivaceous when mature, ovate to limoniform, biapiculate, bilaterally flattened, 5.5–6(–6.5) × 4–5 × 3.5–4 μm, with an apical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, about 55–61 mm diam in 7 d at 25 °C, with yellowish white to pale yellow, producing honey to pale luteous exudates diffusing into the medium; reverse pale luteous. Colonies on PCA with entire edge, about 54–60 mm diam in 7 d at 25 °C, olivaceous buff, greenish olivaceous in the centre because of the formation of ascomata, without coloured exudates; reverse olivaceous buff. Colonies on MEA white to pale yellow with an entire and pale olivaceous buff edge, about 45–51 mm diam in 7 d at 25 °C, forming floccose aerial hyphae, without coloured exudates, reverse ochraceous. Colonies on DG18 white to buff with an entire edge, about 11–17 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates; reverse pale buff.
Specimen examined: USA, isolated from dust by Whitfield & K. Mwange in 2009 (holotype CBS H-22847, culture ex-type CBS 142033 = DTO 318-I7).
Notes: Based on the examined isolate, this species seems to be the best example to reflect the description of D. funicola (= Ch. funicola) given by von Arx et al. (1986), showing seta-like together with repeatedly dichotomously branched terminal hairs. The isolate of D. pseudofunicola grew slower than the isolates of D. funicola. As this species is only known by the type, more isolates are waited for a better delimitation of this species.
Dichotomopilus subfunicola (X.W. Wang & L. Cai) X. Wei Wang & Samson, comb. nov. MycoBank MB818846. Fig. 35, Fig. 36.
Fig. 35.
Dichotomopilus subfunicola (CBS 812.73). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper parts of terminal ascomatal hairs. I–J. Asci. K. Ascospores. Scale bars: E–F = 100 μm; G–K = 10 μm.
Fig. 36.
Dichotomopilus subfunicola (CBS 794.83). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper parts of terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium subfunicola X.W. Wang & L. Cai, Mycol. Prog. 13: 723. 2014.
Ascomata superficial, ostiolate, greenish olivaceous to citrine olivaceous in reflected light, subglobose to ellipsoid, 145–270 μm high, 120–250 μm diam. Ascomatal wall composed of brown, irregular or elongate cells (textura intricata or epidermoidea). Terminal hairs composed of two types: (1) wider, dark brown and erect, 3.5–6 μm diam near the base, tapering and fading towards tips, seta-like or dichotomously branched at wide to acute angles starting near the base, verrucose; (2) thinner, umber to brown, 2.5–4.5 μm diam near the base, dichotomously branched profusely at wide to acute angles starting near the base and often forming a loose net to enfold ascospores (CBS 812.73), or only composed of the thinner type ones (CBS 794.83). Lateral hairs simply branched or seta-like, tapering and fading towards tips. Asci fasciculate, with 8 irregularly-arranged ascospores, clavate, ovate to broadly ovate or pyriform, spore-bearing portion 12.5–18.5 × 7.5–12 μm, stalks 4.5–9 μm long, evanescent quickly. Ascospores olivaceous when mature, ovate, bilaterally flattened, 5.5–6.5(–7.5) × 4–5 × 3.5–4(–4.5) μm, with an apical germ pore at the more attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with an entire edge, about 46–54 mm diam in 7 d at 25 °C, with pale yellow, floccose aerial hyphae, producing pale luteous, pale citrine, citrine green to greenish olivaceous exudates diffusing into the medium; reverse pale luteous, citrine, greyish yellow-green to citrine green. Colonies on PCA pale yellow to greenish olivaceous because of the sparse aerial hyphae and ascomata, with entire or slightly lobate edge, about 55–62 mm diam in 7 d at 25 °C, without coloured exudates; reverse uncoloured. Colonies on MEA with lobate edge, about 41–47 mm diam in 7 d at 25 °C, forming profuse and pale yellow mycelium, greenish olivaceous because of the formation of ascomata (CBS 812.73), or with white to pale yellow aerial hyphae forming a few crenated concentric circles near the edge (CBS 794.83), without coloured exudates, reverse pale luteous to ochraceous. Colonies on DG18 pale luteous, with slightly lobate or crenated edge, about 7–16 mm diam in 7 d at 25 °C, with white aerial hyphae, without coloured exudates; reverse pale luteous.
Specimens examined: New Guinea, isolated from pistol belt by E.T. Reese, culture CBS 812.73. Switzerland, Engadin, isolated from paper by M. Dreyfuss, culture CBS 794.83.
Notes: Dichotomopilus subfunicola is another species close to D. funicola. There is phylogenetic variation within the species, but no strong evidence allowed us to segregate it into two different species. This species also showed high morphological intra-species variation, especially in terminal ascomatal hairs. Isolates of the species often form two types of ascomatal hairs, but some isolates produce only one type (CBS 794.83, Fig. 36). Wang et al. (2014) differentiated D. subfunicola from D. funicola by broader (ovate) asci. Examination of more isolates showed a morphological diversity in the shape of the asci, and this indicates that this character is not suitable for differentiation between those two species.
Dichotomopilus variostiolatus (Carter) X. Wei Wang & Samson, comb. nov. MycoBank MB818847. Fig. 37, Fig. 38, Fig. 39.
Fig. 37.
Dichotomopilus variostiolatus (CBS 179.84T). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G–J. Terminal ascomatal hairs from basal to upper parts. K. Asci. L. Ascospores. Scale bars: D–E = 100 μm; F–L = 10 μm.
Fig. 38.
Dichotomopilus variostiolatus (DTO 319-A2). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Upper part of a terminal ascomatal hair. H. Net structure formed by terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–E = 100 μm; H= 20 μm; F–G, I–J = 10 μm.
Fig. 39.
Dichotomopilus variostilatus (DTO 319-B9). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hair. I. Upper part of a branched terminal ascomatal hair. J–K. Asci. L. Ascospores. Scale bars: E–F = 100 μm; G–L = 10 μm.
Basionym: Chaetomium variostiolatum Carter, Canad. J. Bot. 61: 2603. 1983.
Ascomata superficial, or covered by white to pale yellow aerial hyphae, ostiolate, olivaceous grey dark brick to brown vinaceous in reflected light due to ascomatal hairs, globose or subglobose, 150–250 μm diam. Ascomatal wall brown, textura intricata or epidermoidea in surface view. Ascomatal hairs seta-like or flexuous, smooth to finely verrucose, brown, 4–6.5 μm diam near the base, fading and tapering towards the tips (CBS 179.84), or olivaceous to brown, 2–3.5 μm diam near the base, dichotomously branched profusely at wide angles starting near the base and forming a nearly globose net to enfold ascospores, and often with several longer hairs out of the net (DTO 319-A2), or composed of both types: (1) thinner, olivaceous to brown, 1.6–3.5 μm diam near the base, dichotomously branched profusely at acute to wide angles starting near the base, often constricted at septa, verrucose; (2) wider, dark brown and erect, 3–5 μm diam near the base, tapering and fading towards tips, seta-like or branched near the top, the branches often constricted at septa, verrucose (DTO 319-B9). Lateral hairs simply branched or seta-like, tapering and fading towards tips, sometimes covered by pale yellow soluble crystals. Asci fasciculate, clavate, fusiform, pyriform or obovate, with 8 irregularly arranged ascospores, spore-bearing part 11–24 × 7–12.5 μm, stalks 7–20 μm long, evanescent quickly. Ascospores brown when mature, ovate, (5–)5.5–6.5(–7) × (3.5–)4–4.5(–5) × 3–4 μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with lobate edge, about 34–46 mm diam (CBS 179.84 and DTO 319-B9) or spreading rapidly, over 70 mm diam (DTO 319-A2) in 7 d at 25 °C, with a relatively thick layer of white mycelium varying usually in thickness (CBS 179.84), or white to pale yellow at the beginning because of the aerial hyphae, gradually olivaceous grey because of the formation of ascomata (DTO 319-A2), or with sparse pale yellow aerial hyphae (DTO 319-B9), producing luteous, amber, citrine green to citrine exudates diffusing into the medium; reverse pale luteous, greyish olivaceous or greyish yellow-green. Colonies on PCA translucent, with an entire or crenated edge, about 29–47 mm diam (CBS 179.84 and DTO 319-B9) or over 70 mm diam (DTO 319-A2) in 7 d at 25 °C, with sparse and floccose aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA with an entire or lobate to irregularly crenated edge, about 34–40 mm diam(CBS 179.84 and DTO 319-B9), or about 54–60 mm diam (DTO 319-A2) in 7 d at 25 °C, with floccose and white aerial hyphae together with several radiated furrows and a dark ring around the central point because of the formation of ascomata (CBS 179.84 and DTO 319-B9), or white to greyish yellow-green because of the profuse aerial hyphae and ascomata below (DTO 319-A2) without coloured exudates; reverse luteous to ochraceous. Colonies on DG18 white to buff with an entire, a slightly crenated or lobate to edge, about 9–18 mm diam in 7 d at 25 °C, with a white floccose ring composed of aerial hyphae near the edge (CBS 179.84),or without aerial hyphae (DTO 319-A2 and DTO 319-B9), without coloured exudates, reverse uncoloured.
Specimens examined: New Guinea, collected from tarpaulin by E.T. Reese, ex-type culture CBS 179.84. Thailand, isolated from dust, cultures DTO 319-B9; DTO 319-C1. USA, isolated from dust, culture DTO 319-A2.
Notes: Dichotomopilus variostiolatus was originally described to possess unbranched seta-like terminal hairs on the basis of the ex-type culture CBS 179.84 (Carter 1983). This study confirmed the examination of Carter (Fig. 37), proving a stable morphology of the ex-type culture. At the same time, several indoor isolates were found to cluster with the ex-type. These isolates produce either dichotomously branched (Fig. 38) or together with seta-like (Fig. 39) terminal hairs, indicating a broad range of the species population.
Humicola Traaen, Nytt Mag. Natur. 52: 33. 1914.
Type species: Humicola fuscoatra Traaen
Two indoor taxa of this genus were recognised in this study. They are clustered closely with the type species of Humicola, H. fuscoatra (ex-type CBS 118.14) in a strongly supported monophyletic lineage (Fig. 1).
Humicola olivacea X. Wei Wang & Samson, sp. nov. MycoBank MB818848. Fig. 40.
Fig. 40.
Humicola olivacea (CBS 142031 = DTO 319-C7, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Colonies on SNA. C. Conidia on the surface of medium SNA, top view; D–E. Conidia and conidiophores mounted in lactic acid. Scale bars: D–E = 10 μm.
Etymology: Name refers to the colony colour of this fungus.
Somatic hyphae hyaline to honey, 1–2.5 μm wide. Aleurioconidia usually produced singly or in chains on 2–8 μm long or very reduced lateral hyphae, olivaceous brown, subglobose to broad obovate, attenuated at the base, (7.5–)8–9.5(–11) × (7–)7.5–9(–10) μm. Description of the micromorphology was based on the culture on SNA.
Culture characteristics: Colonies on OA with an entire edge, about 49–55 mm diam in 7 d at 25 °C, greenish olivaceous because of the formation of a large number of conidia, with a thin layer of aerial hyphae; reverse black. Colonies on PCA greenish olivaceous to grey olivaceous, with an entire edge, about 37–43 mm diam in 7 d at 25 °C, with sparse white to grey aerial hyphae; reverse black with grey edge. Colonies on MEA white, with an entire edge, 47–53 mm diam in 7 d at 25 °C, forming radiating furrows, with white and relatively sparse floccose aerial hyphae; reverse olivaceous buff to greenish olivaceous. Colonies on DG18 olivaceous with an entire and buff edge, about 13–19 mm diam in 7 d at 25 °C, with olivaceous aerial hyphae; reverse hazel with buff edge. Colonies on SNA with an entire edge, about 41–47 mm diam in 7 d at 25 °C, translucent, without aerial hyphae, grey olivaceous in the centre; reverse olivaceous in the centre.
Specimen examined: USA, isolated from dust by K. Seifert (holotype CBS H-22848, culture ex-type CBS 142031 = DTO 319-C7).
Notes: This species is morphologically similar to H. glauca, H. lutea and H. repens (Bertoldi 1976), especially in shape and size of aleurioconidia, but can be distinguished by the colour of aleurioconidia, the colony morphology and the formation of aleurioconidia easily in chains. The ITS sequence data also supported the differences between this species and the three morphologically similar species (data not shown here).
Humicola sp. Fig. 41.
Fig. 41.
Humicola sp. 2 (DTO 319-B7). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Colonies on SNA. C. Conidia on the surface of medium SNA, top view; D–E. Conidia and conidiophores mounted in lactic acid. Scale bars: D–E = 10 μm.
Somatic hyphae hyaline to subhyaline, 1–3 μm wide. Aleurioconidia produced terminally or laterally singly or two in chains on hyaline to pale brown hyphae which are very reduced till up to 16 μm long, smooth, globose, obovoid, pyriform to clavate, pale brown to brown, (7.5–)8.5–11.5(–13.5) × (5.5–)7.5–9(–11) μm.
Culture characteristics: Colonies on OA with an entire or irregularly undulated edge, about 52–58 mm diam in 7 d at 25 °C, partially with thick white and floccose aerial hyphae, immersed hyphae grey to black; reverse black. Colonies on PCA white to honey, with an entire edge, about 48–54 mm diam in 7 d at 25 °C, translucent, with thick white aerial hyphae in the centre; reverse white to smoke grey, black in the centre. Colonies on MEA with an entire edge, 51–57 mm diam in 7 d at 25 °C, with thick, white to honey floccose aerial hyphae; reverse black. Colonies on DG18 white, with an entire edge, about 18–24 mm diam in 7 d at 25 °C, with relatively thin white aerial hyphae; reverse pale luteous, black in the centre. Colonies on SNA white, with an entire edge, about 56–62 mm diam in 7 d at 25 °C, translucent with sparse and uneven white aerial hyphae; reverse partially black.
Specimens examined: Mexico, isolated from dust, cultures DTO 318-G9; DTO 318-H1. South Africa, isolated from dust, culture DTO 319-B7.
Notes: These isolates are morphologically similar to H. piriformis especially in the shape of their aleurioconidia. However, it differs in having larger aleurioconidia than H. piriformis (7.0–7.5 μm diam). The ITS sequences of H. aurea, H. glauca, H. lutea, H. piriformis and H. repens are identical and our indoor isolates differ only by one base pair. ITS sequences seemed not to be informative enough to distinguish the aleurioconidial fungi. For example, the ITS sequences of H. grisea and Trichocladium asperum were identical (data not shown here), but the morphology of these two species are distinct (Hambleton et al. 2005). Cultures of all the related species and more research are needed to re-evaluate these related species, and then to make an identification decision for this taxon.
Humicola is a well-known hyphomycetous genus possessing smooth-walled and usually aseptate aleurioconidia forming attached to lateral or terminal conidiogenous cells (Bertoldi, 1976, Hambleton et al., 2005). Further study is strongly encouraged to clarify the relationships of the Humicola species included in this study to the other Humicola species as well as the members of Farrowia (Hawksworth 1975) and some traditional species of Chaetomium such as C. homopilatum which have humicola-like asexual morphs.
Melanocarpus Arx, Stud. Mycol. 8:17. 1975.
Type species: Melanocarpus albomyces (Cooney & R. Emers.) Arx
Melanocarpus tardus X. Wei Wang & Samson, sp. nov. MycoBank MB818849. Fig. 42.
Fig. 42.
Melanocarpus tardus (CBS 541.76, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B–C. Mature ascomata on OA, top view; D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D–F = 20 μm; G–I = 10 μm.
Etymology: Name refers to the restricted growth of this fungus on the agar media.
Ascomata superficial, or embedded in white aerial mycelium, often aggregated, non-ostiolate, black in reflected light, glabrous or with a few hypha-like and hyaline hairs, globose, 50–170 μm diam. Ascomatal wall brown, ochraceous or fulvous when young, dark brown to black when mature, textura epidermoidea or intricata in surface view. Asci fasciculate, ovate to broadly ovate, spore-bearing portion 11–16.5 × 9–13.5 μm, stalks 4–9.5 μm long, with 8 irregularly-arranged ascospores, evanescent quickly before the ascospores mature. Ascospores brown when mature, ovate to broadly ovate, bilaterally flattened, 7–8(–8.5) × (6–)6.5–7.5 × 5–6 μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA growing slowly, about 2–7 mm diam in 7 d at 25 °C, with an entire edge, with white, floccose and compact aerial mycelium, without coloured exudates, or producing honey to greenish olivaceous exudates diffusing into the medium when becoming old; reverse uncoloured. Colonies on PCA with an entire edge, about 2–8 mm diam in 7 d at 25 °C, buff, slightly floccose, without coloured exudates; reverse uncoloured. Colonies on MEA with a lobate edge, about 3–8 mm diam in 7 d at 25 °C, with white and floccose mycelium, without coloured exudates; reverse uncoloured. The growth of colonies on DG18 was restricted, less than 2 mm after 7 d at 25 °C, without coloured exudates; reverse uncoloured.
Specimen examined: Switzerland, St. Gallen, isolated from cotton jacket by E. Müller in Sep. 1976 (holotype CBS H-22849, culture ex-type CBS 541.76).
Notes: The ex-type culture CBS 541.76 is deposited in the CBS collection as T. minuta. Thielavia minuta was originally described to have rapidly spreading colonies. Furthermore, the ascomata are covered by a dense layer of curved brown and curved hairs, and the ascospores are ovate shaped, measuring 6.5–8 × 5–5.5 μm (Cain 1961). CBS 541.76 exhibits very restricted growth on agar media, and the ascomata are glabrous or nearly so, and contain conspicuously bilaterally flattened ascospores. This combination of characteristics does not fit with that of T. minuta and therefore a new species was introduced for this isolate.
Although Melanocarpus tardus and the type species of the genus Melanocarpus, Me. albomyces are on two very long branches in the phylogenetic tree (Fig. 1), they clustered together with high statistic support. We prefer to keep this species as a member of Melanocarpus rather than introducing a novel monotypic genus to accommodate it.
The genus Melanocarpus was considered to possess non-ostiolate ascomata, and differs from Thielavia in having spreading colonies; obovate, spherical or oblate ascospores and chrysonilia-like asexual morph (von Arx et al. 1988). The isolate CBS 541.76 does not produce spreading colonies and chrysonilia-like asexual morph. The addition of this species into Melanocarpus prompts the re-evaluation of this genus.
Ovatospora X. Wei Wang, Samson & Crous, gen. nov. MycoBank MB818850. Fig. 43, Fig. 44.
Fig. 43.
Morphological diversity of Ascomata in the genus Ovatospora. A. O. brasiliensis (CBS 140.50). B. O. pseudomollicella (CBS 251.75T). C. O. medusarum (CBS 148.67T, young ascoma). D. O. medusarum (CBS 148.67T, mature ascoma). E. O. senegalensis (CBS 728.84T). F. O. unipora (CBS 109.83T). Scale bars: A–F = 100 μm.
Fig. 44.
Morphological diversity of ascospores and asci in the genus Ovatospora. Ascospores: A. O. brasiliensis (CBS 140.50). B. O. pseudomollicella (CBS 251.75T). C. O. senegalensis (CBS 728.84T). D. O. medusarum (CBS 148.67T). E. O. unipora (CBS 109.83T). Asci: F. O. pseudomollicella (CBS 251.75T). G. O. medusarum (CBS 148.67T). H. O. senegalensis (CBS 728.84T). I. O. unipora (CBS 109.83T). Scale bars: A–I = 10 μm.
Etymology: Name refers to ovate to broad ovate ascospores of all the known species in this genus.
Type species: Ovatospora brasiliensis (Batista & Pontual) (= Ch. brasiliense).
Ascomata superficial, ostiolate, subglobose or ovate with brown walls of textura angularis in surface view. Terminal hairs usually coiled, sometimes with coiled branches. Lateral hairs flexuous. Asci fasciculate, cylindrical with 8 uniseriate ascospores, or clavate with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores brown when mature, broadly ovate, bilaterally flattened, rounded at one end, with an apical germ pore at another attenuate or apiculate end. Asexual morph unknown.
Ovatospora brasiliensis (Batista & Pontual) X. Wei Wang & Samson, comb. nov. MycoBank MB818851. Fig. 45.
Fig. 45.
Ovatospora brasiliensis (CBS 140.50). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Upper part of a terminal ascomatal hair. H. Basal part of terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–E = 100 μm; F–J = 10 μm.
Basionym: Chaetomium brasiliense Batista & Pontual, Bol. Agr. Com. Pernambuco 15: 70. 1948.
Ascomata superficial, pale olivaceous grey to mouse grey in reflected light due to ascomatal hairs, globose or subglobose, ostiolate, 85–135 μm high, 75–110 μm diam. Ascomatal wall brown, textura angularis, sometimes mixed with textura intricata in surface view. Terminal hairs undulate to loosely coiled with erect or flexuous lower part, conspicuously rough (granulate), greyish sepia to brown, septate, 2–3.5 μm diam in the undulate or coiled upper portion. Lateral hairs flexuous, tapering and fading towards the tips. Asci fasciculate, cylindrical, spore-bearing part 35–45 × 5–7.5 μm, stalks 8–18 μm long, with 8 uniseriate ascospores, evanescent before ascospores become mature. Ascospores olivaceous brown when mature, ovate, bilaterally flattened, (6.5–)7–7.5(–8) × (5.5–)6–6.5(–7) × (4.5–)5–5.5(–6) μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire or slightly undulate edge, about 41–47 mm diam in 7 d at 25 °C, usually irregularly concentric because of the concentric formation of ascomata between the floccose rings consisting of pale grey to pale olivaceous grey or honey-coloured aerial mycelium, with pigmented hyphae immersed in medium, and with dark exudates diffusing into the medium, reverse black. Colonies on PCA translucent, with entire edge, about 36–42 mm diam in 7 d at 25 °C, with olivaceous buff and floccose aerial hyphae mixed with ascomata, without coloured exudates; reverse olivaceous or olivaceous grey. Colonies on MEA with slightly undulate edge, about 32–38 mm diam in 7 d at 25 °C, with floccose, concentric and buff to white aerial mycelium, with pigmented hyphae immersed in medium, without coloured exudates, reverse black with pale edge. Colony growth on DG18 with entire edge, about 16–22 mm diam in 7 d at 25 °C, with white aerial mycelium appearing irregularly petaloid texture, without coloured exudates; reverse uncoloured.
Specimen examined: India, Calcutta, isolated from moist jute cloth by S.N. Basu, culture CBS 140.50.
Notes: Ovatospora brasiliensis formed a sister lineage to O. mollicella and can be distinguished by producing zonate colonies and smaller ascospores (7–7.5 × 6–6.5 × 5–5.5 vs. 8–9.5 × 7–8 × 6–7 μm, von Arx et al. 1986). No ex-type culture is available. Typification of this species awaits obtaining a culture or isolate morphologically consistent with the holotype.
Ovatospora pseudomollicella X. Wei Wang & Samson, sp. nov. MycoBank MB818852. Fig. 46.
Fig. 46.
Ovatospora pseudomollicella (CBS 251.75, ex-type culture). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B–C. Mature ascomata on OA, top view; D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Upper parts of terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E–F = 100 μm; G–J = 10 μm.
Etymology: Name refers to the similarity to Ovatospora mollicella (= Ch. mollicellum).
Ascomata superficial, pale smoke grey to pale olivaceous grey in reflected light due to ascomatal hairs, subglobose to ovate, ostiolate, 100–135 μm high, 80–120 μm diam. Ascomatal wall brown, textura angularis in surface view. Terminal hairs loosely coiled to regularly coiled with erect or flexuous lower part, conspicuously rough (granulate), greyish sepia to brown, septate, 1.5–2.5 μm diam in the undulate or coiled upper portion. Lateral hairs flexuous or undulate. Asci fasciculate, cylindrical, spore-bearing part 40–51 × 6.5–8.5 μm, stalks 7–20 μm long, with 8 uniseriate ascospores, evanescent before ascospores become mature. Ascospores olivaceous brown when mature, ovate, bilaterally flattened, (7–)7.5–8.5(–9) × (5.5–)6–7.5(–8) × 5–6(–7) μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: Colonies on OA with entire edge, about 43–49 mm diam in 7 d at 25 °C, with a floccose, white to pale grey mycelium, often with one or a few irregular concentric rings, with pigmented hyphae immersed in medium, and with mouse grey to black exudates diffusing into the medium, reverse black. Colonies on PCA with entire edge, about 33–39 mm diam in 7 d at 25 °C, with floccose and white to olivaceous buff aerial hyphae mixed with ascomata, without coloured exudates; reverse hazel to olivaceous. Colonies on MEA with slightly undulate edge, about 41–37 mm diam in 7 d at 25 °C, with floccose, white and irregularly petaloid rather than concentric aerial mycelium, with pigmented hyphae immersed in medium, without coloured exudates, reverse black with pale edge. Colony growth on DG18 with slightly crenated edge, about 18–24 mm diam in 7 d at 25 °C, pale vinaceous to vinaceous buff with white aerial mycelium, without coloured exudates; reverse rosy vinaceous to flesh and saffron in the centre.
Specimen examined: India, Uttar Pradesh, isolated from air by Kamal (holotype CBS H-22850, culture ex-type CBS 251.75).
Notes: Phylogenetic inference indicated that O. pseudomollicella clustered close to but separated from O. mollicella (CBS 583.83). This species can be distinguished by the size of its ascospores (7.5–8.5 × 6–7.5 × 5–6 μm), which are larger than those of O. brasiliensis (7–7.5 × 6–6.5 × 5–5.5 μm), and smaller than those of the ex-type of O. mollicella (8–9.5 × 7–8 × 6–7 μm; von Arx et al. 1986).
The previous study (Carter 1982) indicated that the species of this genus, together with the members of Arcopilus and Farrowa (Hawksworth 1975), had long stipitate ascogonial coils, while most of the other species in the Chaetomiaceae had irregular ascogonial coils. This might be worth noticing when observing the formation of young ascomata.
Subramaniula Arx, Proc. Indian Acad. Sci., Plant Sci. 94: 344. 1985.
Type species: Subramaniula thielavioides (Arx, Mukerji & N. Singh) Arx
Synonym: Achaetomium thielavioides Arx, Mukerji & N. Singh, Persoonia 10: 144. 1978.
Notes: The genus Subramaniula was originally proposed to accommodate species with urniform and nearly glabrous ascomata with a translucent wall and a wide ostiole surrounded by a hyaline collar (von Arx 1985b). A recent study (Ahmed et al. 2016) indicated the close relationships between S. thielavioides and several chaetomium-like species. The phylogenetic analyses in this study confirmed their results. These data greatly expanded the number of species in this genus. Subramaniula remains to be redescribed, and the description of one species from the indoor environment is given below.
Subramaniula cristata (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818853. Fig. 47.
Fig. 47.
Subramaniula cristata (DTO 324-H8). A. Colonies from left to right on OA, PCA, MEA and DG18 after 7 d incubation. B. Mature ascomata on OA, top view; C–E. Mature ascomata on OA, side view. F–H. Ascomata mounted in lactic acid. I. Structure of ascomatal wall in surface view. J. Upper part of a terminal ascomatal hair. K. Asci. L. Ascospores. Scale bars: F–H = 100 μm; I–L = 10 μm.
Basionym: Chaetomium cristatum Ames, Mycologia 41: 639. 1949.
Ascomata superficial, ostiolate, pale olivaceous grey or pale mouse grey to mouse grey in reflected light owing to ascomatal hairs, subglobose or ovate, 240–390 μm high, 200–300 μm diam. Ascomatal wall brown, textura angularis in surface view. Terminal hairs erect, finely warty, dark brown, 4.5–5.5 μm diam near the base, apically irregularly-curved and branched repeatedly, forming a network consisting of thinner (1.5–2.3 μm diam), olivaceous or fawn, flexuous or undulate branches. Lateral hairs brown, seta-like, fading and tapering towards the tips. Asci fasciculate, fusiform or clavate, spore-bearing part 23–40 × 9–12 μm, stalks 12–30 μm long, with 8 irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal-fusiform, (9–)9.5–10.5(–11) × (5–)5.5–6.5(–7) μm, with a subapical germ pore. Asexual morph absent.
Culture characteristics: Colonies on OA with an entire edge, about 34–40 mm diam in 7 d at 25 °C, without aerial hyphae, with smoke grey to olivaceous grey exudates diffusing into the medium, reverse pale olivaceous grey to olivaceous. Colonies on PCA translucent, with numerous vinaceous buff ascomata in the central area and a slightly lobate edge, about 33–39 mm diam in 7 d at 25 °C, without aerial hyphae, without coloured exudates; reverse uncoloured. Colonies on MEA pale vinaceous with brick perisphere, about 18–24 mm diam in 7 d at 25 °C, non-sporulating, reverse vinaceous with salmon edge. Colonies on DG18 with an entire or slightly undulate edge, about 3–9 mm diam in 7 d at 25 °C, buff and slightly floccose, non-sporulating, without coloured exudates; reverse uncoloured.
Specimens examined: China, Beijing, isolated from air by A.J. Chen, cultures DTO 324-H7 and DTO 324-H8.
Notes: Subramaniula cristata morphologically fits with S. cuniculorum (Ch. cuniculorum). Ames (1963) synonymised this species to Ch. cuniculorum. This decision was followed by von Arx et al. (1986). The phylogenetic analysis indicated that our indoor isolates clustered with the ex-type of S. cristata. They were close to but separated from the reference isolate of S. cuniculorum. Subramaniula cuniculorum was originally collected from Germany in 1869. No ex-type culture of S. cuniculorum is available now. The reference isolate CBS 800.83 was from Spain. Typification of S. cuniculorum and distinguishing this species from S. cristata await comparison with the holotype of S. cuniculorum.
Additional new combinations from non-indoor environments:
Amesia gelasinospora (Aue & Müller) X. Wei Wang & Samson, comb. nov. MycoBank MB818854.
Basionym: Chaetomium gelasinosporum Aue & Müller, Ber Schweiz. Bot. Ges. 77:193. 1967.
Arcopilus aureus (Chivers) X. Wei Wang & Samson, comb. nov. MycoBank MB818855.
Basionym: Chaetomium aureum Chivers, Proc. Am. Acad. Arts Sci. 48: 87. 1912
Arcopilus cupreus (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818856.
Basionym: Chaetomium cupreum Ames, Mycologia 41: 642. 1949.
Arcopilus fusiformis (Chivers) X. Wei Wang & Samson, comb. nov. MycoBank MB818857.
Basionym: Chaetomium fusiforme Chivers, Proc. Am. Acad. Arts Sci. 48: 87. 1912.
Arcopilus flavigenus (van Warmelo) X. Wei Wang & Samson, comb. nov. MycoBank MB818858.
Basionym: Chaetomium flavigenum van Warmelo, Mycologia 58: 847. 1966.
Botryotrichum spirotrichum (R.K. Benjamin) X. Wei Wang & Samson, comb. nov. MycoBank MB818860.
Basionym: Magnusia spirotricha R.K. Benjamin, Aliso 3: 199. 1955.
Synonyms: Kernia spirotricha (R.K. Benjamin) Aliso 3: 344. 1956
Chaetomidium spirotricha (R.K. Benjamin) Malloch & Cain, Canad. J. Bot. 49: 867. 1971.
Thielavia spirotricha (R.K. Benjamin) Malloch & Cain, Mycologia 65: 1069. 1973.
Emilmuelleria spirotricha (R.K. Benjamin) Arx, Sydowia 38: 6. 1985.
Collariella quadrangulata (Chivers) X. Wei Wang & Samson, comb. nov. MycoBank MB818861.
Basionym: Chaetomium quadrangulatum Chivers, Proc. Am. Acad. Arts Sci. 48: 85. 1912.
Collariella robusta (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818872.
Basionym: Chaetomium robustum Ames, Monograph of the Chaetomiaceae: 35. 1963.
Collariella virescens (Arx) X. Wei Wang & Samson, comb. nov. MycoBank MB819488.
Basionym: Achaetomiella virescens Arx, Genera of Fungi: 247. 1970.
Synonym: Chaetomium virescens (Arx) Udagawa, Trans. Mycol. Soc. Japan 21: 34. 1980.
Dichotomopilus dolichotrichus (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818866.
Basionym: Chaetomium dolichotrichum Ames, Mycologia 37: 145. 1945.
Dichotomopilus erectus (Skolko & J.W. Groves) X. Wei Wang & Samson, comb. nov. MycoBank MB818867.
Basionym: Chaetomium erectum Skolko & J.W. Groves, Canad. J. Res., Section C 26: 277. 1948.
Dichotomopilus fusus (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818868.
Basionym: Chaetomium fusum Ames, Monograph of the Chaetomiaceae: 25. 1963.
Dichotomopilus ramosissimus (X.W. Wang & L. Cai) X. Wei Wang & Samson, comb. nov. MycoBank MB818869.
Basionym: Chaetomium ramosissimum X.W. Wang & L. Cai, Mycol. Prog. 13: 725. 2014.
Dichotomopilus reflexus (Skolko & J.W. Groves) X. Wei Wang & Samson, comb. nov. MycoBank MB818870.
Basionym: Chaetomium reflexum Skolko & J.W. Groves, Canad. J. Res., Section C 26: 279. 1948.
Ovatospora medusarum (Meyer & Lanneau) X. Wei Wang & Samson, comb. nov. MycoBank MB818871.
Basionym: Chaetomium medusarum Meyer & Lanneau, Bull. Soc. Mycol. Fr. 83: 318. 1967.
Ovatospora mollicella (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818873.
Basionym: Chaetomium mollicellum Ames, Monograph of the Chaetomiaceae: 30. 1963.
Ovatospora senegalensis (Ames) X. Wei Wang & Samson, comb. nov. MycoBank MB818874.
Basionym: Chaetomium senegalense Ames, Monograph of the Chaetomiaceae: 36. 1963.
Ovatospora unipora (Aue & Müller) X. Wei Wang & Samson, comb. nov. MycoBank MB818875.
Basionym: Chaetomium uniporum Aue & Müller, Ber Schweiz. Bot. Ges. 77: 187. 1967.
Subramaniula anamorphosa (S.A. Ahmed, et al.) X. Wei Wang & Samson, comb. nov. MycoBank MB818876.
Basionym: Chaetomium anamorphosum S.A. Ahmed, et al., Fungal Diversity 76: 18. 2016.
Subramaniula cuniculorum (Fuckel) X. Wei Wang & Samson, comb. nov. MycoBank MB818877.
Basionym: Chaetomium cuniculorum Fuckel, Symb. Mycol.: 89. 1869.
Subramaniula flavipila X. Wei Wang & Samson, nom. nov. MycoBank MB818878.
Basionym: Chaetomium irregulare Sörgel, Nova Hedwigia 12: 386. 1966.
Etymology: Name refers to the yellow to luteous ascomatal hairs of this fungus.
Notes: Non Subramaniula irregularis P. Cannon & D. Hawksworth, Trans. Br. Mycol. Soc. 87: 56. 1986. The name S. irregularis already exists, therefore the new name is proposed.
Subramaniula fusispora (G. Smith) X. Wei Wang & Samson, comb. nov. MycoBank MB818879.
Basionym: Chaetomium fusisporum G. Smith, Trans. Br. Mycol. Soc. 44: 46. 1961.
Key to the most common Chaetomiaceae species (or species complex) from the indoor environments
-
1.
Asexual morph present ........... 2
-
1.
Asexual morph absent .................. 3
-
2.
Ascomata absent or rare; conidiophores usually produced together with brown serile setae; conidia subglobose, usually hyaline, smooth to slightly roughened, 11–17.5 μm diam ................ Botryotrichum piluliferum
-
2.
Ascomata numerous, terminal ascomatal hairs repeatedly dichotomously branched, usually less than 5 μm diam near the base; Ascospores limoniform, bilaterally flattened, 11.5–14 × 8.5–10 × 7–8 μm, with an apical germ pore asexual morphs acremonium-like ..................... Chaetomium elatum
-
3.
Terminal ascomatal hairs repeatedly dichotomously branched or seta-like, often composed of two types of hairs different in length; ascospores ovate to elongate ovate, bilaterally flattened, usually less than 7.5 μm long (Dichotomopilus indicus or D. funicola species complex (including D. funicola, D. pseudofunicola, D. subfunicola and D. variostiolatus) ........... 4
-
3.
Terminal ascomatal hairs not repeatedly dichotomously branched, ascospores limoniform, bilaterally flattened, with an apical germ pore .......................... 5
-
4.
Shorter terminal hairs, if present, usually forming a net structure to enfold ascospres ........... D. funicola species complex
-
4.
Shorter terminal hairs, if present, inconspicuous due to the masses of ascospores, not easy to observe ............. Dichotomopilus indicus
-
5.
Ascomata subglobose to obovate with a dark collar-like apex around the ostiolar pore; terminal hairs spirally coiled, ascospores limoniform, bilaterally flattened, 6–7 × 5.5–6.5 × 4.5–5.5 μm, with an apical germ pore ........ Collariella bostrychodes
-
5.
Ascomata without a dark collar-like apex around the ostiolar pore; ascospores 8.5–11 × 7–9 × 5.5–7 μm ......... 5
-
6.
Terminal hairs flexuous, undulate to slightly coiled ......... Chaetomium cochliodes
-
6.
Terminal hairs spirally coiled, usually with coils regularly tapering in diameter .............. Chaetomium globosum
Discussion
Chaetomium spp., together with several other genera such as Stachybotrys, Aspergillus and Penicillium, are of concern because of their ability to grow in the indoor environment and their association with production of bioactive metabolites (Brasel et al., 2005, Samson et al., 2010, Andersen et al., 2016, Došen et al., 2016). Depending on the type of water damaged building material, Chaetomium sensu lato are found in 16–66 % of environmental samples (Flannigan and Miller, 2011, Andersen et al., 2016). Our survey shows a high species diversity of Chaetomiaceae in the indoor environments worldwide and based on phylogenetic analysis and morphological examination 30 indoor species lineages were recognised. These species were scattered throughout the Chaetomiaceae and this finding prompted us to re-evaluate the phylogeny of Chaetomium and related genera.
Traditionally, Chaetomium was defined to possess superficial and typically ostiolate ascomata covered by more or less developed hairs or setae and connected to the substrate by rhizoidal hyphae. The asci develop in basal fascicles, are stalked and have a thin and evanescent wall devoid of apical structures. The majority of species produce asci containing eight ascospores, which are single-celled, smooth and pigmented with germ pores and without sheaths (von Arx et al. 1986). This generic concept covers quite a high diversity of morphology. For example, ascomatal hairs can be straight (seta-like), flexuous, arcuate, undulate, circinate, spirally coiled or variously branched, the asci obovate, clavate, fusiform to cylindrical, and the ascospores oblate, ovate, limoniform, fusiform, lunate, triangular to irregular, laterally flattened or not, with one, two or more germ pores which can be apically, sub-apically or even laterally formed on ascospores. Most species do not produce an asexual morph, while some species have a humicola-like, botryotrichum-like, or acremonium-like asexual state. Contrary to the genus Chaetomium, the concepts of some other genera were quite narrow. For example, Emilmuelleria was characterised by its distinct tufts of spirally-coiled ascomatal appendages arranged usually at the opposite sides of non-ostiolate ascomata (von Arx 1985a); Subramaniula was distinguished from Chaetomium by its urniform and nearly glabrous ascomata with a translucent wall and a wide ostiole surrounded by a hyaline collar (von Arx, 1985b, von Arx et al., 1988). The ascospores of Myceliophthora (= Corynascus, with asexual morph) and Corynascella (asexual morph unknown) have more than one germ pore per ascospore, and other non-ostiolate genera usually produce ascospores with only one germ pore.
The concept of Chaetomium has been challenged in the study of the non-ostiolate genus Chaetomidium (Greif et al., 2009, Wang et al., 2016). Based on a six-locus analysis, three species of Chaetomidium, including its type C. fimeti, are closely related to the type species of Chaetomium, C. globosum. As these three Chaetomidium species are all within the C. globosum species complex, the genus Chaetomidium was rejected and the three non-ostiolate species were transferred into the genus Chaetomium (Wang et al. 2016).
The phylogenetic inference in this study clearly demonstrated that the traditionally defined Chaetomium as noted above was polyphyletic. The indoor members of Chaetomium were segregated into 10 monophyletic lineages by several other genera in the family (Fig. 1). Six indoor species fell into the C. globosum complex which was designated here as Chaetomium sensu stricto, including 36 species with limoniform to globose or irregular and bilaterally flattened ascospores as delimited in our previous study (Wang et al. 2016). Chaetomium murorum clustered with three asexual species of Botryotrichum and non-ostiolate Emilmuelleria in a strongly-supported monophyletic clade, which was designated here as the expanded genus Botryotrichum. This result also refuted the presence or absence of ascomatal ostioles to be a criterion for distinguishing genera in the Chaetomiaceae. Species of the traditional genus Subramaniula grouped with several species previously classified in Chaetomium, for example S. cristata (= C. cristatum), S. cuniculorum (= C. cuniculorum), S. fusispora (C. fusisporum) and S. flavipila (= C. irregular). Based on these data, the genus Subramaniula was expanded to include species with typical chaetomium-like ascomata and ascomatal hairs. More data are needed to determine the morphological delimitation of the genera Botryotrichum and Subramaniula supported by the molecular data in this study.
Two taxa obtained from the indoor environment produced humicola-like conidia. Based on a preliminary analysis of ITS sequences of asexual Humicola species, only a few species clustered together with H. fuscoatra, the type species of this genus. The other Humicola species available were placed in several different clades, indicating that the asexual genus Humicola is polyphyletic. More research is needed to clarify the relationship of Humicola sensu stricto to the other humicola-like species as well as those chaetomium-like species with humicola-like asexual morph. The indoor species Me. tardus was on a long branch within the highly supported Melanocarpus clade. Further research is required to determine the phylogenetic relationships of the species with in this genus.
Representatives of the genera Achaetomium, Myceliophthora, Thielavia, and Corynascella were included in our phylogenetic study. These data contributed to the delimitation of genera in Chaetomiaceae and the introduction of five new genera. The genera Achaetomium and Myceliophthora were strongly supported as monophyletic lineages in this study. Thielavia was known as the second largest genus in the family (von Arx et al., 1988, Kirk et al., 2008). Five species of Thielavia were included here in our analysis to represent this genus. The phylogeny confirmed the monophyly of this Thielavia clade. Further research with a larger sampling is required to re-evaluate this genus in more detail. Corynascella was represented only by its type species. Further data are required to determine the phylogenetic relationships between the type species and the other species of Corynascella.
Our results provided an insight in the phylogeny of the family, and in the diversity of indoor Chaetomiaceae (Table 2). Of the 145 isolates obtained in this survey, 30 species were recognised in 10 different genera, presenting a much higher species diversity of indoor Chaetomiaceae than previous studies. Chaetomium globosum was the predominant species in the indoor environments as also noted in the previous studies (Vesper et al., 2007, Ayanbimpe et al., 2010, Straus, 2011, McMullin et al., 2013, Miller and McMullin, 2014). This species accounted for 51 % of the total obtained isolates (74/145). Chaetomium cochliodes is the second predominant species (17/145), mainly because of its abundance in the indoor environments in the USA. In addition, Ch. elatum (6/145) and B. piluliferum (5/145) were also frequently encountered in samples obtained from indoor environments. At the genus level, members of Chaetomium sensu stricto accounted for 69 % of the total isolates obtained (100 isolates in six species). Dichotomopilus was found to be the second most common genus (15 isolates, seven species), followed by Collariella (seven isolates, four species), Botryotrichum (eight isolates, three species), Amesia (five isolates, three species) and Humicola (four isolates, two species). Members of the four other genera were found to be rare in the indoor environments.
Secondary metabolites have been used as valuable additional taxonomic features in several fungal genera such as Penicillium, Aspergillus, Alternaria, Fusarium (Frisvad et al. 2008). It has been known that, in order to adapt to diverse environments, the species of Chaetomium sensu lato are capable of producing various secondary metabolites, which display a wide range of biological activities (Udagawa et al., 1979, Sekita et al., 1981, Ding et al., 2006, Ge et al., 2008, Momesso et al., 2008, Phonkerd et al., 2008, Kharwar et al., 2011, Yamada et al., 2012, Zhang et al., 2012, Lu et al., 2013, Awad et al., 2014, Yan et al., 2014). Some of them are mycotoxins and can cause health hazards (Polizzi et al., 2009, Mason et al., 2010, Andersen et al., 2011, Miller and McMullin, 2014, Wang et al., 2015). In this study profiles of metabolites were analysed in the majority of indoor chaetomium-like species using advanced analytical chemical methods to ensure correct identity of the secondary metabolites. The resulting metabolite profiling shows that it is possible to distinguish Chaetomium sensu stricto and Dichotomopilus from each other and from other genera based on secondary metabolites. It is even possible to discriminate between C. globosum and other species based on the pattern of metabolites. Chaetomium globosum seems to be the only species that produces the combination of chaetoviridin A, chaetoglobosin A, rotiorinol and cochliodones, however, chemical analyses of more isolates from the other closely related Chaetomium species are necessary to verify the result. Furthermore, chaetoviridin A, chaetoglobosin A and cochliodones are the major metabolites produced on building materials with Chaetomium growth (Došen et al. in press). Likewise, analyses of more isolates of C. cochloides are needed to determine which metabolite pattern that is species specific in order to distinguish it from C. pseudofimeti. Production of sterigmatocystin, the precursor to aflatoxin, was only found in H. olivacea in this study. Another study by Rank et al. (2011) did detect sterigmatocystin in B. piluliferum (NRRL 38180), but it was not detected in the B. piluliferum isolate (DTO 254-B8) used in this study. Also in this case an extended analysis of isolates is needed. As only a limited number of isolates and species in the Chaetomiaceae were analysed for their metabolite profile in general, more research is encouraged in order to understand the correlation between the studied taxa, their metabolite profiles and the effects of these products on human health.
In this study we also showed the morphological diversity that exists in some species. Taken together with the morphological data from our earlier studies (Wang et al., 2014, Wang et al., 2016), we show that Ch. globosum, Ch. elatum, B. murorum, the D. funicola species complex (including D. funicola, D. pseudofunicola, D. subfunicola and D. variostiolatus) and D. indicus all exhibited intra-species morphological variation. For the D. funicola species complex, almost each of the examined isolates presented different morphology in the terminal ascomatal hairs. A similar problem in morphological identification also occurs in D. indicus. The examined isolates of this species showed morphological differences not only in the terminal ascomatal hairs, but sometimes even in the shape and size of ascospores. These results implied an active differentiation of morphology within these species lineages. This makes it very complicated to identify these species only based on morphology. A molecular based identification will help to solve this problem. Several Chaeotmiaceae species share ITS sequences, and for routine identification, β-tubulin (tub2) is recommended. On the other hand, it needs to be noted that not all species are represented with tub2 sequences in the public databases and future studies should aim to complete this omission.
It has been demonstrated that the presence of C. globosum in the indoor environment is one of the important contributors to the development of symptoms of rhinitis, asthma and other health problems (Vesper et al., 2007, Apetrei et al., 2009, Polizzi et al., 2009, Mason et al., 2010, Miller and McMullin, 2014). This species is also the most common human pathogen mainly associated with onychomycosis (Naidu et al., 1991, Stiller et al., 1992, Aspiroz et al., 2007, Latha et al., 2010, Tullio et al., 2010, Hwang et al., 2012, Lagacé and Cellier, 2012, Kim et al., 2013). In addition, some other indoor chaetomium-like species were also reported in clinical cases, such as D. funicola (= C. funicola, Koch & Haneke 1965), O. brasiliensis (= C. brasiliense, Hubka et al. 2011) and Am. atrobrunnea (= C. atrobrunneum, Guppy et al., 1998, de Hoog et al., 2013). These species, which grow in our living and working environment, deserve more attention in future.
Acknowledgements
The authors are very grateful to Dr Konstanze Bensch for her kind recommendation of names for the novel taxa. We acknowledge the culture collection of the CBS-KNAW for providing cultures. We would also like to think Dr Keith Seifert for providing data used in identification of Humicola species. This work was mainly supported by the Alfred Sloan Foundation Programme on the Microbiology of the Built Environment (Grant No. G-2014-14529), partly supported by the National Natural Science Foundation of China (Project No. 30570007), the Ministry of Science and Technology of P.R. China (No. 2006FY120100) and the VILLUM foundation.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Contributor Information
X.W. Wang, Email: x.wang@cbs.knaw.nl.
R.A. Samson, Email: r.samson@cbs.knaw.nl.
References
- Abbott S.P., Sigler L., McAleer R. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. Journal of Clinical Microbiology. 1995;33:2692–2698. doi: 10.1128/jcm.33.10.2692-2698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.A., Khan Z., Wang X.W. Chaetomium-like fungi causing opportunistic infections in humans: a possible role for extremotolerance. Fungal Diversity. 2016;76:11–26. [Google Scholar]
- Ames L.M. 1963. A monograph of the Chaetomiaceae. U.S. Army Research and Development, Series 2. [Google Scholar]
- Amend A.S., Seifert K.A., Samson R. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13748–13753. doi: 10.1073/pnas.1000454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B., Došen I., Lewinska A.M. Pre-contamination of new gypsum wallboard with potentially harmful fungal species. Indoor Air. 2016 doi: 10.1111/ina.12298. In press. [DOI] [PubMed] [Google Scholar]
- Andersen B., Frisvad J.C., Søndergaard I. Associations between fungal species and water-damaged building materials. Applied and Environmental Microbiology. 2011;77:4180–4188. doi: 10.1128/AEM.02513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei I.C., Draganesc G.E., Popescu I.T. Possible cause of allergy for the librarians: books manipulation and ventilation as sources of fungus spores spreading. Aerobiologia. 2009;25:159–166. [Google Scholar]
- Asgari B., Zare R. The genus Chaetomium in Iran, a phylogenetic study including six new species. Mycologia. 2011;103:863–882. doi: 10.3852/10-349. [DOI] [PubMed] [Google Scholar]
- Aspiroz C., Gene J., Rezusta A. First Spanish case of onychomycosis caused by Chaetomium globosum. Medical Mycology. 2007;45:279–282. doi: 10.1080/13693780601164280. [DOI] [PubMed] [Google Scholar]
- Awad N.E., Kassem H.A., Hamed M.A. Bioassays guided isolation of compounds from Chaetomium globosum. Journal de Mycologie Médicale. 2014;24:e35–e42. doi: 10.1016/j.mycmed.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Ayanbimpe G.M., Wapwera S.D., Kuchin D. Indoor air mycoflora of residential dwellings in Jos metropolis. African Health Sciences. 2010;10:172–176. [PMC free article] [PubMed] [Google Scholar]
- Barron M.A., Sutton D.A., Veve R. Invasive mycotic infections caused by Chaetomium perlucidum, a new agent of cerebral phaeohyphomycosis. Journal of Clinical Microbiology. 2003;41:5302–5307. doi: 10.1128/JCM.41.11.5302-5307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldi M de. New species of Humicola: an approach to genetic and biochemical classification. Canadian Journal of Botany. 1976;54:2755–2768. [Google Scholar]
- Brasel T.L., Martin J.M., Carriker C.G. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Applied and Environmental Microbiology. 2005;71:7376–7388. doi: 10.1128/AEM.71.11.7376-7388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain R.F. Studies of soil fungi III. New species of Coniochaeta, Chaetomidium, and Thielavia. Canadian Journal of Botany. 1961;39:1231–1239. [Google Scholar]
- Carter A. University of Toronto; Canada: 1982. A taxonomyic study of the ascomycete genus Chaetomium Kunze. Ph.D. dissertation. [Google Scholar]
- Carter A. Three new species in the genus Chaetomium. Canadian Journal of Botany. 1983;61:2603–2607. [Google Scholar]
- Chivers A.H. A monograph of the genera Chaetomium and Ascotricha. Memoirs of the Torrey Botanical Club. 1915;14:155–240. [Google Scholar]
- Corda A.C.J. 1840. Icones fungorum hucusque cognitorum. Vol. 4. Praha. [Google Scholar]
- Cunningham C.W. Can three incongruency tests predict when data should be combined? Molecular Biology and Evolution. 1997;14:733–740. doi: 10.1093/oxfordjournals.molbev.a025813. [DOI] [PubMed] [Google Scholar]
- Daniels J. Chaetomium piluliferum sp. nov., the perfect state of Botryotrichum piluliferum. Transactions of the British Mycological Society. 1961;44:79–86. [Google Scholar]
- de Hoog G.S., Ahmed S.A., Najafzadeh M.J. Phylogenetic findings suggest possible new habitat and routes of infection of human eumyctoma. PLoS Neglected Tropical Diseases. 2013;7(5):e2229. doi: 10.1371/journal.pntd.0002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G., Song Y.C., Chen J.R. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. Journal of Natural Products. 2006;69:302–304. doi: 10.1021/np050515+. [DOI] [PubMed] [Google Scholar]
- Došen I., Nielsen K.F., Clausen G. Potentially harmful secondary metabolites produced by indoor Chaetomium species on artificially and naturally contaminated building materials. Indoor Air. 2016 doi: 10.1111/ina.12290. In press. [DOI] [PubMed] [Google Scholar]
- Dreyfuss M. Taxonomische Untersuchungen innerhalb der Gattung Chaetomium. Sydowia. 1976;28:50–133. [Google Scholar]
- Flannigan B., Miller J.D. Microbial growth in indoor environments. In: Flannigan B., Samson R.A., Miller J.D., editors. Microorganisms in home and indoor work environments. diversity, health impacts, investigation and control. 2nd edn. CRC Press; Boca Raton, FL: 2011. pp. 57–107. [Google Scholar]
- Frisvad J.C., Andersen B., Thrane U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycological Research. 2008;112:231–240. doi: 10.1016/j.mycres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Ge H.M., Zhang W.Y., Ding G. Chaetoglobins A and B, Two unusual alkaloids from endophytic Chaetomium globosum culture. Chemical Communications. 2008;45:5978–5980. doi: 10.1039/b812144c. [DOI] [PubMed] [Google Scholar]
- Gonianakis M., Neonakis I., Darivianaki E. Airborne Ascomycotina on the island of Crete: seasonal patterns based on an 8-year volumetric survey. Aerobiologia. 2005;21:69–74. [Google Scholar]
- Greif M.D., Stchigel A.M., Huhndorf S.M. A re-evaluation of genus Chaetomidium based on molecular and morphological characters. Mycologia. 2009;101:554–564. doi: 10.3852/08-200. [DOI] [PubMed] [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueidan C., Roux C., Lutzoni F. Using multigene phylogeny analysis to assess generic delineation and character evolution in Verrucariaceae (Verrucariales, Ascomycota) Mycological Research. 2007;111:1145–1168. doi: 10.1016/j.mycres.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Guppy K.H., Thomas C., Thomas K. Cerebral fungal infections in the immunocompromised host: A literature review and a new pathogen – Chaetomium atrobrunneum. Neurosurgery (Baltimore) 1998;43:1463–1469. doi: 10.1097/00006123-199812000-00122. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hambleton S., Nickerson N.L., Seifert K.A. Leohumicola, a new genus of heat-resistant hyphomycetes. Studies in Mycology. 2005;53:29–52. [Google Scholar]
- Hawksworth D.L. Farrowia, a new genus in the Chaetomiaceae. Persoonia. 1975;8:167–185. [Google Scholar]
- Hubka V., Mencl K., Skorepova M. Phaeohyphomycosis and onychomycosis due to Chaetomium spp., including the first report of Chaetomium brasiliense infection. Medical Mycology. 2011;49:724–733. doi: 10.3109/13693786.2011.572299. [DOI] [PubMed] [Google Scholar]
- Hoppin E.C., McCoy E.L., Rinaldi M.G. Opportunistic mycotic infection caused by Chaetomium in a patient with acute leukemia. Cancer. 1983;52:555–556. doi: 10.1002/1097-0142(19830801)52:3<555::aid-cncr2820520328>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hwang S.M., Suh M.K., Ha G.Y. Onychomycosis due to nondermatophytic molds. Annals of Dermatology. 2012;24:175–180. doi: 10.5021/ad.2012.24.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharwar R.N., Mishra A., Gond S.K. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Natural Product Reports. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- Kildgård S., Månsson M., Došen I. Accurate dereplication of bioactive secondary metabolites from marine-derived fungi by UHPLC-DAD-QTOFMS and a MS/HRMS Library. Marine Drugs. 2014;12:3681–3705. doi: 10.3390/md12063681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.M., Lee M.H., Suh M.K. Onychomycosis caused by Chaetomium globosum. Annals of Dermatology. 2013;25:232–236. doi: 10.5021/ad.2013.25.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W. 10th edn. CABI; UK: 2008. Ainsworth & Bisby’s Dictionary of the Fungi; pp. 1–771. [Google Scholar]
- Koch H.A., Haneke H. Chaetomium funicolum Cooke als moglicher Erreger einer tiefen Mykose. Mkosen. 1965;9:23–28. [Google Scholar]
- Kunze G., Schmidt J.K. Vol. 1. Germany; Leipzig: 1817. Mykologische Hefte. [Google Scholar]
- Lagacé J., Cellier E. A case report of a mixed Chaetomium globosum/Trichophyton mentagrophytes onychomycosis. Medical Mycology Case Reports. 2012;1:76–78. doi: 10.1016/j.mmcr.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latha R., Sasikala R., Muruganandam N. Onychomycosis due to ascomycete Chaetomium globosum: a case report. Indian Journal of Pathology and Microbiology. 2010;53:566–567. doi: 10.4103/0377-4929.68279. [DOI] [PubMed] [Google Scholar]
- Li J., Zhao X.M., Wang X.W. Growth temperature of Chaetomium species and its taxonomic value. Mycosystema. 2012;31:213–222. [Google Scholar]
- Lodha B.C. Studies on coprophilous fungi I. Chaetomium. The Journal of the Indian Botanical Society. 1964;43:121–140. [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Phylogeny and systematics of the genus Calonectria. Studies in Mycology. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.Y., Zhang Y.S., Li L. Chaetochromones A and B, two new polyketides from the fungus Chaetomium indicum (CBS.860.68) Molecules. 2013;18:10944–10952. doi: 10.3390/molecules180910944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason-Gamer R., Kellogg E. Testing for phylogenetic conflict among molecular datasets in the tribe Tiriceae (Graminae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- Mason S., Cortes D., Horner W.E. Detection of gaseous effluents and by-products of fungal growth that affect environments (RP-1243) HVAC & R Research. 2010;16:109–121. [Google Scholar]
- Mazzucchetti G. Microfungi della cellulosa eddla carta attivita’s e inquadramento sistematico – Il genere “Chaetomium”. Pubblicazioni Dell’ ente nazionale Per La Cellulosa e Per La Carta, Roma. 1965 [Google Scholar]
- McMullin D.R., Sumarah M.W., Miller J.D. Chaetoglobosins and azaphilones produced by Canadian strains of Chaetomium globosum isolated from the indoor environment. Mycotoxin Research. 2013;29:47–54. doi: 10.1007/s12550-012-0144-9. [DOI] [PubMed] [Google Scholar]
- Millner P.D. Radial growth responses to temperature by 58 Chaetomium species, and some taxonomic relationships. Mycologia. 1977;69:492–502. [Google Scholar]
- Millner P.D., Motta J.J., Lentz P.L. Ascospores, germ pores, ultrastructure, and thermophilism of Chaetomium. Mycologia. 1977;69:720–733. [Google Scholar]
- Miller A.N., Huhndorf S.M. Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi) Molecular Phylogenetics and Evolution. 2005;35:60–75. doi: 10.1016/j.ympev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Miller J.D., McMullin D.R. Fungal secondary metabolites as harmful indoor air contaminants: 10 years on. Applied Microbiology and Biotechnology. 2014;98:9953–9966. doi: 10.1007/s00253-014-6178-5. [DOI] [PubMed] [Google Scholar]
- Momesso L.D., Kawano C.Y., Ribeiro P.H. Chaetoglobosins produced by Chaetomium globosum, endophytic fungus found in association with Viguiera robusta Gardn (Asteraceae) Química Nova. 2008;31:1680–1685. [Google Scholar]
- Naidu J., Singh S.M., Pouranik M. Onychomycosis caused by Chaetomium globosum Kunze. Mycopathologia. 1991;113:31–34. doi: 10.1007/BF00436384. [DOI] [PubMed] [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest v. 2. Programme distributed by the author. [Google Scholar]
- O'Donnell K. Fusarium and its near relatives. In: Reynolds R., Taylor J.W., editors. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CABI; UK: 1993. pp. 225–233. [Google Scholar]
- O'Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Phonkerd N., Kanokmedhakul S., Kanokmedhakul K. Bis-spiro-azaphilones and azaphilones from the fungi Chaetomium cochliodes VTh01 and C. cochliodes CTh05. Tetrahedron. 2008;64:9636–9645. [Google Scholar]
- Polizzi V., Delmulle B., Adams A. JEM spotlight: fungi, mycotoxins and microbial volatile organic compounds in mouldy interiors from water-damaged buildings. Journal of Environmental Monitoring. 2009;11:1849–1858. doi: 10.1039/b906856b. [DOI] [PubMed] [Google Scholar]
- Rambaut A. FigTree v. 1.3.1. 2009. http://tree.bio.ed.ac.uk/software/ Computer program and documentation distributed by the author at.
- Rank C.N., Larsen K.F., Varga T.O. Distribution of sterigmatocystin in filamentous fungi. Fungal Biology. 2011;115:406–420. doi: 10.1016/j.funbio.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Saccardo P.A. Sylloge Fungorum. 1882;1:1–768. [Google Scholar]
- Saccardo P.A. Sylloge Fungorum. 1886;4:1–807. [Google Scholar]
- Samson R.A., Flannigan B., Flannigan M.E. Elsevier; Amsterdam, The Netherlands: 1994. Health implications of fungi in indoor environments. [Google Scholar]
- Samson R.A., Houbraken J., Thrane U. CBS Laboratory Manual Series 2. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherland: 2010. Food and indoor fungi. [Google Scholar]
- Sekita S., Yoshihira K., Natori S. Mycotoxin production by Chaetomium spp. and related fungi. Canadian Journal of Microbiology. 1981;27:766–772. doi: 10.1139/m81-119. [DOI] [PubMed] [Google Scholar]
- Seth H.K. A monograph of the genus Chaetomium. Beihefte zur Nova Hedwigia. 1970;37:1–133. [Google Scholar]
- Skolko A.J., Groves J.W. Notes on seed-borne fungi V. Chaetomium species with dichotomously branched hairs. Canadian Journal of Research. 1948;26:269–280. [Google Scholar]
- Skolko A.J., Groves J.W. Notes on seed-borne fungi VII. Chaetomium. Canadian Journal of Botany. 1953;31:779–809. [Google Scholar]
- Sörgel G. Zum problem der trennung von arten bei pilzen, dargestellt am beispiel der ascomycetengattung Chaetomium. Archives of Microbiology. 1960;36:51–66. [Google Scholar]
- Stiller M.J., Rosenthal S., Summerbell R.C. Onychomycosis of the toenails caused by Chaetomium globosum. Journal of the American Academy of Dermatology. 1992;26:775–776. doi: 10.1016/s0190-9622(08)80558-0. [DOI] [PubMed] [Google Scholar]
- Straus D.C. The possible role of fungal contamination in sick building syndrome. Frontiers in Bioscience. 2011;3:562–580. doi: 10.2741/e270. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio V., Banche G., Allizond V. Non-dermatophytemoulds as skin and nail foot mycosis agents: Phoma herbarum, Chaetomium globosum and Microascus cinereus. Fungal Biology. 2010;114:345–349. doi: 10.1016/j.funbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Udagawa S., Muroi T., Kurata H. The production of chaetoglobosins, sterigmatocystin, O-methylsterigmatocystin, and chaetocin by Chaetomium spp. and related fungi. Canadian Journal of Microbiology. 1979;25:170–177. doi: 10.1139/m79-027. [DOI] [PubMed] [Google Scholar]
- Vesper S., McKinstry C., Ashley P. Quantitative PCR analysis of molds in the dust from homes of asthmatic children in North Carolina. Journal of environmental Monitoring. 2007;9:826–830. doi: 10.1039/b704359g. [DOI] [PubMed] [Google Scholar]
- Visagie C.M., Hirooka Y., Tanney J.B. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Studies in Mycology. 2014;78:63–139. doi: 10.1016/j.simyco.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arx J.A. Emilmuelleria, a new genus of Ascomycota. Sydowia. 1985;38:6–10. [Google Scholar]
- von Arx J.A. On Achaetomium and a new genus Subramaniula. Proceedings of the Indian Academy of Science (Plant Science) 1985;94:341–345. [Google Scholar]
- von Arx J.A., Dreyfuss M., Müller E. A reevaluation of Chaetomium and Chaetomiaceae. Persoonia. 1984;12:169–179. [Google Scholar]
- von Arx J.A., Figueras M.J., Guarro J. Sordariaceous ascomycetes without ascospore ejaculation. Beihefte zur Nova Hedwigia. 1988;94:1–104. [Google Scholar]
- von Arx J.A., Guarro J., Figueras M.J. The Ascomycete genus Chaetomium. Beihefte zur Nova Hedwigia. 1986;84:1–162. [Google Scholar]
- Wang M.H., Li L., Jiang T. Stereochemical determination of tetrahydropyran-substituted xanthones from fungus Chaetomium murorum. Chinese Chemical Letters. 2015;26:1507–1510. [Google Scholar]
- Wang X.W., Lombard L., Groenewald J.Z. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia. 2016;36:83–133. doi: 10.3767/003158516X689657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Wang X.L., Liu F.J. Phylogenetic assessment of Chaetomium indicum and allied species, with the introduction of three new species and epitypification of C. funicola and C. indicum. Mycological Progress. 2014;13:719–732. [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., editors. PCR protocols: a guide to methods and applications. Academic Press; New York, USA: 1990. pp. 315–322. [Google Scholar]
- WHO . World Health Organization Regional Office for Europe; Copenhagen: 2009. WHO guidelines for indoor air quality: dampness and mould. [PubMed] [Google Scholar]
- Yamada T., Jinno M., Kikuchi T. Three new azaphilones produced by a marine fish-derived Chaetomium globosum. The Journal of Antibiotics. 2012;65:413–417. doi: 10.1038/ja.2012.40. [DOI] [PubMed] [Google Scholar]
- Yan W., Ge H.M., Wang G. Pictet-Spengler reaction-based biosynthetic machinery in fungi. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18138–18143. doi: 10.1073/pnas.1417304111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Li H.Q., Zong S.C. Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Reviews in Medical Chemistry. 2012;12:127–148. doi: 10.2174/138955712798995066. [DOI] [PubMed] [Google Scholar]
- Zopf W. Zur Entwicklungsgeschichte der Ascomyceten: Chaetomium. Nova Acta der Kaiserlich Leopoldinisch-Carolinisch Deutschen Akademie der Naturforscher. 1881;42:199–292. [Google Scholar]